Abstract
Submarine groundwater discharge (SGD) is the submarine seepage of all fluids from coastal sediments into the overlying coastal seas. It has been well documented that the SGD may contribute a great deal of allochthonous nutrients to the coastlines. It is, however, less known how much carbon enters the ocean via the SGD. Nutrients (NO3, NO2, NH4, PO4, SiO2), alkalinity and dissolved inorganic carbon (DIC) in the submarine groundwater were measured at 20 locations around Taiwan for the first time. The total N/P/Si yields from the SGD in Taiwan are respectively 3.28 ± 2.3 × 104, 2.6 ± 1.8 × 102 and 1.89 ± 1.33 × 104 mol/km2/a, compared with 9.5 ± 6.7 × 105 mol/km2/a for alkalinity and 8.8 ± 6.2 × 105 mol/km2/a for DIC. To compare with literature data, yields for the major estuary across the Taiwan Strait (Jiulong River) are comparable except for P which is extremely low. Primary production supported by these nutrient outflows is insufficient to compensate the DIC supplied by the SGD. As a result, the SGD helps making the coastal waters in Taiwan and Jiulong River heterotrophic.
Similar content being viewed by others
Introduction
Submarine groundwater discharge (SGD) is the submarine seepage of all fluids from coastal sediments into the overlying coastal areas. It has been well documented that the SGD may contribute much nutrients to the coastlines1,2,3,4,5,6,7,8,9. Excessive supply of nutrients may lead to eutrophication, hence affecting the sustainability of the coastal environment10. SGD also contains excess carbon11,12,13,14. It is, however, less known how nutrients and carbon interact after the SGD enters the oceans.
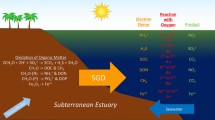
Because the groundwater has been in contact with the sediments for a long period of time it is expected that some of the particulate organic matter in the sediments would have decomposed thus consuming dissolved oxygen (DO) but releasing dissolved inorganic carbon (DIC) and dissolved organic carbon (DOC) along with nutrients. The partial pressure of CO2 (pCO2) would also increase. Part of the DOC would decompose, further increase DIC and pCO2. Some of the CaCO3 in the sediments might also dissolve thus increase the total alkalinity (TA). The groundwater is isolated from the atmosphere but when the groundwater enters the oceans it is expected that the high pCO2 in the SGD might make the receiving coastal water a CO2 source for the atmosphere. Yet, the nutrient supply from the SGD would enhance primary productivity in coastal waters, hence drawing down the pCO2 of surface waters. Whether the SGD would eventually lead to a carbon source or sink into the receiving coastal waters does not have an a priori answer.
Recently the salinity and major ions such as Ca, Mg, K, Na, Cl and SO4 in the submarine groundwater samples around Taiwan have been measured15. However, nutrients and carbon in the SGD have never been reported in the SGD from this part of the world. In fact, only a handful of studies have reported nutrients and carbon in the SGD in China7,16,17,18,19. In this study, SGD samples were collected from 20 locations around the subtropical island with an area of 35,873 km2. DO, nutrients (NO3, NO2, NH4, PO4, SiO2), N2O, CH4, DOC, pH, TA, and DIC were measured and pCO2 calculated. For comparison, we also collected data in the Jiulong River in China across the Taiwan Strait. Whether the SGD helps making the coastal waters autotrophic or heterotrophic is evaluated.
