Abstract
Rensch’s rule (RR) postulates that in comparisons across closely related species, male body size relative to female size increases with the average size of the species. This holds true in several vertebrate and also in certain free-living invertebrate taxa. Here, we document the validity of RR in avian lice using three families (Philopteridae, Menoponidae, and Ricinidae). Using published data on the body length of 989 louse species, subspecies, or distinct intraspecific lineages, we applied phylogenetic reduced major axis regression to analyse the body size of females vs. males while accounting for phylogenetic non-independence. Our results indicate that philopterid and menoponid lice follow RR, while ricinids exhibit the opposite pattern. In the case of philopterids and menoponids, we argue that larger-bodied bird species tend to host lice that are both larger in size and more abundant. Thus, sexual selection acting on males makes them relatively larger, and this is stronger than fecundity selection acting on females. Ricinids exhibit converse RR, likely because fecundity selection is stronger in their case.
Similar content being viewed by others
Introduction
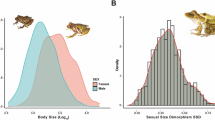
Body size is a fundamental trait of living organisms which influences most aspects of their biology. In sexually reproducing species, body size often differs between sexes. This is referred to as sexual size dimorphism (SSD). Male-biased sexual size dimorphism (MBSSD) refers to taxa the males of which are larger than the females, and female-biased sexual size dimorphism (FBSSD) refers to taxa the females of which are larger. When examining patterns of SSD among closely related animal species, Rensch1 observed that the relative male size (as compared to female size) increases with the average size of the species. In cases of taxa characterized by MBSSD, SSD increases as a consequence of the increasing relative male size. In taxa which exhibit FBSSD, the difference between the sexes diminishes with the increasing size of the species. This phenomenon is known as Rensch’s rule (RR). RR can be neatly visualised by plotting the male against the female sizes of different species on a logarithmic scale2. On the resulting graph, a group of species with a constant relative male size is positioned along trend lines of slope 1. When RR applies, the trend can be characterised by a line with a slope >1, meaning that relative male size increases with the female absolute size. See Fig. 1a for further details. The reversed relationship between relative male size and the size of the species is called Converse Rensch’s rule (CRR). In this case, relative male size decreases with the average size of the species, resulting in a decreasing SSD among MBSSD species and increasing SSD among FBSSD species. This defines a line with a slope <1 on the same graph. See Fig. 1b for further details.
Graphical representation of Rensch’s rule and converse Rensch’s rule. When the logarithm of male body size is plotted against the logarithm of female body size, species with equal male and female sizes (no sexual size dimorphism: No SSD) are located along a line with a slope of 1 going through the origin (grey dashed line). Species deviating from it show SSD proportional to the distance from this line. Species where the males are larger (male-biased sexual size dimorphism: MBSSD) are located above, and species where females are larger (female-biased sexual size dimorphism: FBSSD) located below it. The slopes of the trend lines indicate whether the relative male size changes with the average size of the species. If relative male size does not change with the average size, the trend has a slope of 1 (grey solid lines). If relative male size increases with the average size, the trend has a slope > 1 (a, black solid line). This is called Rensch’s rule (a). Among species where the females are smaller (FBSSD), SSD decreases. When the males’ size exceeds the females’ size (MBSSD), the SSD increases. If relative male size decreases with the average size, the trend has a slope < 1 (b, black solid line). This is called converse Rensch’s rule (b). In this case, males are getting proportionally smaller with the average size of the species, meaning that SSD decreases in MBSSD species and increases in FBSSD species with size.
Although there have been many studies focusing on RR, there is no general consensus about the causes for the allometry for SSD across closely related species. A thorough review by Fairbairn3 gathered a variety of functional hypotheses to explain RR. Dale et al.4 organised the existing explanations into three groups. We adopt this categorisation with the difference that we treat the fecundity selection hypothesis separately from the natural selection hypothesis. Note that the following hypotheses are not necessarily exclusive.
First, the evolutionary constraints hypothesis3 posits that the two sexes react to a different extent to the same selection pressures on body size. One cause of this may be that one sex has more additive genetic variance on body size, allowing it to respond more rapidly to selection. If the selection pressure varies among species, this can give rise to a pattern consistent with RR when males can respond more strongly and to CRR when females do.
