Abstract
The present study tested the efficacy of 24-epibrassinolide (EBL) and calcium (Ca) for mediating salinity tolerance in tomato. Salinity stress affected the morphological parameters of tomato as well as leaf relative water content (LRWC), photosynthetic and accessory pigments, leaf gas exchange parameters, chlorophyll fluorescence and the uptake of essential macronutrients. The salt (NaCl) treatment induced oxidative stress in the form of increased Na+ ion concentration by 146%, electrolyte leakage (EL) by 61.11%, lipid peroxidation (MDA) 167% and hydrogen peroxide (H2O2) content by 175%. Salt stress also enhanced antioxidant enzyme activities including those in the ascorbate–glutathione cycle. Plants treated with EBL or Ca after salt exposure mitigated the ill effects of salt stress, including oxidative stress, by reducing the uptake of Na+ ions by 52%. The combined dose of EBL + Ca reversed the salt-induced changes through an elevated pool of enzymes in the ascorbate–glutathione cycle, other antioxidants (superoxide dismutase, catalase), and osmoprotectants (proline, glycine betaine). Exogenously applied EBL and Ca help to optimize mineral nutrient status and enable tomato plants to tolerate salt toxicity. The ability of tomato plants to tolerate salt stress when supplemented with EBL and Ca was attributed to modifications to enzymatic and non-enzymatic antioxidants, osmolytes and metabolites.
Similar content being viewed by others
Introduction
Worldwide, the availability of agricultural land is shrinking gradually due to non-regulated and non-judicious agricultural practices, the rapid rise in industrialization, urbanization, and biotic and abiotic environmental pressures1. Of the various abiotic pressures, salinity is spreading across the globe due to salt water intrusion as a result of sea level rises in coastal areas, extensive irrigation practices in arid regions, and large-scale soil erosion2. It is estimated that, globally, approximately 7% of the total land area and 20% (~45 million ha) of the gross cultivable area are affected by the presence of high salt concentrations3,4. Salinity reduces crop yields worth billions of dollars every year5 and thus is a major abiotic constraint to crop yield and sustainable agricultural productivity6. Salinity causes ionic, oxidative and osmotic stress which retards plant growth and development7,8,9,10,11. Prolonged and high salt concentrations cause oxidative stress that generates reactive oxygen species (ROS), which oxidize biomolecules such as nucleic acids (DNA/RNA), proteins, lipid bilayer membranes, and enzyme inhibitors12,13,14,15,16. However, plants are equipped with defense mechanisms such as enzymatic and non-enzymatic antioxidants, osmoprotectants including proline and glycine betaine (GB), and enzymes in the ascorbate–glutathione (AsA–GSH) cycle viz. monodehydroascorbate reductase (MDHAR), ascorbate peroxidase (APX), dehydroascorbate reductase (DHAR) and glutathione reductase (GR), which neutralize the effects of salt-generated ROS8,16,17. Plant biologists are looking for alternatives to enhance crop production under increased salt stress. One strategy to combat salt stress is external supplementation of phytohormones.
Brassinosteroids (BRs) belong to a class of polyhydroxylated steroid phytohormones that are implicated in the resistance to a broad spectrum of environmental cues such as heavy metals, drought, pesticides and salinity18,19,20, Exogenous application of the natural BR, 24-epibrassinolide (EBR), has enhanced growth, pigment constituents, photosynthetic attributes, osmolytes and antioxidant enzyme activity in crop plants under abiotic stresses20,21,22,23. Foliar application of EBR to plants controls a range of biochemical and physiological responses to salt stress24,25.
Calcium (Ca) is a principal macronutrient in plants and is a ubiquitous secondary messenger in plant signaling. Ca is involved in the regulation of plant growth and development and the enhancement of abiotic stress tolerance7,26,27,28. It is well known that Ca plays a crucial role in controlling the structure, signaling and function of membranes by making bonds with the phospholipid bilayer, thus stabilizing and promoting the structural integrity of membrane organelles in plants exposed to stress environments26,29. Our earlier study showed that Ca application alleviates heavy metal toxicity in chickpea (Cicer arietinum) and Indian mustard (Brassica juncea) plants using various physiological and biochemical characteristics26,27. Khan, et al.7 showed that a combined treatment of Ca and gibberellin alleviated the effects of salt stress in linseed (Linum usitatissimum) plants better than 150 mM NaCl treatment. Al-Whaibi, et al.30 identified a coordinated role of salicylic acid and Ca that protects wheat (Triticum aestivum) plants from salinity stress. Rahman, et al.31 discovered that Ca supplementation to salt-stressed rice (Oryza sativa) seedlings minimized the impact of salt stress by improving ion homeostasis, and the antioxidant defense and glyoxalase systems. The synergistic role of EBR and Ca before and after seed sowing in the regulation of salinity-induced stress in tomato plants has not been reported. We evaluated the role of EBR and Ca on oxidative stress, antioxidant enzymes, and osmolytes in tomato seedlings. The study showed that EBR and Ca alleviated NaCl toxicity through modifications to antioxidant enzymes, osmolytes, the ascorbate–glutathione cycle and secondary metabolites.
Materials and Methods
Plant material and experimental setup
Healthy, uniform seeds of tomato (Solanum lycopersicon cv. K-21) were surface sterilized for 5 minutes with 5% NaOCl and then washed thoroughly with double-distilled water. The seeds were primed with 24-epibrassinolide (10−7 M) for 8 h and then sown in earthen pots containing a 3:1 mixture of perlite and sand. After germination, plants were thinned to three seedlings per pot. The pots containing seedlings were watered with full strength Hoagland’s nutrient solution (200 mL) every alternate day for 10 days. The salt stress (150 mM NaCl) treatment started 14 days after sowing by applying a modified Hoagland solution every week until day 40. After 14 days of salt stress, calcium (Ca)-in the form of calcium chloride (CaCl2; 50 mM)-was sprayed to plant foliage every alternate day until day 40. Control plants were provided with Hoagland’s nutrient solution only. The experimental pots were positioned in a complete randomized block design in a greenhouse with average day/night temperatures of 26 ± 2 °C, relative humidity of 70–75%, and an average photoperiod of 18 h light/6 h dark. The plants were harvested 40 days after sowing. Data presented in the manuscript is average data from three independent experiments, each treatment was replicated five times. Biochemical parameters and antioxidant enzyme activity were determined in the topmost fully grown young leaves.
Determination of growth and photosynthetic pigment parameters
Shoot and root length was determined by scale. Samples were oven dried at 70 °C for 24 h and then weighed.
Photosynthetic pigment contents were analyzed using the acetone extract method32. Acetone (80%) was used to extract fresh leaf tissues, and absorbance read at 480 nm, 645 nm, 663 nm with a spectrophotometer (Beckman 640D, USA).
Leaf gas exchange measurements viz. net photosynthetic rate (Pn), carbon dioxide assimilation rate (A), stomatal conductance (gs) and transpiration rate (E) were measured on fully expanded horizontal leaves in full and bright sunlight between 10:00 h and 12:00 h using IRGA (LCA-4 model Analytical development Company, Hoddesdon England).
Chlorophyll fluorescence parameters were recorded with a junior PAM chlorophyll fluorometer (H. Walz, Effeltrich, Germany) on fully expanded horizontal tomato leaves33.
Estimation of leaf relative water content (LRWC)
The standard protocol of Yamasaki and Dillenburg34 was adopted to estimate LRWC. Twenty leaf discs were punched from the upper most leaves, and their initial (fresh) weight noted. The discs were kept in double-distilled water for 60 min to become turgid and then weighed. The leaves were then oven dried at 70 °C for 24 h and then weighed. LRWC was calculated using the following formula:
Determination of proline and glycine betaine (GB) content
The acid ninhydrin method of Bates, et al.35 was used to estimate proline content. A 500 mg fresh leaf sample was homogenized in sulfosalicylic acid and then centrifuged at 12,000 g for 8 min. The pellet was discarded, and a 2 mL aliquot of the supernatant added to equal volumes of acid ninhydrin and glacial acetic acid at 100 °C for 60 min. The slurry was placed on ice, and toluene blue was used to extract proline from samples before measuring the absorbance at 520 nm with a spectrophotometer (Beckman 640D, USA). The Pro content was determined from a standard curve and expressed as µM proline g−1 FW.
Glycine betaine content was determined according to the method of Grieve and Grattan36. Dry leaf material (500 mg) was extracted with 20 mL of double-distilled water after shaking at room temperature for 24 h. To the filtered extract, 2 N sulfuric acid was added. A 0.5 mL aliquot was reacted with 0.2 mL cold potassium iodide and centrifuged at 10000 g for 15 min. The supernatant was treated with 1,2-dichloroethane to dissolve the periodide-produced crystals. The reaction mixture was left undisturbed for 3 h before measuring the absorbance at 365 nm with a spectrophotometer (Beckman 640D, USA). The GB content was determined from a standard reference curve.
Determination of oxidative stress biomarkers
Hydrogen peroxide (H2O2) was estimated using the method of Velikova, et al.37. Fresh leaf tissue (500 mg) was macerated with 0.1% trichloroacetic acid (TCA), and the homogenate centrifuged at 12,000 g for 8 min. The supernatant (0.5 mL) was mixed with 0.5 mL each of 100 mM potassium phosphate buffer (pH 7.0) and 1 M potassium iodide. The color was read at 390 nm with a spectrophotometer (Beckman 640D, USA). The H2O2 content was expressed as µM g−1 FW.
The standard protocol of Madhava Rao and Sresty38 was used to measure malondialdehyde (MDA) dependent content and to determine lipid peroxidation. Fresh leaf tissues were ground in 0.1% trichloroacetic acid (TCA) and centrifuged at 10,000 g for 5 min. Then, 4 mL of thiobarbituric acid (TBA) (prepared in 20% TBA) was added to 1 mL of supernatant and boiled at 100 °C for 30 min. The reaction mixture was terminated on an ice bath followed by centrifugation at 10,000 g for 10 min. The intensity of color formation was read at 530 and 600 nm with a spectrophotometer (Beckman 640D, USA).