Results and Discussion
Concentrations of chemical parameters
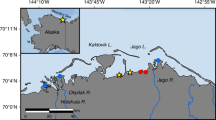
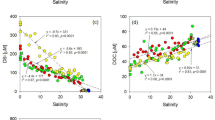
The average concentrations of chemical parameters for a total of 278 submarine groundwater measurements and for the local surface seawaters above the SGD sampling sites are given in Table 1. Salinity data are taken from Chen et al.15. Out of the 20 sampling sites 15 showed evidence of some submarine freshwater outflow (those marked is black on Fig. 1). The salinity of the SGD varies widely between 0.008 and 34.8 with an average of 21.92 ± 11.43, which is considerably lower than the average of the corresponding local surface seawater (32.5 ± 2.42). Previous study15 has indicated that the SGD export from Taiwan is about 1.07 ± 0.7 × 1010 t/a; of which, 0.38 ± 0.48 × 1010 t/a is the freshwater component. These values are, respectively, about 14% and 5.2% of the total river outflow from Taiwan, and fall within the ranges reported elsewhere. We realize that the SGD sampling sites were not distributed evenly and that seasonal data were not obtained for most sites. However, Moosdorf et al.20 provided the only other estimate of annual fresh groundwater discharge from Taiwan at 5,486 m3 per m of coastline. This compares with our freshwater SGD component of 3,200 ± 4,000 m3/m/a. Considering the large uncertainty, the agreement is reasonable. We thus went on to look at chemical species with the understanding that an uncertainty of a factor of two is to be expected. The percentage DO saturation (DO (%)) of the SGD and local surface seawater are plotted vs salinity in Fig. 2. Again, the average DO (%), 67.6 ± 21.9, is considerably lower than that of the local surface seawater (95 ± 6.47; Table 1). In general, the DO (%) in the SGD is lower when the salinity is lower (Fig. 2a; p < 0.001) because of the consumption of DO due to decomposition of organic matter in the subterranean environment isolated from the atmosphere.
Decomposition of organic matter leads to the release of nutrients. As a result, the average NO3 concentration, 27.4 ± 54.4 μM, is much higher than that of the average local surface seawater (4.84 ± 5.08 μM; Table 1). The NO3 in the SGD increases when the salinity decreases (Fig. 2b; p < 0.001). In fact, the average NO3 concentration in the local surface seawater is higher than those generally found in waters surrounding Taiwan21,22,23 (<2 μM). Although there are other sources of NO3 such as riverine input and acid rain24, the SGD has likely played a role.
NO2, NH4 and N2O are all reduced forms of NO3 and the average NH4 concentration (92.4 ± 387 μM) is much higher than that of NO3 (27.4 ± 54.4 μM; Table 1). The presence of NO3 and NO2, however, indicates that much, but not all, of the nitrogen released as a result of organic matter decomposition has been reduced to NH4. Concentrations of NO2, NH4 and N2O are much higher in the SGD compared to those found in the local surface seawater (Table 1). Higher NO2 and N2O values are generally found when the salinity is lower (Fig. 2c, p < 0.01 for NO2, and Fig. 2e, p < 0.025 for N2O, respectively) but the pattern is not as clear in the case of NH4 (p < 0.25; Fig. 2d). For PO4 the average concentration in the SGD (0.88 ± 2.44 μM) is higher than that in the local surface seawater (0.55 ± 0.48 μM; Table 1) with waters surrounding Taiwan having the lowest value21 (<0.4 μM). Worth noting is that the average (NO3 + NO2 + NH4)/PO4 ratio of 136 is much higher than the Redfield ratio of 16. This is consistent with the notion that the surface waters of rivers entering the East China Sea and the South China Sea have an average N/P ratio higher than 10025,27. In addition, P is removed from groundwater more easily than N27.
Along with nitrogen and phosphorus silicate is also a major macronutrient in the oceans, and silicate concentrations in the SGD also increase with decreasing salinity (p < 0.001; Fig. 2g). The average SiO2 concentration in the SGD (64.2 ± 58.3 μM) is also significantly higher than the average concentration in the local surface seawater (10.8 ± 17.9 μM; Table 1). Several high values above 50 μM are found in the local surface seawater compared with the generally low value of <5 μM found in waters surrounding Taiwan21. This is an indication that phytoplankton uptake is not fast enough to consume the SiO2 released by the SGD near its source.