Second, the natural selection hypothesis3 predicts that if the increase in the species’ body size reduces interspecific competition, then it enhances intersexual resource competition, resulting in a niche divergence between the sexes, and this niche divergence finds manifestation in divergences in body size.
Third, the sexual selection hypothesis2,3 assumes correlation — but not a total correspondence — between the body sizes of the sexes. When sexual selection on body size is stronger in one sex than the other, the other sex follows the growth of the first with more sexual selection pressure on it, but it cannot quite keep up, since there is only a correlation between the body sizes of the sexes. If sexual selection acts more heavily on males, RR is expected to turn up, regardless of whether the selection is for larger or smaller body size. When the males are selected to be larger, the female body size does not change as rapidly, resulting in RR. Stronger sexual selection on female body size results in CRR.
The “Fecundity selection” hypothesis5 claims that variation in SSD among species could be caused by a variation in the intensity of fecundity selection acting on female size. In this case, it is hypothesised that males show only a correlated response to changes in female body size. This process results in the emergence of a CRR pattern.
RR seems to hold in many taxa, primarily (but not exclusively) among vertebrates2,6,7,8. Dale et al.4 showed that polygynous bird species follow RR, while in cases of species with reversed sex-roles, the allometry follows CRR. Székely et al.8 argued that selection favours larger males in birds, where a larger size is advantageous in competition for females, while FBSSD develops in bird species where females compete with one another for males.
However, there are controversies concerning the applicability of the rule. The evidence for RR in FBSSD taxa is particularly scarce9. Blanckenhorn et al.10 reviewed the validity of RR in insects. Investigating data from seven insect orders, they found that RR applies to only half of the insect orders and, thus, may not be the norm in insects. CRR also occurs in insects11,12.
While parasitism is one of the most common life strategies on earth13, only a handful of papers have investigated RR among parasites. For instance, Poulin determined that RR applies to parasitic copepods14, but found no evidence for it among parasitic Nematodes15. Recently, Surkova et al.16 found RR among fleas, but not among parasitic mites.
Since parasitic lice (Insecta: Phthiraptera) reproduce sexually and exhibit remarkable sexually selected traits, including size dimorphism17, they constitute a suitable taxon to investigate the applicability of RR in parasites. Lice are obligate ectoparasites which complete their entire life cycle in the host plumage or pelage18. Two suborders of lice are found on avian hosts: Amblycera and Ischnocera. Philopteridae, the only avian lice in the latter suborder, are particularly specialised to move on feathers and hide in plumage19. Birds mainly counter philopterids by preening.
Menoponidae is the largest family in the suborder Amblycera. Menoponids are less specialized, and they can be found on any body parts of the host, although their oviposition and feeding are more restricted to certain areas. They live on the skin, in the fluffy underlayer of the plumage, and also on feather shafts20,21,22. They are more agile than ischnocerans, and they use their mobility to escape from preening18. A few genera are more specialized, for example Actornithophilus and Colpocephalum species can live inside feather shafts, and Piagetiella species can live inside the pouch of pelicans23.
Ricinid lice — also from the suborder Amblycera — are mostly restricted to small-bodied passerines and hummingbirds (Trochilidae), with a few species parasitizing medium-sized passerines like thrushes (Turdus spp.) and orioles (Oriolus spp.). They tend to be relatively large-bodied compared to their hosts24, and the prevalence and intensity of their infestations tend to be low25.
As in most other sexually reproducing animals, the males constitute the more competitive sex among lice and, thus, their body size may be more influenced by intrasexual rivalry. Mating time in louse species can range from 10–15 seconds up to 40 hours, although the latter can be interpreted as mate guarding behaviour by the males. The males of several philopterid lice possess modified antennae, which they use to grasp the female’s thorax during copulation to ensure attachment to the female. This is important, as it prevents rivals from dislodging them during copulation17,19. Another form of male-male competition among these creatures is sperm competition, which is the most widespread form of sexual competition in arthropods26. Larger males can produce greater quantities of sperm, and, therefore, they are more competitive in this context17,27,28.