The method of Dionisio-Sese and Tobita39 was followed to determine electrolyte leakage. Fresh leaf discs in test tubes containing 10 mL of double-distilled water were analyzed for their electrical conductivity (EC0). The sample tubes were boiled at 50 °C and 100 °C for 20 and 10 min, respectively, in a temperature-controlled dry block heater, and the respective electrical conductivities (EC1 and EC2) measured simultaneously. Electrolyte leakage was calculated using the following formula:
Estimation of enzymatic antioxidant activities and the ascorbate-glutathione cycle Preparation of enzyme extract and assay
Fresh leaf material was collected and homogenized in a deep-freezer-cooled pestle and mortar in the presence of 1 mL of ice-cold 100 mM potassium phosphate buffer (pH 7.0) containing 1% of polyvinyl pyrrolidone. The slurry was centrifuged at 12,000 g for 30 min at 4 °C, and the resulting supernatant was used to determine different enzyme activities.
Superoxide dismutase activity (SOD, EC1.15.1.1) was measured using the nitroblue tetrazolium (NBT) reduction method of Dhindsa and Matowe40. The reaction mixture contained 1.5 ml of 100 mM phosphate buffer (pH 7.4), 0.2 ml of 10 mM methionine, 0.1 ml of 50 µM riboflavin, 100 µL extract of enzyme with equal amounts of 1 mM ethylenediaminetetraacetic acid (EDTA) and 70 µM nitro blue tetrazolium chloride (NBT). The mixture was kept under fluorescent tubes for 20 min, before measuring absorbance at 560 nm with a spectrophotometer (Beckman 640D, USA). One unit of SOD activity was defined as the amount of protein causing a 50% decrease of SOD-inhibitable NBT reduction and expressed in EU mg−1 protein.
Catalase (CAT: 1.11.1.6) activity was determined by monitoring the decomposition of H2O2 for 2 min at 240 nm with a spectrophotometer (Beckman 640D, USA)41. CAT activity was expressed in EU mg–1 protein.
Glutathione S-transferase (GST, 2.5.1.18) activity was estimated using the method of Hasanuzzaman and Fujita42. The reaction mixture was prepared by adding 100 mM Tris-HCl, buffer (pH 7.0), 1.0 mM GSH, 1 mM 1-chloro-2,4-dinitrobenzene (CDNB), and enzyme extract and the absorbance read at 340 nm with a spectrophotometer (Beckman 640 D, USA). GST activity was expressed in EU mg−1 protein.
Ascorbate peroxidase (APX, 1.11.1.11) activity was assayed according to Nakano and Asada43. The solution mixture containing potassium phosphate buffer (pH 7.0), ascorbate, H2O2, EDTA, and enzyme extract was prepared, and the H2O2-mediated oxidation of ascorbate was read at 290 nm for 2 min with a spectrophotometer (Beckman 640D, USA). APX activity was expressed in EU mg−1 protein.
Glutathione reductase (GR, 1.6.4.2) activity was measured according to the method of Foster and Hess44. The reaction mixture contained 100 mM potassium phosphate buffer (pH 7.0), 1 mM EDTA, 500 µM GSSG, 150 µM NADPH, and enzyme extract. The change in the intensity of absorbance color was read for 3 min at 340 nm with a spectrophotometer (Beckman 640D, USA). GR activity was expressed in EU mg−1 protein.
Monodehydroascorbate reductase (MDHAR, 1.6.5.4) activity was determined following the method of Miyake and Asada45. MDHAR activity was expressed in µmol NADPH oxidized (EU mg−1 protein).
Dehydroascorbate reductase (DHAR, 1.8.5.1) activity was assayed according to the standard protocol by Nakano and Asada43. The absorbance was recorded at 265 nm with a spectrophotometer (Beckman 640D, USA). DHAR activity was expressed in EU mg−1 protein.
Ascorbate (AsA) and glutathione (GSH) contents were measured according to the methods of Huang, et al.46 and Yu, et al.47. The glutathione content was determined by subtracting oxidized glutathione from total reduced glutathione.
Determination of Na+ and other inorganic ions
Dry plant samples (0.5 g) were digested in a mixture of sulfuric acid and nitric acid (1/5, v/v) using a digestion assembly at 70 °C for 20 h. The solution was then treated with an acid mixture of HNO3/HClO4 (5/1, v/v) to make the solution colorless. The concentration of sodium and other ions was then determined with an atomic absorption spectrophotometer (Perkin-Elmer Analyst Model 300) and expressed in µM g−1 DW.
Determination of flavonoid content
Fresh leaf material (500 mg) was ground and extracted in ethyl alcohol at 25 °C. The colorimetric method of Zhishen, et al.48 was used to estimate flavonoid content. For the calibration curve, catechin extract was used as a standard. The absorbance was recorded at 510 nm with a spectrophotometer (Beckman 640D, USA). Flavonoid content was expressed in mg catechin equivalents g−1 extract.
Statistical analysis
The data were presented as the mean of five replicates ± standard error (SE). The data analysis was done with one-way analysis of variance (ANOVA) through the SPSS software (Version 17). The values at P ≤ 0.05 was treated as significant.
Results
Growth and biomass yield
Shoot length increased by 16.66%, 8.33% and 44.44% in the EBL, Ca and EBL + Ca treated control plants, respectively, relative to the control. Salt stress (150 mM) reduced shoot and root lengths by 59.89% and 39.60%, respectively, relative to the control plants (Table 1). NaCl-treated seedlings supplemented with EBL or Ca enhanced shoot length by 54.28% and 77.53%, respectively, compared to seedlings treated with NaCl alone. Root length increased by 16.98% with EBL and 21.31% with Ca in NaCl-treated seedlings, relative to NaCl alone. However, the combined dose of EBR + Ca to salt-stressed plants increased shoot and root lengths by 134.18% and 67.70%, respectively, compared to plants treated with NaCl alone.
Shoot dry weights decreased by 66.66% in NaCl-treated seedlings relative to control plants. Supplementing NaCl-treated seedlings with EBL or Ca enhanced shoot dry weight by 100.00% and 75.00%, respectively, compared to NaCl alone. The combined dose of EBR + Ca to NaCl-treated seedlings further increased shoot dry weight (by 116.66%) compared to NaCl alone (Table 1). NaCl decreased root dry weights by 60.86% over control plants, however, application of EBL, Ca and EBL + Ca to NaCl stressed plants enhanced root dry weight by 66.66%, 33.33% and 100.00% respectively over NaCl-alone treated plants.
Photosynthetic pigments and leaf gas exchange
The total Chl content declined by 42.75% in NaCl-treated seedlings relative to control (Table 1). Supplementing NaCl-treated seedlings with EBR or Ca improved pigment contents by 35.44% and 31.64%, respectively, relative to NaCl alone. The combined dose of EBL + Ca to NaCl-treated seedlings further increased total Chl content (by 67.08%) compared to NaCl alone.
Carotenoid content increased by 52.94% in NaCl-treated seedlings relative to control plants. The application of EBL or Ca further increased this value by 10.25% and 5.12%, respectively, relative to NaCl alone. The combined dose of EBL + Ca to NaCl-treated seedlings increased the carotenoid content (by 21.79%) compared to NaCl alone (Table 1).
Salt stress reduced leaf gas exchange parameters—Pn, A, gs and E—by 20.31%, 48.02%, 81.32% and 67.45%, respectively, relative to the control. Pretreatment with EBR enhanced Pn by 29.80%, A by 32.11%, gs by 121.44% and E by 30.90% in NaCl-stressed seedlings, relative to NaCl alone, and Ca supplementation followed a similar ameliorating trend. The combined dose of EBL + Ca to NaCl-stressed plants enhanced Pn, A, gs and E by 90.51%, 65.10%, 301.28% and 74.54%, respectively, relative to NaCl alone (Table 2).
Chlorophyll fluorescence parameters
Salt stress reduced the efficiency of PSII (Fv/Fm) by 34.61%, quantum yield of PSII (ΦPSII) by 23.43%, and photochemical efficiency (qp) by 27.47%, respectively, compared to control plants, but increased non-photochemical quenching (NPQ) by 41.79%. Pretreatment with EBR increased Fv/Fm by 43.13%, ΦPSII by 18.36% and qp by 13.63% but decreased NPQ by 18.94% in NaCl-stressed seedlings relative to NaCl alone. Supplementation with Ca enhanced Fv/Fm, ΦPSII, and qp by 35.29%, 10.20% and 9.09%, respectively, and decreased NPQ by 26.31% in NaCl-stressed seedlings relative to NaCl alone (Table 3). The combined dose of EBR + Ca enhanced Fv/Fm, ΦPSII, qp by 72.54%, 65.30%, and 33.33%, respectively, and decreased NPQ by 26.31% in NaCl-stressed seedlings relative to NaCl alone (Table 3).
Leaf relative water content, proline and glycinebetaine
Salt stress reduced LRWC by 28.49% compared to control plants. The application of EBL or Ca to NaCl-stressed plants increased LRWC by 22.23% and 15.42%, respectively, relative to NaCl alone. The combined dose of EBL + Ca increased LRWC by 32.84% in NaCl-stressed seedlings relative to NaCl alone (Table 4).
Salt stress increased the proline and GB contents by 3.48- and 1.55-fold relative to the control plants (Table 4). Pretreatment with EBL further enhanced the proline content by 1.30-fold and GB content by 1.41-fold compared to NaCl alone. Foliar supplementation with Ca further enhanced the proline content by 1.26-fold and GB content by 1.35-fold relative to NaCl alone. The EBL + Ca treatment further enhanced the proline content by 1.41-fold and GB content by 1.58-fold compared to NaCl alone.