In the reduced environment CH4 is generated. We do not have sufficient CH4 data (n = 13) to see a clear trend relative to the salinity but the average CH4 concentration (523.1 ± 1,231 nM; Table 1) in the SGD is clearly higher than that in the local surface seawater (240 ± 554 nM; Table 1). Note the CH4 concentrations in waters around Taiwan are around only 5 nM28. This indicates that the SGD inputs of CH4 do not have sufficient time to oxidize or to be released to the atmosphere near their sources.
Decomposition of organic matter generally lowers pH in the aerobic environment29 such as found in our case so the average pH (7.81 ± 0.29) of the SGD is slightly lower than that of the local surface seawater (8.10 ± 0.14; Table 1; Fig. 3a), which is similar to the pH of the waters surrounding Taiwan30,31,32,33. As for TA and DIC their values increase with the dissolution of calcareous rocks and decomposition of organic matter and it is indeed what was found. The average TA and DIC in the SGD (3,438 ± 1,417 and 3,193 ± 1,373 μmol/kg, respectively) are significantly higher than those found in the local surface seawater (2,343 ± 358 and 2,040 ± 363 μmol/kg, respectively; Table 1) and higher values are found at lower salinities (p < 0.001 in both cases; Fig. 3b,c). These TA and DIC values are more than 1,000 μmol/kg higher than those found in waters near Taiwan33,34,35.
The SGD has a high average pCO2 of 4,729 ± 13,163 μatm (Table 1; Fig. 3d) compared with the average of the local surface seawater (477 ± 479 μatm). The pCO2 shows a weak negative (p < 0.05; Fig. 3d) correlation with salinity. These values are higher than the pCO2 of surface waters found near Taiwan31,33,35,36. But, whether the surface seawater receiving the SGD is heterotrophic, i.e., whether the water is a source or sink of CO2 depends on the balance between carbon-consuming primary production and the excess DIC supplied by the SGD. The average C/N and C/P ratios of particulate matter in NW Pacific marginal seas are 8.8 and 152, respectively36,37. Based on this stoichiometry the average amount of nitrogen and phosphorus supplied by the SGD in Taiwan for each kg of water may consume 1,070 μmol/kg and 133 μmol/kg DIC, respectively. These values are much lower than the average excess DIC supported by the SGD. That is to say, primary production supported by the nutrient input from the SGD is insufficient to compensate for the high DIC and pCO2 supplied by the SGD. As a result, the SGD around Taiwan leads to a CO2 source for the atmosphere. Similar situation applies to the Jiulong and Pearl River Estuaries. Finally, decomposition of DOC also releases CO2. Indeed, the higher pCO2 values in local surface seawaters (477 ± 479 μatm; Table 1) relative to the atmosphere (~400 μatm) support this conclusion. Similar conclusion has been reported for the Pearl River Estuary16 and elsewhere38,39.
The number of DOC data is also small (n = 31) but there seems to be a trend showing high values at low salinities (p < 0.25; Fig. 3e). The average DOC in the submarine groundwater (114 ± 112 μM) is slightly higher than that in the local seawater (84 ± 27 μM; Table 1). The waters surrounding Taiwan generally have a DOC concentration below 75 μM40,41. Note Fig. 3e seems to indicate that the DOC is removed, hence becoming a source of nutrients and pCO2.
It is critical to point out that the C/N and C/P values of the SGD (Fig. 3f,g) are much higher than the Redfield Ratio. To re-iterate, the excess nutrients supplied by the SGD are insufficient to consume the excess carbon thus the SGD helps making the coastal waters heterotrophic.
Of note is that the SGD-derived DIC flux is greater than the TA flux in the Pearl River estuary, indicating that the SGD serves to reduce the CO2 buffering capacity of the local seawater29. Yet, submarine groundwaters around Taiwan the TA flux is slightly higher than the DIC flux. As a result, the SGD from Taiwan serves to increase slightly the CO2 buffering capacity of the local seawater. Even so, the high pCO2 and the high C/N and C/P ratios of Taiwan’s SGD makes it a contributor of heterotrophic nearshore waters.