Generally, females are the larger sex in lice18. Harnos et al.24 showed that females of the Philopteridae, Menoponidae, and Ricinidae families follow Harrison’s rule (HR)29. This rule postulates that larger hosts tend to harbour larger parasites. In the case of females of the philopterid lice Columbicola columbae, fertility is positively related to body size30. The authors proposed a microevolutionary mechanism to explain the emergence of HR. When C. columbae find themselves on relatively smaller hosts, host defences (preening) select them for smaller sizes better able to fit in the interbarb spaces. On relatively larger hosts, fecundity selection selects for larger females.
The purpose of our present study is to test whether RR applies to avian lice. Since the epidemiological and morphological characteristics of different louse taxa exhibit markedly different relationships to host characters17,24, first, we investigate three major families of avian lice; the ischnoceran family Philopteridae and the amblyceran families Menoponidae and Ricinidae. Harnos et al.24 also compared host-parasite body size allometries across the four philopterids guilds (called ‘wing lice’, ‘body lice’, ‘head lice’, and ‘generalists’) formerly outlined by Johnson et al.31 Since this categorization is challenged by recent studies on the Brueelia-complex, where a species-level categorization is required32,33, we prefer to discontinue comparisons between ‘ecomorph’ categories until a widely accepted new categorization will be published. In the second part of our study, we analyse RR in menoponid and philopterid lice separately from three different host orders. In the hope of gaining more insight into the underlying mechanism behind RR in avian lice, we also provide descriptive statistics linking SSD and the body size of lice to the body mass of their hosts.
Methods
Data collection
Data were obtained from species descriptions and are identical with the dataset recently used by Harnos et al.24, although that study used only female total body length values. Most of the body length data refer to species, however, when available, data regarding distinct subspecies or distinct populations associated with different host species were included as separate louse lineages. In cases of multiple measurements of the same parasite species (or subspecies, or lineage) from different sources in the literature, we averaged the values. Louse body size was expressed as total body length of slide-mounted specimens. Slide-mounting is a well standardized method for preserving and measuring lice34, thus its potential distorting effects are expected to be similar across samples. Research efforts may differ across host taxa, potentially introducing a certain degree of bias in our data set. Table 1 and Table 2 contain the sample sizes.
To analyse RR separately for different host orders, information on host taxonomy was obtained from IOC World Bird List v 8.235. The vast majority of ricinid lice in our dataset are from passeriform birds (97 out of 106 records), therefore, this louse family was excluded. In the cases of philopterids and menoponids, we chose the three most common host orders of the two families. The dataset with references to sources are available in the Supporting Information as comma separated value files (see Supplementary Data S1).
Louse phylogeny
The molecular phylogeny of lice is poorly understood; therefore, we adapted the louse tree of Harnos et al.24 without any further modifications. This tree is basically a compilation based on published taxonomies25,36,37,38,39,40,41,42,43,44,45 and interpreted as an approximation of the true phylogeny of avian lice. The phylogeny of lice (see Supplementary Data S2) in CAIC format is available in the Supporting Information.
Statistical analyses
We fitted phylogenetic reduced major axis regression46,47 (pRMA) for log-transformed male vs. female body lengths separately for the three louse families, and for philopterids and menoponids from three different host orders. Deviation from isometry was accepted when the slope of the fitted line significantly (P value ≤ 0.05) differed from 1. We also estimated phylogenetic signal expressed as Pagel’s λ48. All analyses were carried out in R 3.4.349. We used a jackknife method to investigate the influence of each observation on the slopes of the fitted lines. We refitted all pRMA models by leaving out each observation one at a time, and we recorded the results for each model, calculated the difference in the slope estimates, and observed if the significance of its deviation from isometry changed. We applied the ape 5.0 package50 to import and handle phylogenetic trees, the phytools 0.6–44 package51 to fit pRMAs, the RcmdrMisc 1.0–5 package52 to calculate descriptive statistics, and the ggplot2 2.2.1 package53 to create a visual rending of the data. The R code we used is available in the Supporting Information (see Supplementary Code S3).
Results
Descriptive statistics
The means and standard deviations of male and female body lengths, the relative male sizes (expressed as the ratio of male to female body length), host body masses, and the sample sizes are reported in Table 1.