Hydrogen peroxide, malondialdehyde content and electrolyte leakage
Salt stress increased the production of H2O2, MDA, and EL by 174.67%, 166.66% and 448% relative to the control plants (Table 4). However, pretreatment with EBR decreased the production of H2O2 by 48.17%, MDA by 42.93% and EL by 20.29% relative to NaCl alone. Similarly, foliar supplementation with Ca reduced the production of H2O2 by 38.15%, MDA by 35.50% and EL by 13.07% relative to NaCl alone. The combined dose of EBR + Ca was more effective at reducing stress in NaCl-stressed seedlings than the individual applications of EBL or Ca, with reductions of 65.85%, 58.87% and 51.47% in H2O2, MDA, and EL production, respectively, compared to NaCl alone.
Activity of antioxidant enzymes and enzymes of ascorbate–glutathione cycle
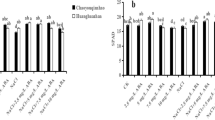
Salt stress elevated SOD and CAT activities by 61.43 and 74.34%, respectively, relative to control seedlings. Pretreatment with EBR further increased SOD activity by 23.63% and CAT activity by 33.32% in NaCl-stressed seedlings compared to NaCl alone. Foliar supplementation with Ca increased SOD and CAT activities by 23.18% and 35.66%, respectively, relative to NaCl alone. The combined dose of EBL + Ca increased SOD activity by 56.68% and CAT activity by 60.97% relative to NaCl alone (Fig. 1A,B).
Salt stress increased GST content by 93.76% relative to the control seedlings, which further increased by 27.16%, 18.54% and 42.46% with the EBL, Ca and EBL + Ca treatments, respectively, relative to NaCl alone (Fig. 1C).
The activity of the four enzymes in the AsA–GSH pathway—APX, GR, MDHAR, and DHAR—differed in their response to salt stress (Fig. 2A–D). Salt stress increased APX and GR activities by 86.05% and 91.89%, respectively, compared to control seedlings, but MDHAR and DHAR activities decreased by 32.00% and 30.19%, respectively (Fig. 1B). Pretreatment with EBL further enhanced the activity of APX by 46.13%, GR by 61.05%, MDHAR by 21.00% and DHAR by 16.90% in NaCl-stressed seedlings relative to NaCl alone. Supplementation of Ca to NaCl-stressed seedlings increased the activity of APX by 37.67%, GR by 47.51%, MDHAR by 39.35% and DHAR by 42.39% relative to NaCl alone. The combined dose of EBL + Ca further enhanced APX, GR, MDHAR and DHAR activities by 108.54%, 140.47%, 39.35% and 42.39%, respectively, in NaCl-stressed seedlings relative to NaCl alone.
Non-enzymatic antioxidants
Salt stress reduced the ascorbate (AsA) content by 39.53% compared to the control. However, AsA content increased by 33.84% with EBL pretreatment and 28.84% with Ca supplementation in NaCl-stressed seedlings compared to NaCl alone. The combined dose of EBL + Ca further enhanced AsA content by 53.84% in NaCl-stressed seedlings relative to NaCl alone (Fig. 3A).
Pretreatment of seeds with EBL (10−7 M) and foliar application of Ca (50 mM) maintained (A) AsA, (B) GSH, (C) GSSG, (D) GSH/GSSG ratio and (E) flavonoid content in tomato seedlings under NaCl stress. Data presented are means ± SE (n = 5). Different letters indicate significant differences at P ≤ 0.05.
Salt stress increased GSH production by 50.01% relative to control seedlings. GSH production further increased by 22.72% with EBL pretreatment and 19.96% with Ca supplementation in NaCl-stressed seedlings relative to NaCl alone. The combined dose of EBR + Ca in NaCl-stressed seedlings further increased GSH production by 33.33% relative to NaCl alone (Fig. 3B).
Salt stress decreased GSSG content by 23.27% relative to control seedlings. However, EBL, Ca and EBL + Ca supplementation increased GSSG content in NaCl-stressed seedlings by 34.10%, 20.63% and 63.22%, respectively, relative to NaCl alone (Fig. 3C).
Salt stress increased the redox state (ratio of GSH/GSSG) by 112.35% relative to the control seedlings. The ratio of GSH/GSSG in NaCl-stressed seedlings in the EBR, Ca and EBR + Ca treatments decreased by 94.42%, 0.75% and 18.19% relative to NaCl alone (Fig. 3D).
Flavonoids
Salt stress reduced flavonoid content by 52.64% relative to control seedlings. However, supplementation with EBL, Ca and EBL + Ca to NaCl-stressed seedlings further enhanced flavonoid content by 15.31%, 8.12% and 56.66%, respectively, relative to NaCl alone (Fig. 3E).
Nutrient elements
Salt stress increased the Na+ ion concentration by 146.29% and Na+/K+ ratio by 773% and decreased K+ and Ca+ ion concentrations by 71.80% and 44.54%, respectively, relative to the control. EBL supplementation reduced the Na+ concentration by 32.67% and Na+/K+ ratio by 75.23%, and increased the K+ and Ca2+ concentrations by 107.75% and 74.91% in the NaCl-stressed seedlings relative to NaCl alone. Supplementation with Ca followed a similar trend. However, the combined dose of EBL + Ca to NaCl-stressed seedlings reduced the Na+ concentration and Na/K ratio by 51.84% and 84.57%, respectively, and increased the K+ and Ca2+ concentrations by 211.40% and 141.01%, respectively, relative to NaCl alone (Table 5).
Discussion
Growth and biomass yield
Salt stress reduced the growth and biomass yield (shoot dry weight) of tomato seedlings, and the findings are consistent with our earlier results in chickpea49. Salt stress also reduces plant growth and biomass yield in tomato50, wheat51 and rice52 etc. and possibly due to the inhibition of nutrient uptake to plants. Supplementation with EBR and Ca, individually or combined, enhanced the recovery of plant growth related parameters. Supplementation with EBR has increased growth and yield parameters in various plants under salt stress, including pepper (Capsicum annuum)53, pea (Pisum sativum)54 and Indian mustard23. Similarly, Ca supplementation has alleviated the inhibitory growth effects of salt stress in species such as linseed7, rice31, pistachio55 and Indian mustard56. EBL increased growth in Vigna radiata under salt stress by enhancing the photosynthetic rate and carbonic anhydrase activity57. EBR plays a role in H+-ATPase activation58 that is directly responsible for the activation of cell wall loosening enzymes and therefore improving growth. According to Haubrick and Assmann59, brassinosteroids are involved in cell elongation and germination due to their interaction with other growth regulators. The growth promoting and ameliorating ability of EBR may be due to the modulation of cellulose biosynthesis and enhanced rates of cell division and cell elongation which ultimately lead to enhanced plant growth60. Calcium supplementation reduced the effects of cadmium stress on growth and biomass yield in Indian mustard, which was attributed to the uptake of mineral elements by Ca26.
Pigments and leaf gas exchange
The photosynthetic potential of plants reflects their overall performance, which is manifested by various biomass and growth parameters. Salt stress negatively impacts chlorophyll content, leading to its impaired biosynthesis and accelerated pigment degradation61. Salt-driven stress also destabilizes pigments associated with the chlorophyll protein complex and reduces the amount of photosynthetic pigments by accelerating the activity of chlorophyllase1. Plants supplemented with EBR or Ca increased their photosynthetic potential in the absence of salinity. However, combined effect of EBR + Ca showed maximum photosynthetic efficiency under both control as well as salt stress. Supplementation with EBL to NaCl-stressed plants enhanced chlorophyll synthesis, which is likely due to its role in regulating stress-causing agents62. According to Li, et al.63, enhanced expression of the BR biosynthetic pathway enhances the activity of Calvin cycle enzymes that boost photosynthesis. Under salt stress, EBR and Ca reduced the uptake of Na+ ions in tomato seedlings and increased the activity of enzymes associated with carbon reactions of photosynthesis. All these positive effects of EBR and Ca alleviated the inhibition of photosynthetic capacity under salt stress. These findings are consistent with those of Hayat, et al.57 in V. radiata, Ahmad, et al.20 in C. arietinum, Ahmad, et al.26 in B. juncea and Rahman, et al.31 in O. sativa. EBL supplementation can assist mineral uptake especially Mg2+ during mercury (Hg) stress20, which may explain the increase in chlorophyll content in tomato seedlings under salt stress as Mg2+, being the central element of the chlorophyll molecule, decreased under NaCl stress. Choudhary, et al.64 reported that EBL supplementation enhanced photosynthetic pigments in radish (Raphanus sativus) under chromium stress. Lechowski and Białczyk65 reported that Ca served as a second messenger for cytokinin action in boosting chlorophyll synthesis.
Carotenoids play a major role in the photosynthetic reaction center by regulating photo protection against auto-oxidation66. The enhanced biosynthesis of carotenoids during salt stress in the present study may be due to carotenoids acting as an antioxidant and assisting in oxidative stress management27,49. Increased carotenoid biosynthesis with EBR supplementation is also due to phytoene synthase (PSY), a key enzyme in its biosynthetic pathway18. Additionally, supplementation of EBR and Ca further increased the carotenoid content, which reduced the oxidative damage caused by salt toxicity; this is supported by studies on two varieties of pepper53, and chickpea20.
The reduction in leaf gas exchange parameters [CO2 assimilation rate (A), transpiration rate (E) and stomatal conductance (gs)] under NaCl stress in the present study agrees with similar studies on maize (Zea mays)67, cucumber (Cucumis sativus)68 and mung bean (V. radiata)69. Similarly, salinity stress reduced photosynthesis (PN) and gs in mung bean57 and cucumber22. Salt-induced osmotic stress results in stomatal closure thereby slowing the transpiration rate. Stomatal closure also leads to a reduction in photosynthetic rate as the fixation of CO2 decreases. Salt-induced reductions in PN have been attributed to protein dysfunction due to altered enzyme activities and negative feedback by reduced sink activity70. Supplementation with EBL enhanced gas exchange parameters in the present study and is supported by studies on salt-stressed watermelon (Citrullus lanatus)71 and water-stressed cowpea (Vigna unguiculata)72 and drought stressed capsicum (C. annuum)73. The activity of Rubisco enzyme was activated by brassinosteroids74 and may be due to an enhanced CO2 assimilatory rate75. BRs have also enhanced the expression of specific genes in B. juncea under pesticide stress18. Application of Ca enhanced gas exchange parameters in a perennial lawn species (Zoysia japonica) under drought stress76. Ca helps plants to maintain relative water contents and stomatal conductance27 thus preventing damage from cytoplasm dehydration77.