The percentage saturation for aragonite (Fig. 3h) and calcite (Fig. 3i) reaches a mean value of four and six, respectively for the local surface seawater but are slightly lower in the SGD. There is no doubt that the higher TA, DIC and pCO2 of the SGD compared to the local seawater is due to the dissolution of calcareous rocks and decomposition of organic matter in the groundwater. The decomposition of DOC must also be at play hence increasing pCO2. Since the submarine groundwaters do not become anoxic sulfate reduction probably has not occurred to a great extent. Figure 4 shows ΔHCO3 plotted vs ΔCa (local seawater is taken to be with HCO3 = 2.3 mM and Ca = 10.3 mM at a salinity of 35; Ca data taken from Chen et al.)16. The samples falling around the HCO3/Ca = 2 line reflect the dissolution of CaCO3. Much of the data shows an excess of HCO3 and Ca but the pattern is not obvious.
Figure 5 shows the saturation state of aragonite and calcite plotted vs pH. Lower saturation state corresponds to lower pH, indicating that the decomposition of organic matter leads to the dissolution of calcareous rocks. The end result, however, is that the submarine groundwater is mostly highly super saturated, especially those with a pH above 7.5. The saturation state of aragonite and calcite even reach 12 and 20, respectively.
Fluxes of nutrients and carbon
Since the properties of groundwater are not expected to show much seasonal variation as compared to the flux (e.g. Szymczycha et al.39) the above conclusions represent reasonable averages. Based on the rudimentary SGD flux value reported by Chen et al.15 the annual amount of nitrogen, phosphorus, silicate, TA and DIC export due to the SGD around Taiwan are 1.18 ± 0.83 × 109, 9.3 ± 6.5 × 106, 0.68 ± 0.48 × 109, 3.43 ± 2.4 × 1010 and 3.17 ± 2.22 × 1010 mol, respectively (Table 2). Based on the river flow (http://gweb.wra.gov.tw/wrweb/) and the N, P data (http://wgshow.epa.gov.tw/) of the 25 largest rivers in Taiwan the total N and P fluxes are 1.12 × 1010 and 0.12 × 1010 mol/a, respectively. Simply stated, the SGD outflow is as much as 10.5% of the river outflow for N but only 0.78% for P.
The Jiulong River catchment across the Taiwan Strait from Taiwan is 40.8% of Taiwan’s size. The annual SGD discharge of Jiulong River is 0.213 ± 0.058 × 1010 m3 (Wang et al.)17 which is 21.3% of the total discharge for Taiwan estimated by Chen et al.15. By way of comparison, the annual discharge of N, P, Si, TA and DIC for Jiulong River are 0.58–1.21 × 109, 2.9–6.1 × 105, 0.96–2.0 × 109, 0.75–1.6 × 1010 and 0.88–1.82 × 1010 mol, respectively, based on the concentration and water discharge data of Wang et al.17 (Table 2). Recently Hong et al.19 also presented annual fluxes for N, Si and DIC at 1.83–1.95 × 109, 2.94–3.14 × 109 and 2.14–2.65 × 1010 mol, respectively (Table 2), comparable to the results of Wang et al.17.