As mentioned above, our sample may be biased, i.e. it may not necessarily represent the true distribution of lice across host body size classes. In this sample, the mean host mass is the largest among the philopterids, with a considerable standard deviation. Host masses tend to be lower for menoponid lice, though they still cover a wide range. As expected, ricinids were found only on small-bodied birds. The means of relative male sizes are similar between louse families. Menoponids have a somewhat shorter body length than philopterids, while ricinids are the largest among the three families.
In our sample, the differences between menoponids and philopterids grouped by three different host orders suggest that larger-bodied bird orders (Passeriformes < Charadriiformes < Galliformes) harbour lice with larger mean male and female body length and also a slightly larger mean relative male length. The mean host masses of philopterids and menoponids marginally differ in the three orders, with menoponids found on slightly larger hosts.
Table 2 shows the louse species (or subspecies, or lineage) closest to the 2.5%, 50% (median), and 97.5% quantiles of relative male size (ratio of male to female body length) for each group investigated. Relative male size, male and female body lengths, and host size and species are also reported. On a family level, philopterid and menoponid lice seem to show a pattern consistent with RR; with the increase of female body length, the male body lengths increase faster (thus the relative male size increases), with increasing host body weights. Ricinids, on the other hand, seem to exhibit a CRR pattern. Relative male size decreases as the female size increases, and host body masses also decrease with the increase of relative male size. Grouped by host orders, philopterids and menoponids from Passeriformes also show a trend consistent with RR, while in other groups, these descriptive statistics do not clearly match either RR or CRR.
Results of pRMA models
Results of the pRMA regressions, the estimated phylogenetic signals, and sample sizes are reported in Table 3. For visual representations of the data and the fitted lines, see Fig. 2 for families, Fig. 3 for philopterids and menoponids from different host orders. On a family level, both philopterid and menoponid lice show male-female allometric relationships consistent with RR. Ricinid lice exhibit a CRR trend (allometric slope < 1), which is surprising. Grouping the lice by host orders, we observed that RR applies to menoponids from Passeriformes and philopterids from both Charadriiformes and Galliformes. In all cases in which allometries consistent with RR were confirmed, the estimated slopes have numerically similar values, ranging from 1.11 to 1.16.
Allometric relationships of philopterid and menoponid lice from passeriform, charadriiform, and galliform birds. Allometric relationship between log-transformed male and female body lengths (µm) with isometric slopes (dashed lines) and fitted phylogenetic reduced major axis regression lines (solid lines) by louse families and host orders.
Jackknife diagnostics of pRMA models
In each pRMA model where deviation from isometry was confirmed, leaving out any single observation (data point) from the regression model changed neither the significance of the results nor the general direction of the slope (whether it is smaller or larger than 1). The effects of the most influential points on the slope — expressed as a percentage of the slope estimate — are reported in Table 3. In the cases of these models, the maximal effects range from 0.46% to 3.09%.
In some models where the deviation from isometry was not confirmed, certain observations can have a notable influence on the estimated slope. In these cases, leaving out particular observations from the model can result in a significant (P value ≤ 0.05) deviation from isometry. In the regression model of menoponids from Galliformes 9 out of 34 have this property.
Discussion
We have shown that two major taxa of avian ectoparasites, philopterid and menoponid lice, clearly obey RR. Host order level analysis in these families also confirmed RR separately in the case of Menoponidae from Passeriformes and Philopteridae from Charadriiformes and Galliformes. In contrast, however, ricinids follow CRR, where males get relatively smaller (as compared to females) with increasing size of the species.
Deviance from isometry was not proven in some cases. Philopterids from Passeriformes show a numerically similar allometric trend compared to menoponids from the same host order. The model explains less variance in the data among philopterids from these birds, perhaps indicating weaker mechanisms behind RR than among menoponids. Although not significant, the steep slope of menoponids from Galliformes and the slope of Menoponidae from Charadriiformes, which are consistent with CRR, are interesting trends. They indicate that accounting for host taxonomy and life history traits is a promising direction in investigating the underlying causes for RR.
Although without formal comparison, in all cases where RR was supported, the allometric slopes showed similar values. Based on this, it would be hard to come up with different interpretations of the results. Philopterids altogether show a somewhat steeper, but generally similar allometric trend compared to menoponids.