Chlorophyll fluorescence
Chlorophyll fluorescence is a widely used alternative method to quantify tolerance and acclimation of plants to environmental extremes78. Salt stress decreased the Fv/Fm value in the present study, which is consistent with the findings in Indian mustard23. In our study, salt stress reduced ΦPSII and qP in tomato seedlings, which was also reported in salt-stressed eggplant (Solanum melongena)79 and water-stressed cowpea (Vigna unguiculata)72. Salt stress can affect PSII electron transport80 and their photoinhibition results in destruction in antenna pigments. Salt stress blocks electron transfer from the primary acceptor (plastoquinone, QA) at the secondary (plastoquinone, QB) at the acceptor side of PSII leading to a decline in the Fv/Fm ratio81. Supplementation with EBR reversed the negative effects of NaCl on the Fv/Fm ratio, ΦPSII, qP and NPQ in tomato seedlings, which is consistent with findings in Indian mustard23, cucumber22,24. Brassinosteroids maintained the Fv/Fm ratio, ΦPSII and qp in wheat82 and eggplant79 under NaCl stress and also reduced PSII photoinhibition in wheat83. In the present study, EBR supplementation to salt-stressed tomato seedlings reduced NPQ, which is similar to the findings in Solanum melongena (L.) plants79. Application of Ca enhanced the Fv/Fm ratio, ΦPSII, qP and reduced NPQ in the salt-stressed tomato seedlings in the present study. This positive role of Ca may be attributed to the mineral ion homeostasis and uptake of water under NaCl stress31. These results suggest that EBR and Ca protect the PSII from over-excitation and maintain the structural integrity of the thylakoid membrane.
Leaf relative water content, proline and glycinebetaine
Salt stress significantly reduced LRWC in tomato seedlings in the present study, which is likely due to salt-induced constraints to the availability and uptake of water and injury to root system architecture27,49. Supplementation with EBR and Ca had a positive effect on LRWC under salt stress by reducing membrane injury and improving the water balance. A similar response has been observed with exogenous Ca application in linseed (Linum usitatissimum)7, Indian mustard (B. juncea8) and rice (O. sativa31) and EBR supplementation in ryegrass (Lolium rigidum25) and pepper (C. annuum84). Supplementation of 24-EBL to Cd-stressed common bean (Phaseolus vulgaris) enhanced proline content and LRWC85 thus efficient water uptake is related to EBL action. Calcium application also assists water and mineral uptake as it controls the transport of Na+ ions27.
To combat the negative effects of salt stress, plants trigger the production of osmolytic cytosolutes86. Proline and GB helped to alleviate salt stress in Indian mustard87, rice14, mung bean13, linseed7, mulberry11. Both of these osmolytes help in cell osmoregulation under salinity13,49. Proline shields the photosynthetic machinery and acts as a molecular chaperone, energy storage, and protects membranes and enzyme activity88,89. Proline can also reduce oxidative stress by neutralizing the effect of free radicals, bringing down the elevated levels of H2O2 and MDA, and increasing the activities of those enzymes associated with ROS scavenging90. GB detoxifies excess ROS, maintains proper functioning of photosynthetic machinery, and modulates gene activation related to stress17,91. GB also plays an adaptive role in controlling osmotic adjustment and protecting cellular and subcellular constituents, protecting machinery at the transcriptional and translational level, and as a molecular chaperone in the folding of enzymes to protect proteins from damage induced by various abiotic stresses92. GB prevents the inactivation of Rubisco and the oxygen-evolving complex of PSII93. Hence, in our study, proline and GB maintained their elevated levels in tomato seedlings during salt stress, and these levels increased further with supplementation of EBR and Ca. Epibrassinolide enhances the proline content in many plant species under stress94,95. Yusuf, et al.96 reported an increase in proline content and proline metabolism enzymes’ activities after application of epibrassinolide to salt-stressed wheat plants. Enhanced synthesis of proline and GB is related to the restoration of photosynthetic efficiency and photoassimilate production, plant growth and reduces salt mediated oxidative stress7,14. Foliar application of proline in B. juncea also mitigated the salt driven changes and overwhelmed the salt stress changes in growth, photosynthesis, and yield parameters97. Ca is an important signaling molecule and is involved in proline biosynthesis98. According to Yoo, et al.99, a calcium-signaling unit (calmodulin) activates the transcription factor MYB2 (myeloblastosis), which in turn activates many downstream genes including pyrroline-5-carboxlyate synthetase 1 (P5CS1). Parre, et al.100 reported overexpression of the P5CS1 gene under salt stress in Arabidopsis by another Ca-signaling component, phospholipase “C”.
Hydrogen peroxide, malondialdehyde content and electrolyte leakage
H2O2 and MDA production and EL are indicators of oxidative stress biomarkers under abiotic stress14,20. Tomato plants under salt stress produced more MDA and H2O2 than control plants, which consequently increased EL; these results are similar to those in wheat101, and chickpea1,27,49. Supplementation with EBR and Ca in salt-stressed tomato seedlings reduced these values indicating the efficacy of EBR and Ca to alleviate salt-induced oxidative stress. These findings agree with previous studies on O. sativa21,31,102, tomato103. Supplementation with EBR reduced salt-driven oxidative stress in tomato seedlings more so in combination with Ca than an individual application. EBL supplementation enhanced membrane stability in iron-deficient peanuts104, and decreased the production of H2O2 in chickpea seedlings under mercury stress20 which in turn reduced lipid peroxidation and electrolyte leakage. Thus, EBL can be directly related to membrane protection under salinity stress. Ca bonds to the phospholipid bilayer of cellular membranes thus stabilizing the lipid bilayer and providing structural integrity during stress105. Similar findings related to the protective role of Ca and EBR during saline and other stress environments have been substantiated in Indian mustard56, wheat96, mung bean106, chickpea27, and tomato107. Therefore, a combined dose of EBR + Ca could serve as an effective practice to reduce salt-induced oxidative stress.
Antioxidants and the ascorbate–glutathione cycle
Plants have evolved efficient resistance mechanisms, such as enzymatic and non-enzymatic antioxidants, which regulate oxidation reactions and protect plant cells from oxidative damage by scavenging ROS108. SOD is considered the first line of defense against abiotic-induced oxidative stress. It catalyzes the dismutation reaction of the superoxide anion (\({{\rm{O}}}_{2}^{\cdot -}\)) into oxygen (O2) and H2O2 and this oxidant H2O2 is reduced by APX, CAT and GPX to water109. In the present study, both APX and CAT activity increased in tomato seedlings in response to salt stress, which is a similar response in other species like O. sativa110, C. arietinum1, B. juncea3,4,26.
CAT has a high turnover rate, but with lower affinity for H2O2 than APX. It is generally accepted that CAT involves the removal of overproduced H2O2 during oxidative stress27,49. NaCl enhanced SOD, CAT, APX and GR activities in chickpea1, tomato111 and wheat112, Acacia113. Application of EBR further enhanced antioxidant enzyme activity in the present study, which is consistent with the findings in cucumber22,24, mung bean57, wheat114 under NaCl stress. The H2O2 produced is detoxified by APX into H2O using AsA as the substrate. The increased GR activity after salt stress provides GSH which reduces DHAR to dehydroascorbate (DHA) to AsA via the AsA–GSH cycle. GSH is oxidized to GSSG and subsequently recycled by GR, thus, the ratio of GSH/GSSG is important for sustaining the cell redox state115. GR activity is enhanced by EBL supplementation and protects the photosynthetic machinery from superoxide radicals by maintaining the NADP+ concentration for electron transport57. According to Yuan, et al.116, EBL enhances GR activity to maintain the GSH/GSSG ratio for normal cell functioning. Upregulation of GR activity with EBL application protects the photosynthetic apparatus from the toxic effects of superoxide radicals by maintaining optimal concentrations of NADP+ for electron transport57.
Ascorbate and glutathione are powerful antioxidants117 and they serve as redox buffering agents and prevent oxidation of the plasma membrane118. Glutathione and ascorbate can donate electrons for key enzymes such as APX and GPX119. Other enzymes in the ascorbate–glutathione cycle (MDHAR, DHAR, GR) also play a role in the management of oxidative stress tolerance120,121. In the present study, the GSH/GSSG ratio, DHAR and MDHAR activities, and AsA content in salt-stressed tomato seedlings declined, but EBR and Ca restored and upregulated the enzyme activities. EBR enhanced the synthesis of GSH and AsA through the activation of MDHAR and DHAR enzymes thus providing a maximum supply of AsA to APX and GSSG to GR103. Increased MDHAR and DHAR activities with EBL supplementation have also been reported in B. juncea122, Acacia gerrardii113 and Solanum lycopersicum123. Salt-stressed tomato seedlings supplemented with Ca and EBR had much higher MDHAR and DHAR activities than the controls, which suggests that the radicals produced by APX during the H2O2 reduction reaction to water were transferred immediately back into AsA via MDHAR or through spontaneous disproportion processes123.
GSH plays a significant role in maintaining the GSH/GSSG ratio in the conversion of GSSG to GSH. The combined application of EBR + Ca increased GSH production thereby converting more GSSG to its reduced form and creating a reduced redox homeostatic environment. Glutathione S-transferase is an enzyme which enhances plants’ survival under salt stress conditions124. In the present study GST increased in all the treatments viz, NaCl, NaCl + EBR, NaCl + EBR + Ca in tomato seedlings, which indicates a promising role of thiols in the detoxification of salinity stress. EBR enhanced the activity of GST and suppressed lipid peroxy-radicals in tomato plants under hydrocarbon stress123. Thus it may be concluded that from above discussion that EBR and Ca modulated the AsA-GSH cycle to a redox state in presence of salt stress which plays a profound role in imparting salt stress tolerance to tomato. The whole summary of antioxidants has been apprehensively depicted in.