Our study in Taiwan (Table 2) results in a SGD discharge of about 1.18 ± 0.83 × 109 mol/a N (NO3 + NO2 + NH4), which is equivalent to a yield of 3.28 ± 2.3 × 104 mol/a N per square kilometer of the total catchment area. This value is similar to the annual yield of Jiulong River at 3.90–8.23 × 104 mol/km2/a calculated based on the size of the catchment area and the flux of N reported by Wang et al.17. The flux of P resulted from this study for Taiwan is 9.3 ± 6.5 × 106 mol/a, and the yield is 260 ± 180 mol/km2/a. The P flux and yield for Jiulong River at 2.9–6.1 × 105 and 20–41 mol/km2/a (Table 2), respectively, are surprisingly low. As reported above, the N/P ratio obtained from this study for the submarine groundwater is 136. The flux data of Wang et al.17 for Jiulong River translate to a N/P ratio of 2000 which is extremely high although we realize that P is removed from groundwater. Our work for the Jiulong River (Table 3) results in an N (n = 11) to P (n = 9) ratio of 51 in the river basin and an N (n = 7) to P (n = 7) ratio of 27 in the estuary. Of note is that the average groundwater N and P concentrations calculated from the data of Wang et al.17 are 495 and 22.75 μM, respectively. The resulting N/P ratio is only 21.8, similar to what we found in the Jiulong River estuary but way below the reported ratio of 2,000 for the SGD by these authors.
Liu et al.16 reported the total annual flux of P for the Pearl River at 30–680 × 106 mol and Liu et al.42 obtained similar values. Liu et al.42 also reported the N fluxes for the Pearl River at 3.65–157 × 109 and 0.95–40 × 109 mol in summer and winter, respectively. They reported the Si fluxes at 1.9–91.3 × 109 and 0.51–23.4 × 109 mol in summer and winter, respectively (Table 2). The Pearl River (Fig. 1) has a large catchment area of 453,700 km2 which is located at the same latitude as southern Taiwan. The P flux of Liu et al.16 translates to a yield of 66–1,500 mol/km2/a and the results of Liu et al.42 are similar (Table 2). These results are comparable to those from Taiwan but much higher than those from the Jiulong River. The annual DIC flux of Liu et al.16 for the Pearl River is 15.3–34.7 × 1010 mol and the yield is 3.37–7.65 × 105 mol/km2 which is comparable with our result in Taiwan. The reported SGD N and P in the literature are also given in Table 2, and the ranges are high. Our fluxes per m2 for N and P in Taiwan are at the low end of these reported values.
The total flux of Si for Taiwan is 0.68 ± 0.48 × 109 mol/a compared with the larger flux of 0.96–2.0 × 109 mol/a (Wang et al.)17 or 2.94–3.14 × 109 mol/a (Hong et al.)19 reported for Jiulong River. As for the yield the value of 6.53–13.6 × 104 mol/km2/a calculated based on the flux data of Wang et al.17, and the value of 0.65–0.69 × 104 mol/km2a based on the data of Hong et al.19 bracket the yield of Taiwan at 1.89 ± 1.33 × 104 mol/km2/a. Liu et al.42 reported the total flux and yield of Si for the Pearl River. Although their total fluxes are high their yields also bracket our results for Taiwan. The Si fluxes reported for a subtropical bay in south China43 are slightly higher than those for Taiwan (Table 2).
The TA and DIC fluxes for Taiwan are 3.43 ± 2.4 × 1010 and 3.17 ± 2.22 × 1010 mol/a, respectively. These values compare with 0.75–1.6 × 1010 and 0.88–1.82 × 1010 mol/a, respectively, for Jiulong Rvier based on the data of Wang et al.17 (Table 2). The yield of TA for Taiwan at 9.5 ± 6.7 × 105 mol/km2/a is slightly higher than those for Jiulong River, at 5.1–10.9 × 105 mol/km2/a. The reported TA fluxes elsewhere bracket our results (Table 2). The yield of DIC for Taiwan is 8.81 ± 6.17 × 105 mol/km2/a which falls between the slightly lower value of 6–12.4 × 105 mol/km2/a (Wang et al.)17 and the slightly higher value of 14.6–18 × 105 (Hong et al.)19 for the Jiulong River. The Pearl River basin also has an abundance of calcareous rocks. The DIC yield (3.37–7.65 × 105 mol/km2/a), nevertheless, is smaller compared to our result in Taiwan. This is perhaps because the weathering is weaker in the less steep Pearl River basin. This points to the difficulty of comparing the total flux or yield. It is yet not possible to compare data per unit area of the ocean floor or per unit length of the coastal line. In terms of flux per m2 of the seepage area, however, our DIC flux falls in the range reported elsewhere as shown in Table 2. Our DOC flux (9.5 ± 9.3 mol/m2/a) is also comparable with those reported in the literature (Table 2).