The non-exclusive alternative hypotheses explaining RR and Converse RR may more or less apply to our findings. Lice can respond quickly to selection pressures on body size30, but the genetics of their body size in relation to sex is not known. Furthermore, we lack knowledge about possible niche divergences between the sexes.
Sexual selection is known to be an influential agent of evolution in several taxa of parasites54,55, including parasitic lice17,27,28. Given that larger-bodied host species tend to have more prevalent and more abundant infestations of menoponid and philopterid lice56,57,58, we expect that the males in these populations tend to coexist with more rivals and also face an increased level of outbreeding due to a higher chance of multiple infections. This strengthens intrasexual competition59, and it also probably exerts a selection pressure favouring larger males. Our descriptive statistics in Table 2 empirically support this view; in many cases (namely in the Philopteridae and Menoponidae families together and separately from the Passeriformes), relative male size tends to increase with host body size. This probably indicates that in menoponids and philopterids, sexual selection due to male-male rivalry exerts stronger selection pressure on male size than fecundity selection exerts on female size. Contrarily, CRR observed in ricinid lice may indicate that fecundity selection is stronger on female body size than sexual selection is on male body size.
Based on our findings, it appears that similar selection pressures shape the evolution of SSD across avian lice, except for the family of Ricinidae.
Data Availability
All data analysed in the study are available in the Supporting Information.
References
Rensch, B. Evolution above the species level. (Methuen and Co. 1959).
Fairbairn, D. J. & Preziosi, R. F. Sexual Selection and the Evolution of Allometry for Sexual Size Dimorphism in the Water Strider, Aquarius remigis. Am. Nat. 144, 101–118 (1994).
Fairbairn, D. J. Allometry for sexual size dimorphism: pattern and process in the coevolution of body size in males and females. Annu. Rev. Ecol. Syst. 28, 659–687 (1997).
Dale, J. et al. Sexual selection explains Rensch’s rule of allometry for sexual size dimorphism. Proc. R. Soc. B Biol. Sci. 274, 2971–2979 (2007).
Head, G. Selection on Fecundity and Variation in the Degree of Sexual Size Dimorphism Among Spider Species (Class Araneae). Evolution 49, 776–781 (1995).
Abouheif, E. & Fairbairn, D. J. A comparative analysis of allometry for sexual size dimorphism: assessing Rensch’s rule. Am. Nat. 149, 540–562 (1997).
Smith, R. J. & Cheverud, J. M. Scaling of Sexual Dimorphism in Body Mass: A Phylogenetic Analysis of Rensch’s Rule in Primates. Int. J. Primatol. 23, 1095–1135 (2002).
Székely, T., Freckleton, R. P. & Reynolds, J. D. Sexual selection explains Rensch’s rule of size dimorphism in shorebirds. Proc. Natl. Acad. Sci. USA 101, 12224–12227 (2004).
Webb, T. J. & Freckleton, R. P. Only half right: species with female-biased sexual size dimorphism consistently break Rensch’s rule. PLoS ONE 2, 1–10 (2007).
Blanckenhorn, W. U., Meier, R. & Teder, T. Rensch’s rule in insects: patterns among and within species. in Sex, size and gender roles: evolutionary studies of sexual size dimorphism (Oxford University Press 2007).
Garcia-Navas, V., Noguerales, V., Cordero, P. J. & Ortego, J. Ecological drivers of body size evolution and sexual size dimorphism in short-horned grasshoppers (Orthoptera: Acrididae). J. Evol. Biol. 30, 1592–1608 (2017).
Guillermo-Ferreira, R., Novaes, M. C., Lecci, L. S. & Bispo, P. C. Allometry for Sexual Size Dimorphism in Stoneflies Defies the Rensch’s Rule. Neotrop. Entomol. 43, 172–175 (2014).
Poulin, R. & Morand, S. Parasite biodiversity. (Smithsonian Institution 2014).
Poulin, R. Sexual Size Dimorphism and Transition to Parasitism in Copepods. Evolution 50, 2520–2523 (1996).
Poulin, R. Covariation of sexual size dimorphism and adult sex ratio in parasitic nematodes. Biol. J. Linn. Soc. 62, 567–580 (1997).
Surkova, E. N. et al. Sexual size dimorphism and sex ratio in arthropod ectoparasites: contrasting patterns at different hierarchical scales. Int. J. Parasitol. https://doi.org/10.1016/j.ijpara.2018.05.006 (2018).