Flavonoids
Plants that accumulate higher flavonoid content have greater salt tolerance than plants with low flavonoid-accumulating capacity125. Flavonoid content increases in many plants subjected to salt stress, and being electron donating agents, they have antioxidative properties to scavenge ROS126. Salt stress enhanced flavonoid content in black nightshade (Solanum nigrum127) and common bean (Phaseolus vulgaris)128. Oxidative stress imparts pressure on the flavonoid pathway to enhance flavonoid synthesis129. Abiotic stress frequently enhances flavonoid content to protect plants from osmotic and oxidative stress27. Flavonoids inhibit the lipoxygenase enzyme that is responsible for the conversion of polyunsaturated fatty acids to oxygen-containing derivatives130. Accumulation of these flavonoids helps to decrease lipid peroxidation and strengthen membrane protection131. Application of brassinosteroids enhanced the flavonoid content in heart-leaved moonseed (Tinospora cordifolia) with the highest concentration reported in leaves132, tomato123 and tea plants133, Similarly, Ca supplementation enhanced the flavonoid content in chickpea27. Enhanced flavonoid content in Ca-supplied NaCl-stressed seedlings is attributed to (i) restricted uptake of Na+ ions by Ca, and (ii) induction of gene expression for polyphenol biosynthesis76. Thus, EBR and Ca synergistically increase the ROS scavenging capacity which may correspond to the increase in flavonoid content under salt stress in the present study.
Ion accumulation
Under saline conditions, Na+ ions surround the rhizosphere ready to enter the root; this causes a large electrochemical gradient of ions, which results in an influx of Na+ ions via membrane-located channels and transporters on the plasma membrane134,135. The antagonistic effect that persists between Na+ and essential minerals ions, such as K+ and Ca2+, at their site of uptake results in an ion imbalance by altering Ca2+ and the ratios of Na+/K+ under salt stress. In our study, we reported an influx of excess Na+ ions which elevated their endogenous concentration to trigger K+ efflux, reflected in the low K+ content, resulting in disturbed ion homeostasis which may displace Ca2+ by Na+. The salt ion influx in to the cell and mineral ion leakage from the cell may also lead to higher ROS accumulation. However, both EBR and Ca maintained mineral ion homeostasis by increasing Ca2+ and K+ concentrations and decreasing the Na+/K+ ratio under salt stress and control conditions, thereby conferring salt tolerance by regulating ROS production. Ca and K are required by plants for various enzymatic activities, and a deficiency of these elements under NaCl stress will inhibit protein synthesis and reduce growth136. Supplementation with EBR enhanced Ca2+ and K+ concentrations and the K+/Na+ ratio in wheat under salt stress136. Supplementation of Ca2+ externally enhanced the uptake of mineral elements thus improves photosynthesis137, and at the same time hampers Na+ uptake.
Conclusion and future prospectus
The present study revealed that salt stress has a negative impact on growth and photosynthetic pigments in tomato seedlings. It also increases the production of ROS which causes lipid peroxidation and electrolyte leakage. Pretreatment of seeds with EBL and supplementation of Ca enhanced growth and the pigment system and decreased ROS accumulation through the scavenging activities of antioxidants. EBL and Ca reversed the negative impact of NaCl stress through the modulation of physiological attributes, biochemical parameters, and enzymatic and non-enzymatic activities of antioxidants. Our results demonstrate that supplementation with EBR and Ca to restrain salt stress could pave the way forward to boost salt-stress tolerance in salt-challenged fields which is vital to future crop productivity.
References
Rasool, S., Ahmad, A., Siddiqi, T. O. & Ahmad, P. Changes in growth, lipid peroxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta Physiol. Plant. 35, 1039–1050, https://doi.org/10.1007/s11738-012-1142-4 (2013).
Dassanayake, M. & Larkin, J. C. Making Plants Break a Sweat: the Structure, Function, and Evolution of Plant Salt Glands. Front. Plant Sci. 8, 406 (2017).
Ahmad, P. et al. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L) through antioxidative defense system. Front. Plant Sci. 6, https://doi.org/10.3389/fpls.2015.00868 (2015).
Fatma, M., Masood, A., Per, T. S. & Khan, N. A. Nitric Oxide Alleviates Salt Stress Inhibited Photosynthetic Performance by Interacting with Sulfur Assimilation in Mustard. Front. Plant Sci. 7, https://doi.org/10.3389/fpls.2016.00521 (2016).
Shabala, S. & Cuin, T. A. Potassium transport and plant salt tolerance. Physiol. Plant. 133, 651–669, https://doi.org/10.1111/j.1399-3054.2007.01008.x (2008).
Wan, H. et al. Genome-Wide Association Study Reveals the Genetic Architecture Underlying Salt Tolerance-Related Traits in Rapeseed (Brassica napus L.). Front. Plant Sci. 8, https://doi.org/10.3389/fpls.2017.00593 (2017).
Khan, M. N., Siddiqui, M. H., Mohammad, F., Naeem, M. & Khan, M. M. A. Calcium chloride and gibberellic acid protect linseed (Linum usitatissimum L.) from NaCl stress by inducing antioxidative defence system and osmoprotectant accumulation. Acta Physiol. Plant. 32, 121–132, https://doi.org/10.1007/s11738-009-0387-z (2010).
Khan, M. N., Siddiqui, M. H., Mohammad, F. & Naeem, M. Interactive role of nitric oxide and calcium chloride in enhancing tolerance to salt stress. Nitric Oxide 27, 210–218, https://doi.org/10.1016/j.niox.2012.07.005 (2012).
Ahmad, P., Ozturk, M. & Gucel, S. Oxidative damage and antioxidants induced by heavy metal stress in two cultivars of mustard (Brassica juncea L.) plants. Fresenius Environ. Bull. 21, 2953–2961 (2012).
Ahmad, P., Azooz, M. & Prasad, M. N. V. Ecophysiology and responses of plants under salt stress. (Springer Science & Business Media, 2012).
Ahmad, P., Ozturk, M., Sharma, S. & Gucel, S. Effect of sodium carbonate-induced salinity–alkalinity on some key osmoprotectants, protein profile, antioxidant enzymes, and lipid peroxidation in two mulberry (Morus alba L.) cultivars. J. Plant Interact. 9, 460–467, https://doi.org/10.1080/17429145.2013.855271 (2014).
Ahmad, P. Oxidative damage to plants, antioxidant networks and signaling. (Academic Press, 2013).
Khan, M. I. R., Asgher, M. & Khan, N. A. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol. Biochem. 80, 67–74, https://doi.org/10.1016/j.plaphy.2014.03.026 (2014).
Hasanuzzaman, M. et al. Exogenous Proline and Glycine Betaine Mediated Upregulation of Antioxidant Defense and Glyoxalase Systems Provides Better Protection against Salt-Induced Oxidative Stress in Two Rice (Oryza sativa L.) Varieties. BioMed Research International 2014, 1–17, https://doi.org/10.1155/2014/757219 (2014).
Shabala, L. et al. Cell-Type-Specific H+ -ATPase Activity in Root Tissues Enables K+ Retention and Mediates Acclimation of Barley (Hordeum vulgare) to Salinity Stress. Plant Physiol. 172, 2445–2458, https://doi.org/10.1104/pp.16.01347 (2016).
Hossain, M. S., ElSayed, A. I., Moore, M. & Dietz, K.-J. Redox and Reactive Oxygen Species Network in Acclimation for Salinity Tolerance in Sugar Beet. J. Exp. Bot. 68, 1283–1298, https://doi.org/10.1093/jxb/erx019 (2017).
Khan, M. I. R., Iqbal, N., Masood, A. & Khan, N. A. Variation in Salt Tolerance of Wheat Cultivars: Role of Glycinebetaine and Ethylene. Pedosphere 22, 746–754, https://doi.org/10.1016/s1002-0160(12)60060-5 (2012).
Sharma, A. et al. Pre-sowing Seed Treatment with 24-Epibrassinolide Ameliorates Pesticide Stress in Brassica juncea L. through the Modulation of Stress Markers. Front. Plant Sci. 7, https://doi.org/10.3389/fpls.2016.01569 (2016).
Rajewska, I., Talarek, M. & Bajguz, A. Brassinosteroids and Response of Plants to Heavy Metals Action. Front. Plant Sci. 7, https://doi.org/10.3389/fpls.2016.00629 (2016).
Ahmad, P. et al. Modification of Osmolytes and Antioxidant Enzymes by 24-Epibrassinolide in Chickpea Seedlings Under Mercury (Hg) Toxicity. J. Plant Growth Regul. 37, 309–322, https://doi.org/10.1007/s00344-017-9730-6 (2017).
Sharma, I., Ching, E., Saini, S., Bhardwaj, R. & Pati, P. K. Exogenous application of brassinosteroid offers tolerance to salinity by altering stress responses in rice variety Pusa Basmati-1. Plant Physiol. Biochem. 69, 17–26, https://doi.org/10.1016/j.plaphy.2013.04.013 (2013).
Fariduddin, Q., Mir, B. A., Yusuf, M. & Ahmad, A. 24-epibrassinolide and/or putrescine trigger physiological and biochemical responses for the salt stress mitigation in Cucumis sativus L. Photosynthetica 52, 464–474, https://doi.org/10.1007/s11099-014-0052-7 (2014).
Wani, A. S., Hayat, S., Ahmad, A. & Tahir, I. Efficacy of brassinosteroid analogues in the mitigation of toxic effects of salt stress in Brassica juncea plants. J. Environ. Biol. 38, 27–36, https://doi.org/10.22438/jeb/38/1/ms-196 (2017).
Fariduddin, Q., Khalil, R. R. A. E., Mir, B. A., Yusuf, M. & Ahmad, A. 24-Epibrassinolide regulates photosynthesis, antioxidant enzyme activities and proline content of Cucumis sativus under salt and/or copper stress. Environ. Monit. Assess. 185, 7845–7856, https://doi.org/10.1007/s10661-013-3139-x (2013).