Conclusions
The concentrations, fluxes and yields of N, P, Si, TA and DIC for the SGD in Taiwan have been reported for the first time, and these values are broadly comparable with the data in the literature. The nutrients supplied by the SGD are insufficient to compensate the DIC supported at the same time. As a result, the SGD around Taiwan leads to a source of CO2 for the atmosphere in the coastal seas. Similar situation exists in the Jiulong and Pearl River estuaries in Southeast China, and perhaps in other coastal regions around the world as well.
Methods
Geologically Taiwan is relatively young. The collision of the Philippine Arc and the Asian continent gave rise to the Central Range of Taiwan, and the orogenesis is still going44. The population is 23 million. The western part of Taiwan is mainly covered by undeformed sediments, and is heavily populated. Less populated is Southern Taiwan where the coasts are largely covered by coral reefs. Eastern Taiwan has a coastal range, and the less populated coasts are mainly rocky.
Preliminary sampling of the SGD in Taiwan was performed from 2004 to 2016. Twenty sampling sites around the coastal areas Taiwan are shown in Fig. 1. Measurements of SGD fluxes were reported in Chen et al.15. Submarine groundwater samples for chemical analysis were drawn by a device designed by Zhang and Satake45 mostly on the sandy coast. At one site 350 m off SW Taiwan divers collected freshwater (S = 0.008) at a water depth of 8 m. The corresponding local surface seawater sample was also collected. Samples for NO3, NO2, NH4 and PO4 were collected in Jiulong River and its estuary in 2008. Preserved samples, with saturated HgCl2 added, were brought back and measured in the laboratory with details given in Chen21, Yang et al.41 and Tseng et al.46 and 201728. The percentage DO saturation (DO (%)) was calculated based on the solubility equation of Chen47. The HCO−3 and pCO2 were calculated based on pH and TA using the CO2SYS program.
References
Burnett, W. C., Taniguchi, M. & Oberdorfer, J. Measurement and significance of the direct discharge of groundwater into the coastal zone. J Sea Res 46, 109–116 (2001).
Burnett, W. C., Bokuniewicz, H., Huettel, M., Moore, W. S. & Taniguchi, M. Groundwater and pore water inputs to the coastal zone. Biogeochemistry 66, 3–33 (2003).
Church, T. M. An underground route for the water cycle. Nature 380, 579–580 (1996).
Gu, H. Q., Moore, W. S., Zhang, L., Du, J. Z. & Zhang, J. Using radium isotopes to estimate the residence time and the contribution of submarine groundwater discharge (SGD) in the Changjiang effluent plume, East China Sea. Cont Shelf Res 35, 95–107 (2012).
Rodellas, V., Garcia-Orellana, J., Masque, P., Feldman, M. & Weinstein, Y. Submarine groundwater discharge as a major source of nutrients to the Mediterranean Sea. P Natl Acad Sci USA 112, 3926–3930 (2015).
Taniguchi, M., Burnett, W. C., Cable, J. E. & Turner, J. V. Investigation of submarine groundwater discharge. Hydrol Process 16, 2115–2129 (2002).
Wang, X. L. et al. An estimation of nutrient fluxes via submarine groundwater discharge into the Sanggou Bay-A typical multi-species culture ecosystem in China. Mar Chem 167, 113–122 (2014).
Zhang, J. & Satake, H. Submarine groundwater seepage in Toyama. Aquabiology 24, 294–301 (2002).