Rózsa, L., Tryjanowski, P. & Vas, Z. 4 Under the changing climate: how shifting geographic distributions and sexual selection shape parasite diversification. Parasite Divers. Diversif. Evol. Ecol. Meets Phylogenetics 58 (2015).
Johnson, K. P. & Clayton, D. H. The biology, ecology, and evolution of chewing lice. Ill. Nat. Hist. Surv. Spec. Publ. 24, 449–476 (2003).
Clayton, D. H., Bush, S. E. & Johnson, K. P. Coevolution of life on hosts: integrating ecology and history. (University of Chicago Press 2015).
Ash, J. S. A Study of the Mallophaga of Birds with Particular Reference to Their Ecology. Ibis 102, 93–110 (1960).
Mey, E. 20. Ordnung Phthiraptera, Tierläuse, Lauskerfe. in Lehrbuch der Speziellen Zoologie. Band I: Wirbellose Tiere. 5. Teil: Insecta 308–330 (Spektrum Akademisher Verlag 2003).
Baum, H. Biologie und okologie der Amselfederlouse. Angew. Parasitol. 129–175 (1968).
Galloway, T. D., Proctor, H. C. & Mironov, S. V. Chewing Lice (Insecta: Phthiraptera: Amblycera, Ischnocera) and Feather Mites (Acari: Astigmatina: Analgoidea, Pterolichoidea): Ectosymbionts of Grassland Birds in Canada. Arthropods Can. Grassl. 3, 139–188 (2014).
Harnos, A. et al. Size matters for lice on birds: Coevolutionary allometry of host and parasite body size. Evolution (2017).
Nelson, B. C. A revision of the New world species of Ricinus (Mallophaga) occurring on Passeriformes (Aves) Berkley. (Los Angeles, London. University of California Press 1972).
Andersson, M. B. Sexual selection. (Princeton University Press 1994).
Tryjanowski, P., Adamski, Z., Dylewska, M., Bulkai, L. & Rózsa, L. Demographic correlates of sexual size dimorphism and male genital size in the lice Philopterus coarctatus. J. Parasitol. 95, 1120–1124 (2009).
Pap, P. L., Adam, C., Vágási, C. I., Benkő, Z. & Vincze, O. Sex ratio and sexual dimorphism of three lice species with contrasting prevalence parasitizing the house sparrow. J. Parasitol. 99, 24–30 (2013).
Harrison, L. Mallophaga from Apteryx, and their significance; with a note on the genus Rallicola. Parasitology 88–100 (1915).
Villa, S. M. et al. Body size and fecundity are correlated in feather lice (Phthiraptera: Ischnocera): implications for Harrison’s rule. Ecol. Entomol. 43, 394–396
Johnson, K. P., Shreve, S. M. & Smith, V. S. Repeated adaptive divergence of microhabitat specialization in avian feather lice. BMC Biol. 10, 52 (2012).
Gustafsson, D. R. & Bush, S. E. Morphological revision of the hyperdiverse Brueelia -complex (Insecta: Phthiraptera: Ischnocera: Philopteridae) with new taxa, checklists and generic key. Zootaxa 4313, 1–443 (2017).
Bush, S. E. et al. Unlocking the black box of feather louse diversity: A molecular phylogeny of the hyper-diverse genus Brueelia. Mol. Phylogenet. Evol. 94, 737–751 (2016).
Palma, R. L. Slide-mounting of lice: a detailed description of the Canada balsam technique. N. Z. Entomol. 6, 432–436 (1978).
Gill, F. & Donsker, D. IOC World Bird List v 8, 2 (2018).
Rheinwald, G. D M Ricinus De Geer, 1778. Revision der ausseramerikanischen Arten. Mitteilungen Aus Dem Hambg. Zooloichen Mus. Inst. 181–326 (1968).
Lonc, E. Phenetic classification of Ricinidae (Phthiraptera: Amblycera). Pol. Pismo Entomol. 59, 403–491 (1990).
Smith, V. S. Basal ischnoceran louse phylogeny (Phthiraptera: Ischnocera: Goniodidae and Heptapsogasteridae). Syst. Entomol. 25, 73–94 (2000).