Sun, S., An, M., Han, L. & Yin, S. Foliar application of 24-epibrassinolide improved salt stress tolerance of perennial ryegrass. HortScience 50, 1518–1523 (2015).
Ahmad, P. et al. Alleviation of Cadmium Toxicity in Brassica juncea L. (Czern. & Coss.) by Calcium Application Involves Various Physiological and Biochemical Strategies. Plos One 10, e0114571, https://doi.org/10.1371/journal.pone.0114571 (2015).
Ahmad, P. et al. Calcium and Potassium Supplementation Enhanced Growth, Osmolyte Secondary Metabolite Production, and Enzymatic Antioxidant Machinery in Cadmium-Exposed Chickpea (Cicer arietinum L.). Front. Plant Sci. 7, https://doi.org/10.3389/fpls.2016.00513 (2016).
Cao, X.-Q. et al. Biotic and Abiotic Stresses Activate Different Ca2+ Permeable Channels in Arabidopsis. Front. Plant Sci. 8, https://doi.org/10.3389/fpls.2017.00083 (2017).
Sharma, D., Jamra, G., Singh, U. M., Sood, S. & Kumar, A. Calcium Biofortification: Three Pronged Molecular Approaches for Dissecting Complex Trait of Calcium Nutrition in Finger Millet (Eleusine coracana) for Devising Strategies of Enrichment of Food Crops. Front. Plant Sci. 7, https://doi.org/10.3389/fpls.2016.02028 (2017).
Al-Whaibi, M. H., Siddiqui, M. H. & Basalah, M. O. Salicylic acid and calcium-induced protection of wheat against salinity. Protoplasma 249, 769–778, https://doi.org/10.1007/s00709-011-0322-1 (2012).
Rahman, A., Nahar, K., Hasanuzzaman, M. & Fujita, M. Calcium Supplementation Improves Na+/K+ Ratio, Antioxidant Defense and Glyoxalase Systems in Salt-Stressed Rice Seedlings. Front. Plant Sci. 7, https://doi.org/10.3389/fpls.2016.00609 (2016).
Arnon, D. I. Copper enzymes in isolated chloroplasts, polyphenoxidase in Beta vulgaris. Plant Physiol. 24, 1–15, https://doi.org/10.1104/pp.24.1.1 (1949).
Li, P.-M., Cai, R.-G., Gao, H.-Y., Peng, T. & Wang, Z.-L. Partitioning of excitation energy in two wheat cultivars with different grain protein contents grown under three nitrogen applications in the field. Physiol. Plant. 129, 822–829, https://doi.org/10.1111/j.1399-3054.2007.00880.x (2007).
Yamasaki, S. & Dillenburg, L. R. Measurements of leaf relative water content in Araucaria angustifolia. Revista Brasilleira de fisiologia vegetal 11, 69–75 (1999).
Bates, L. S., Waldren, R. P. & Teare, I. D. Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207, https://doi.org/10.1007/bf00018060 (1973).
Grieve, C. M. & Grattan, S. R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70, 303–307, https://doi.org/10.1007/bf02374789 (1983).
Velikova, V., Yordanov, I. & Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 151, 59–66, https://doi.org/10.1016/s0168-9452(99)00197-1 (2000).
Madhava Rao, K. V. & Sresty, T. V. S. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 157, 113–128, https://doi.org/10.1016/s0168-9452(00)00273-9 (2000).
Dionisio-Sese, M. L. & Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 135, 1–9, https://doi.org/10.1016/s0168-9452(98)00025-9 (1998).
Dhindsa, R. S. & Matowe, W. Drought Tolerance in Two Mosses: Correlated with Enzymatic Defence Against Lipid Peroxidation. J. Exp. Bot. 32, 79–91, https://doi.org/10.1093/jxb/32.1.79 (1981).
Aebi, H. In Methods Enzymol. Vol. 105, (eds Colowick, S. P. & Kaplan, N. O.) 121–126 (Elsevier, 1984).
Hasanuzzaman, M. & Fujita, M. Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 22, 584–596, https://doi.org/10.1007/s10646-013-1050-4 (2013).
Nakano, Y. & Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22, 867–880 (1981).
Foster, J. G. & Hess, J. L. Responses of Superoxide Dismutase and Glutathione Reductase Activities in Cotton Leaf Tissue Exposed to an Atmosphere Enriched in Oxygen. Plant Physiol. 66, 482–487, https://doi.org/10.1104/pp.66.3.482 (1980).
Miyake, C. & Asada, K. Thylakoid-bound ascorbate peroxidase in spinach chloroplasts and photoreduction of its primary oxidation product monodehydroascorbate radicals in thylakoids. Plant Cell Physiol. 33, 541–553 (1992).
Huang, C. et al. Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J. Exp. Bot. 56, 3041–3049, https://doi.org/10.1093/jxb/eri301 (2005).
Yu, C.-W., Murphy, T. M. & Lin, C.-H. Hydrogen peroxide-induced chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct. Plant Biol. 30, 955, https://doi.org/10.1071/fp03091 (2003).
Zhishen, J., Mengcheng, T. & Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 64, 555–559, https://doi.org/10.1016/s0308-8146(98)00102-2 (1999).
Ahmad, P. et al. Nitric Oxide Mitigates Salt Stress by Regulating Levels of Osmolytes and Antioxidant Enzymes in Chickpea. Front. Plant Sci. 7, https://doi.org/10.3389/fpls.2016.00347 (2016).
Li, H., Zhu, Y., Hu, Y., Han, W. & Gong, H. Beneficial effects of silicon in alleviating salinity stress of tomato seedlings grown under sand culture. Acta Physiol. Plant. 37, https://doi.org/10.1007/s11738-015-1818-7 (2015).
Jan, A. U., Hadi, F., Midrarullah, Nawaz, M. A. & Rahman, K. Potassium and zinc increase tolerance to salt stress in wheat (Triticum aestivum L.). Plant Physiol. Biochem. 116, 139–149, https://doi.org/10.1016/j.plaphy.2017.05.008 (2017).
Mostofa, M. G., Saegusa, D., Fujita, M. & Tran, L.-S. P. Hydrogen Sulfide Regulates Salt Tolerance in Rice by Maintaining Na+/K+ Balance, Mineral Homeostasis and Oxidative Metabolism Under Excessive Salt Stress. Front. Plant Sci. 6, https://doi.org/10.3389/fpls.2015.01055 (2015).
Abbas, S., Latif, H. & Elsherbiny, E. A. Effect of 24-epibrassinolide on the physiological and genetic changes on two varieties of pepper under salt stress conditions. Pak. J. Bot 45, 1273–1284 (2013).
Shahid, M. et al. Brassinosteroid (24-Epibrassinolide) Enhances Growth and Alleviates the Deleterious Effects Induced by Salt Stress in Pea (‘Pisum sativum’L.). Aust. J. Crop. Sci. 5, 500 (2011).
Hojjatnooghi, F., Mozafari, V., Tajabadipour, A. & Hokmabadi, H. Effects of salinity and calcium on the growth and chemical composition of pistachio seedlings. J. Plant Nutr. 37, 928–941 (2014).
Yousuf, P. Y., Ahmad, A., Hemant Ganie, A., Aref, I. M. & Iqbal, M. Potassium and calcium application ameliorates growth and oxidative homeostasis in salt-stressed indian mustard (Brassica juncea) Plants. Pak. J. Bot 47, 1629–1639 (2015).
Hayat, S., Hasan, S. A., Yusuf, M., Hayat, Q. & Ahmad, A. Effect of 28-homobrassinolide on photosynthesis, fluorescence and antioxidant system in the presence or absence of salinity and temperature in Vigna radiata. Environ. Exp. Bot. 69, 105–112, https://doi.org/10.1016/j.envexpbot.2010.03.004 (2010).
Cerana, R. et al. Effects of a brassinosteroid on growth and electrogenic proton extrusion in Azuki bean epicotyls. Physiol. Plant. 59, 23–27, https://doi.org/10.1111/j.1399-3054.1983.tb06565.x (1983).
Haubrick, L. L. & Assmann, S. M. Brassinosteroids and plant function: some clues, more puzzles. Plant, Cell and Environment 29, 446–457, https://doi.org/10.1111/j.1365-3040.2005.01481.x (2006).
Sharma, M. & Laxmi, A. Jasmonates: Emerging Players in Controlling Temperature Stress Tolerance. Front. Plant Sci. 6, https://doi.org/10.3389/fpls.2015.01129 (2016).
Neelam, S. & Subramanyam, R. Alteration of photochemistry and protein degradation of photosystem II from Chlamydomonas reinhardtii under high salt grown cells. J. Photochem. Photobiol. B: Biol. 124, 63–70, https://doi.org/10.1016/j.jphotobiol.2013.04.007 (2013).
Kanwar, M. K. et al. Isolation and characterization of 24-Epibrassinolide from Brassica juncea L. and its effects on growth, Ni ion uptake, antioxidant defense of Brassica plants and in vitro cytotoxicity. Acta Physiol. Plant. 35, 1351–1362, https://doi.org/10.1007/s11738-012-1175-8 (2013).
Li, X.-J. et al. Overexpression of a brassinosteroid biosynthetic gene Dwarf enhances photosynthetic capacity through activation of Calvin cycle enzymes in tomato. BMC Plant Biol. 16, https://doi.org/10.1186/s12870-016-0715-6 (2016).
Choudhary, S. P., Kanwar, M., Bhardwaj, R., Yu, J.-Q. & Tran, L.-S. P. Chromium Stress Mitigation by Polyamine-Brassinosteroid Application Involves Phytohormonal and Physiological Strategies in Raphanus sativus L. Plos One 7, e33210, https://doi.org/10.1371/journal.pone.0033210 (2012).
Lechowski, Z. & Białczyk, J. Calcium mediated cytokinin action on chlorophyll synthesis in isolated embryo of Scots pine. Biol. Plant. 35, https://doi.org/10.1007/bf02921119 (1993).
Gururani, M. A., Venkatesh, J. & Tran, L. S. P. Regulation of Photosynthesis during Abiotic Stress-Induced Photoinhibition. Molecular Plant 8, 1304–1320, https://doi.org/10.1016/j.molp.2015.05.005 (2015).
Parveen, N. & Ashraf, M. Role of silicon in mitigating the adverse effects of salt stress on growth and photosynthetic attributes of two maize (Zea mays L.) cultivars grown hydroponically. Pak J Bot 42, 1675–1684 (2010).
Wang, S. et al. Silicon enhanced salt tolerance by improving the root water uptake and decreasing the ion toxicity in cucumber. Front. Plant Sci. 6, https://doi.org/10.3389/fpls.2015.00759 (2015).
Mahmood, S. et al. Plant Growth Promoting Rhizobacteria and Silicon Synergistically Enhance Salinity Tolerance of Mung Bean. Front. Plant Sci. 7, https://doi.org/10.3389/fpls.2016.00876 (2016).
Iyengar, E. & Reddy, M. Photosynthesis in highly salt tolerant plants. Handbook of photosynthesis. Marshal Dekar, Baten Rose, USA 909 (1996).
Cheng, W. et al. In Proceedings of the 5th International Conference on Advanced Design and Manufacturing Engineering (Atlantis Press, 2015).
Lima, J. V. & Lobato, A. K. S. Brassinosteroids improve photosystem II efficiency, gas exchange, antioxidant enzymes and growth of cowpea plants exposed to water deficit. Physiol. Mol. Biol. Plants 23, 59–72, https://doi.org/10.1007/s12298-016-0410-y (2017).
Hu, W.-h et al. 24-Epibrassinosteroid alleviate drought-induced inhibition of photosynthesis in Capsicum annuum. Sci. Hort. 150, 232–237, https://doi.org/10.1016/j.scienta.2012.11.012 (2013).
Yu, J. Q. A role for brassinosteroids in the regulation of photosynthesis in Cucumis sativus. J. Exp. Bot. 55, 1135–1143, https://doi.org/10.1093/jxb/erh124 (2004).
Sinha, S. K., Srivastava, H. S. & Tripathi, R. D. Influence of some growth regulators and cations on inhibition of chlorophyll biosynthesis by lead in maize. Bull. Environ. Contam. Toxicol. 51, https://doi.org/10.1007/bf00198887 (1993).
Xu, C., Li, X. & Zhang, L. The Effect of Calcium Chloride on Growth, Photosynthesis, and Antioxidant Responses of Zoysia japonica under Drought Conditions. Plos One 8, e68214, https://doi.org/10.1371/journal.pone.0068214 (2013).
Arshi, A., Abdin, M. Z. & Iqbal, M. Effect of CaCl2 on growth performance, photosynthetic efficiency and nitrogen assimilation of Cichorium intybus L. grown under NaCl stress. Acta Physiol. Plant. 28, 137–147, https://doi.org/10.1007/s11738-006-0040-z (2006).
Choi, H. G., Moon, B. Y. & Kang, N. J. Correlation between Strawberry (Fragaria ananassa Duch.) Productivity and Photosynthesis-Related Parameters under Various Growth Conditions. Front. Plant Sci. 7, https://doi.org/10.3389/fpls.2016.01607 (2016).
Wu, X., Zhu, Z., Li, X. & Zha, D. Effects of cytokinin on photosynthetic gas exchange, chlorophyll fluorescence parameters and antioxidative system in seedlings of eggplant (Solanum melongena L.) under salinity stress. Acta Physiol. Plant. 34, 2105–2114, https://doi.org/10.1007/s11738-012-1010-2 (2012).
Megdiche, W. et al. Photosynthesis and photosystem 2 efficiency of two salt-adapted halophytic seashore Cakile maritima ecotypes. Photosynthetica 46, 410–419, https://doi.org/10.1007/s11099-008-0073-1 (2008).
Shu, S., Guo, S.-R., Sun, J. & Yuan, L.-Y. Effects of salt stress on the structure and function of the photosynthetic apparatus in Cucumis sativus and its protection by exogenous putrescine. Physiol. Plant. 146, 285–296, https://doi.org/10.1111/j.1399-3054.2012.01623.x (2012).
Shahbaz, M., Ashraf, M. & Athar, H.-U.-R. Does exogenous application of 24-epibrassinolide ameliorate salt induced growth inhibition in wheat (Triticum aestivum L.)? Plant Growth Regul. 55, 51–64, https://doi.org/10.1007/s10725-008-9262-y (2008).
Lu, Q. & Lu, C. Photosynthetic pigment composition and photosystem II photochemistry of wheat ears. Plant Physiol. Biochem. 42, 395–402, https://doi.org/10.1016/j.plaphy.2004.02.008 (2004).
Houimli, S. I. M., Denden, M. & El Hadj, S. B. Induction of salt tolerance in pepper (Capsicum annuum) by 24-epibrassinolide. EurAsian Journal of BioSciences 2, 83–90 (2008).
Rady, M. M. Effect of 24-epibrassinolide on growth, yield, antioxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Sci. Hort. 129, 232–237, https://doi.org/10.1016/j.scienta.2011.03.035 (2011).
Per, T. S. et al. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant Physiol. Biochem. 115, 126–140, https://doi.org/10.1016/j.plaphy.2017.03.018 (2017).
Iqbal, N., Umar, S. & Khan, N. A. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J. Plant Physiol. 178, 84–91, https://doi.org/10.1016/j.jplph.2015.02.006 (2015).
Szabados, L. & Savouré, A. Proline: a multifunctional amino acid. Trends Plant Sci. 15, 89–97, https://doi.org/10.1016/j.tplants.2009.11.009 (2010).
Reddy, P. S. et al. Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor (L.) Moench]. Plant Physiol. Biochem. 94, 104–113, https://doi.org/10.1016/j.plaphy.2015.05.014 (2015).
Bhaskara, G. B., Yang, T.-H. & Verslues, P. E. Dynamic proline metabolism: importance and regulation in water limited environments. Front. Plant Sci. 6, https://doi.org/10.3389/fpls.2015.00484 (2015).
Chen, T. H. H. & Murata, N. Glycinebetaine: an effective protectant against abiotic stress in plants. Trends Plant Sci. 13, 499–505, https://doi.org/10.1016/j.tplants.2008.06.007 (2008).
Wani, S. H., Singh, N. B., Haribhushan, A. & Mir, J. I. Compatible Solute Engineering in Plants for Abiotic Stress Tolerance - Role of Glycine Betaine. Curr. Genomics 14, 157–165, https://doi.org/10.2174/1389202911314030001 (2013).
Ohnishi, N. & Murata, N. Glycinebetaine Counteracts the Inhibitory Effects of Salt Stress on the Degradation and Synthesis of D1 Protein during Photoinhibition in Synechococcus sp. PCC 7942. Plant Physiol. 141, 758–765, https://doi.org/10.1104/pp.106.076976 (2006).
Choudhary, S. P., Kanwar, M., Bhardwaj, R., Gupta, B. D. & Gupta, R. K. Epibrassinolide ameliorates Cr (VI) stress via influencing the levels of indole-3-acetic acid, abscisic acid, polyamines and antioxidant system of radish seedlings. Chemosphere 84, 592–600, https://doi.org/10.1016/j.chemosphere.2011.03.056 (2011).
Ramakrishna, B. & Rao, S. S. R. 24-Epibrassinolide alleviated zinc-induced oxidative stress in radish (Raphanus sativus L.) seedlings by enhancing antioxidative system. Plant Growth Regul. 68, 249–259, https://doi.org/10.1007/s10725-012-9713-3 (2012).
Yusuf, M., Fariduddin, Q., Khan, T. A. & Hayat, S. Epibrassinolide reverses the stress generated by combination of excess aluminum and salt in two wheat cultivars through altered proline metabolism and antioxidants. S. Afr. J. Bot. 112, 391–398, https://doi.org/10.1016/j.sajb.2017.06.034 (2017).
Wani, A. S., Ahmad, A., Hayat, S. & Tahir, I. Is foliar spray of proline sufficient for mitigation of salt stress in Brassica juncea cultivars? Environmental Science and Pollution Research 23, 13413–13423, https://doi.org/10.1007/s11356-016-6533-4 (2016).
Verslues, P. E. & Sharma, S. Proline Metabolism and Its Implications for Plant-Environment Interaction. The Arabidopsis Book 8, e0140, https://doi.org/10.1199/tab.0140 (2010).
Yoo, J. H. et al. Direct Interaction of a Divergent CaM Isoform and the Transcription Factor, MYB2, Enhances Salt Tolerance in Arabidopsis. J. Biol. Chem. 280, 3697–3706, https://doi.org/10.1074/jbc.m408237200 (2005).
Parre, E. et al. Calcium Signaling via Phospholipase C Is Essential for Proline Accumulation upon Ionic But Not Nonionic Hyperosmotic Stresses in Arabidopsis. Plant Physiol. 144, 503–512, https://doi.org/10.1104/pp.106.095281 (2007).
Zheng, Y. et al. Potassium nitrate application alleviates sodium chloride stress in winter wheat cultivars differing in salt tolerance. J. Plant Physiol. 165, 1455–1465, https://doi.org/10.1016/j.jplph.2008.01.001 (2008).
Sharma, I., Bhardwaj, R. & Pati, P. K. Stress modulation response of 24-epibrassinolide against imidacloprid in an elite indica rice variety Pusa Basmati-1. Pestic. Biochem. Physiol. 105, 144–153, https://doi.org/10.1016/j.pestbp.2013.01.004 (2013).
Li, M. et al. Brassinosteroid Ameliorates Zinc Oxide Nanoparticles-Induced Oxidative Stress by Improving Antioxidant Potential and Redox Homeostasis in Tomato Seedling. Front. Plant Sci. 7, https://doi.org/10.3389/fpls.2016.00615 (2016).
Song, Y. L. et al. Role of foliar application of 24-epibrassinolide in response of peanut seedlings to iron deficiency. Biol. Plant. 60, 329–342, https://doi.org/10.1007/s10535-016-0596-4 (2016).