Cruickshank, R. H. et al. Phylogenetic analysis of partial sequences of elongation factor 1α identifies major groups of lice (Insecta: Phthiraptera). Mol. Phylogenet. Evol. 19, 202–215 (2001).
Smith, V. S. Avian louse phylogeny (Phthiraptera: Ischnocera): a cladistic study based on morphology. Zool. J. Linn. Soc. 132, 81–144 (2001).
Marshall, I. K. A morphological phylogeny for four families of amblyceran lice (Phthiraptera: Amblycera: Menoponidae, Boopiidae, Laemobothriidae, Ricinidae). Zool. J. Linn. Soc. 138, 39–82 (2003).
Mey, E. Zur taxonomie, verbreitung und parasitophyletischer evidenz des Philopterus-komplexes (Insecta, Phthiraptera, Ischnocera). Ornithol. Anz. 43, 149–203 (2004).
Cicchino, A. C. & Mey, E. On morphology, taxonomy, ecology, and distribution of Bothriometopus Taschenberg (Phthiraptera, Ischnocera, Philopteridae sensu lato). Rudolstädter Naturhistorische Schriften 14, 43–60 (2007).
Hughes, J., Kennedy, M., Johnson, K. P., Palma, R. L. & Page, R. D. Multiple cophylogenetic analyses reveal frequent cospeciation between pelecaniform birds and Pectinopygus lice. Syst. Biol. 56, 232–251 (2007).
Price, R. D. & Johnson, K. P. Three new species of chewing lice (Phthiraptera: Ischnocera: Philopteridae) from Australian parrots (Psittaciformes: Psittacidae). Proc. Entomol. Soc. Wash. (2007).
Clarke, M. R. B. The reduced major axis of a bivariate sample. Biometrika 67, 441–446 (1980).
McArdle, B. H. The structural relationship: regression in biology. Can. J. Zool. 66, 2329–2339 (1988).
Pagel, M. Inferring the historical patterns of biological evolution. Nature 401, 877 (1999).
R Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing 2018).
Paradis, E., Claude, J. & Strimmer, K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 (2004).
Revell, L. J. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223 (2012).
Fox, J. RcmdrMisc: R Commander Miscellaneous Functions. (2016).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis. (Springer-Verlag New York 2009).
Poulin, R. Evolutionary ecology of parasites. (Princeton university press 2011).
Goater, T. M., Goater, C. P. & Esch, G. W. Parasitism: The Diversity and Ecology of Animal Parasites. (Cambridge University Press 2013).
Rothschild, M. & Clay, T. Fleas, flukes and cuckoos. A study of bird parasites. Fleas Flukes Cuckoos Study Bird Parasites (1957).
Poiani, A. Ectoparasitism as a possible cost of social life: a comparative analysis using Australian passerines (Passeriformes). Oecologia 92, 429–441 (1992).
Rózsa, L. Patterns in the abundance of avian lice (Phthiraptera: Amblycera, Ischnocera). J. Avian Biol. 249–254 (1997).
Eberhard, W. G. et al. Sexual Selection and Static Allometry: The Importance of Function. Q. Rev. Biol. 93, 207–250 (2018).
Acknowledgements
The research on which this article is based enjoyed support through the grant “In the light of evolution: theories and solutions” (GINOP-2.3.2-15-2016-00057). The Project is supported by the European Union and co-financed by the European Social Fund (grant agreement no. EFOP-3.6.3-VEKOP-16-2017-00005, project title: Strengthening the scientific replacement by supporting the academic workshops and programs of students, developing a mentoring process). The authors would like to thank Thomas Cooper for proofreading the manuscript.
Author information
Authors and Affiliations
Contributions
L.R. gathered the data used, I.S.P. and A.H. analysed the data and prepared Figures 2–3, I.S.P. drew Figure 1, I.S.P. and L.R. wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Piross, I.S., Harnos, A. & Rózsa, L. Rensch’s rule in avian lice: contradictory allometric trends for sexual size dimorphism. Sci Rep 9, 7908 (2019). https://doi.org/10.1038/s41598-019-44370-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-44370-5
This article is cited by
-
Relationship between body size and sexual size dimorphism in syringophilid quill mites
Parasitology Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.