Hepler, P. K. Calcium: A Central Regulator of Plant Growth and Development. The Plant Cell Online 17, 2142–2155, https://doi.org/10.1105/tpc.105.032508 (2005).
Mir, B. A., Khan, T. A. & Fariduddin, Q. 24-epibrassinolide and spermidine modulate photosynthesis and antioxidant systems in Vigna radiata under salt and zinc stress. International Journal of Advanced Research 3, 592–608 (2015).
Cui, L., Zou, Z., Zhang, J., Zhao, Y. & Yan, F. 24-Epibrassinoslide enhances plant tolerance to stress from low temperatures and poor light intensities in tomato (Lycopersicon esculentum Mill.). Functional & Integrative Genomics 16, 29–35, https://doi.org/10.1007/s10142-015-0464-x (2016).
Farooq, M. A. et al. Methyl Jasmonate Regulates Antioxidant Defense and Suppresses Arsenic Uptake in Brassica napus L. Front. Plant Sci. 7, https://doi.org/10.3389/fpls.2016.00468 (2016).
Carvalho, L. C., Vidigal, P. & Amancio, S. Oxidative stress homeostasis in grapevine (Vitis vinifera L.). Frontiers in Environmental Science 3, https://doi.org/10.3389/fenvs.2015.00020 (2015).
Mishra, P., Bhoomika, K. & Dubey, R. S. Differential responses of antioxidative defense system to prolonged salinity stress in salt-tolerant and salt-sensitive Indica rice (Oryza sativa L.) seedlings. Protoplasma 250, 3–19, https://doi.org/10.1007/s00709-011-0365-3 (2013).
Manai, J., Gouia, H. & Corpas, F. J. Redox and nitric oxide homeostasis are affected in tomato (Solanum lycopersicum) roots under salinity-induced oxidative stress. J. Plant Physiol. 171, 1028–1035, https://doi.org/10.1016/j.jplph.2014.03.012 (2014).
Ali, Q. et al. Seed priming by sodium nitroprusside improves salt tolerance in wheat (Triticum aestivum L.) by enhancing physiological and biochemical parameters. Plant Physiol. Biochem. 119, 50–58, https://doi.org/10.1016/j.plaphy.2017.08.010 (2017).
Abd_Allah, E. F., Alqarawi, A. A., Hashem, A., Wirth, S. & Egamberdieva, D. Regulatory roles of 24-epibrassinolide in tolerance of Acacia gerrardii Benth to salt stress. Bioengineered 9, 61–71, https://doi.org/10.1080/21655979.2017.1297348 (2017).
Tofighi, C., Khavari-Nejad, R. A., Najafi, F., Razavi, K. & Rejali, F. Responses of wheat plants to interactions of 24-epibrassinolide and Glomus mosseae in saline condition. Physiol. Mol. Biol. Plants 23, 557–564, https://doi.org/10.1007/s12298-017-0439-6 (2017).
Dixit, G. et al. Sulfur mediated reduction of arsenic toxicity involves efficient thiol metabolism and the antioxidant defense system in rice. J. Hazard. Mater. 298, 241–251, https://doi.org/10.1016/j.jhazmat.2015.06.008 (2015).
Yuan, L.-Y. et al. Effects of 24-epibrassinolide on ascorbate–glutathione cycle and polyamine levels in cucumber roots under Ca(NO3)2 stress. Acta Physiol. Plant. 35, 253–262, https://doi.org/10.1007/s11738-012-1071-2 (2013).
Foyer, C. H. & Noctor, G. Ascorbate and glutathione: the heart of the redox hub. Plant Physiol. 155, 2–18 (2011).
Pandey, P., Singh, J., Achary, V. M. M. & Reddy, M. K. Redox homeostasis via gene families of ascorbate-glutathione pathway. Frontiers in Environmental Science 3, https://doi.org/10.3389/fenvs.2015.00025 (2016).
Batth, R., Singh, K., Kumari, S. & Mustafiz, A. Transcript Profiling Reveals the Presence of Abiotic Stress and Developmental Stage Specific Ascorbate Oxidase Genes in Plants. Front. Plant Sci. 8, https://doi.org/10.3389/fpls.2017.00198 (2017).
Miller, G. A. D., Suzuki, N., Ciftci-Yilmaz, S. & Mittler, R. O. N. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant, Cell Environ. 33, 453–467, https://doi.org/10.1111/j.1365-3040.2009.02041.x (2010).
de Sousa, A. et al. Oxidative Metabolism of Rye (Secale cereale L.) after Short Term Exposure to Aluminum: Uncovering the Glutathione–Ascorbate Redox Network. Front. Plant Sci. 7, https://doi.org/10.3389/fpls.2016.00685 (2016).
Arora, P., Bhardwaj, R. & Kanwar, M. K. 24-epibrassinolide regulated diminution of Cr metal toxicity in Brassica juncea L. plants. Brazilian Journal of Plant Physiology 22, 159–165, https://doi.org/10.1590/s1677-04202010000300002 (2010).
Ahammed, G. J. et al. Role of brassinosteroids in alleviation of phenanthrene–cadmium co-contamination-induced photosynthetic inhibition and oxidative stress in tomato. J. Exp. Bot. 64, 199–213, https://doi.org/10.1093/jxb/ers323 (2013).
Chan, C. & Lam, H.-M. A Putative Lambda Class Glutathione S-Transferase Enhances Plant Survival under Salinity Stress. Plant Cell Physiol. 55, 570–579, https://doi.org/10.1093/pcp/pct201 (2014).
Wahid, A. & Ghazanfar, A. Possible involvement of some secondary metabolites in salt tolerance of sugarcane. J. Plant Physiol. 163, 723–730, https://doi.org/10.1016/j.jplph.2005.07.007 (2006).
Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Polish Journal of Environmental Studies 15 (2006).
Ben Abdallah, S. et al. Salt stress (NaCl) affects plant growth and branch pathways of carotenoid and flavonoid biosyntheses in Solanum nigrum. Acta Physiol. Plant. 38, https://doi.org/10.1007/s11738-016-2096-8 (2016).
Taïbi, K. et al. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S. Afr. J. Bot. 105, 306–312, https://doi.org/10.1016/j.sajb.2016.03.011 (2016).
Agati, G. et al. The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. J. Plant Physiol. 168, 204–212, https://doi.org/10.1016/j.jplph.2010.07.016 (2011).
Nijveldt, R. J. et al. Flavonoids: a review of probable mechanisms of action and potential applications. The American Journal of Clinical Nutrition 74, 418–425, https://doi.org/10.1093/ajcn/74.4.418 (2001).
Potapovich, A. & Kostyuk, V. Comparative study of antioxidant properties and cytoprotective activity of flavonoids. Biochemistry (Moscow) 68, 514–519 (2003).
Raghu, K. & Rao, R. Effect of brassinosteroids on antioxidants content and radical scavenging activity of Tinospora cordifolia (Willd.) Miers ex Hook. F & Thoms. Journal of Medicinal Plants 4, 117–121 (2016).
Li, X. et al. Brassinosteroids Improve Quality of Summer Tea (Camellia sinensis L.) by Balancing Biosynthesis of Polyphenols and Amino Acids. Front. Plant Sci. 7, https://doi.org/10.3389/fpls.2016.01304 (2016).
De Leon, T. B., Linscombe, S., Gregorio, G. & Subudhi, P. K. Genetic variation in Southern USA rice genotypes for seedling salinity tolerance. Front. Plant Sci. 6, https://doi.org/10.3389/fpls.2015.00374 (2015).
Gu, M. F. et al. Accumulation capacity of ions in cabbage (Brassica oleracea L.) supplied with sea water. Plant Soil Environ. 62, 314–320 (2016).
Shahbaz, M. & Ashraf, M. Influence of exogenous application of brassinosteroid on growth and mineral nutrients of wheat (Triticum aestivum L.) under saline conditions. Pakistan Journal of Botany 39, 513 (2007).
Wei, T. et al. Photosynthesis is improved by exogenous calcium in head-stressed tobacco plants. J Plant Physiol. 168, 2063–2071 (2011).
Acknowledgements
Authors extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding their research group No. (RG-1438-039).
Author information
Authors and Affiliations
Contributions
Parvaiz Ahmad, Mohammed Nasser Alyemeni, Leonard Wijaya designed the experimental work. Pravej Alam and Elsayed Fathi Abd_Allah analyzed the data and helped in writing of introduction and discussion part of the manuscript. Renu Bhardwaj, M.N. Alyemeni and Kadambot H.M. Siddique carried out the statistical analysis and revised the manuscript to present form.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahmad, P., Abd_Allah, E.F., Alyemeni, M.N. et al. Exogenous application of calcium to 24-epibrassinosteroid pre-treated tomato seedlings mitigates NaCl toxicity by modifying ascorbate–glutathione cycle and secondary metabolites. Sci Rep 8, 13515 (2018). https://doi.org/10.1038/s41598-018-31917-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-31917-1
This article is cited by
-
γ-Aminobutyric acid (GABA) and ectoine (ECT) impacts with and without AMF on antioxidants, gas exchange attributes and nutrients of cotton cultivated in salt affected soil
BMC Plant Biology (2023)
-
24-Epibrasinolide Delays Chlorophyll Degradation and Stimulates the Photosynthetic Machinery in Magnesium-Stressed Soybean Plants
Journal of Plant Growth Regulation (2023)
-
Synergistic effects of exogenous glutathione and calcium on ascorbate–glutathione cycle and glutathione-associated enzymes upregulation under lead stress in Brassica napus L
Environmental Science and Pollution Research (2023)
-
24-Epibrassinolide Regulates Functional Components of Nitric Oxide Signalling and Antioxidant Defense Pathways to Alleviate Salinity Stress in Brassica juncea L. cv. Varuna
Journal of Plant Growth Regulation (2023)
-
Allantoin improves salinity tolerance in Arabidopsis and rice through synergid activation of abscisic acid and brassinosteroid biosynthesis
Plant Molecular Biology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.