Zhang, L. et al. Pore water nutrient characteristics and the fluxes across the sediment in the Pearl River estuary and adjacent waters, China. Estuar Coast Shelf S 133, 182–192, https://doi.org/10.1016/j.ecss.2013.08.028 (2013).
Moore, W. S. The effect of submarine groundwater discharge on the ocean. Annu Rev Mar Sci 2, 59–88 (2010).
Cai, W. J., Wang, Y. C., Krest, J. & Moore, W. S. The geochemistry of dissolved inorganic carbon in a surficial groundwater aquifer in North Inlet, South Carolina, and the carbon fluxes to the coastal ocean. Geochim Cosmochim Ac 67, 631–639 (2003).
Goni, M. A. & Gardner, L. R. Seasonal dynamics in dissolved organic carbon concentrations in a coastal water-table aquifer at the forest-marsh interface. Aquat Geochem 9, 209–232 (2003).
Moore, W. S., Blanton, J. O. & Joye, S. B. Estimates of flushing times, submarine groundwater discharge, and nutrient fluxes to Okatee Estuary, South Carolina. J Geophys Res-Oceans 111, https://doi.org/10.1029/2005JC003041 (2006).
Santos, I. R., Burnett, W. C., Dittmar, T., Suryaputra, I. G. N. A. & Chanton, J. Tidal pumping drives nutrient and dissolved organic matter dynamics in a Gulf of Mexico subterranean estuary. Geochim Cosmochim Ac 73, 1325–1339 (2009).
Chen, C. T. A. et al. Submarine groundwater discharge around Taiwan. Acta Oceanlologica Sinica 37, 18–22, https://doi.org/10.1007/s13131-018-1216-2 (2018).
Liu, Q. et al. How significant is submarine groundwater discharge and its associated dissolved inorganic carbon in a river-dominated shelf system? Biogeosciences 9, 1777–1795 (2012).
Wang, G. Z. et al. Net subterranean estuarine export fluxes of dissolved inorganic C, N, P, Si, and total alkalinity into the Jiulong River estuary, China. Geochim Cosmochim Ac 149, 103–114 (2015).
Cai, P. H. et al. Using Ra-224/Th-228 disequilibrium to quantify benthic fluxes of dissolved inorganic carbon and nutrients into the Pearl River Estuary. Geochim Cosmochim Ac 170, 188–203 (2015).
Hong, Q. Q., Cai, P. H., Shi, X. M., Li, Q. & Wang, G. Z. Solute transport into the Jiulong River estuary via pore water exchange and submarine groundwater discharge: New insights from Ra-224/Th-228 disequilibrium. Geochim Cosmochim Ac 198, 338–359 (2017).
Moosdorf, N., Stieglitz, T., Waska, H., Durr, H. H. & Hartmann, J. Submarine groundwater discharge from tropical islands: a review. Grundwasser 20, 53–67, https://doi.org/10.1007/s00767-014-0275-3 (2015).
Chen, C. T. A. Distributions of nutrients in the East China Sea and the South China Sea connection. J Oceanogr 64, 737–751 (2008).
Chen, C. T. A. & Wang, S. L. A salinity front in the southern East China Sea separating the Chinese coastal and Taiwan Strait waters from Kuroshio waters. Cont Shelf Res 26, 1636–1653 (2006).
Naik, H. & Chen, C. T. A. Biogeochemical cycling in the Taiwan Strait. Estuar Coast Shelf S 78, 603–612 (2008).
Chen, C. T. A., Wang, B. J., Hsu, H. C. & Hung, J. J. Rain and lake waters in Taiwan: Composition and Acidity. Terrestrial, Atmospheric and Oceanic Sciences 5, 573–584 (1994).
Chen, C. T. A. & Wang, S. L. Carbon, alkalinity and nutrient budgets on the East China Sea continental shelf. J Geophys Res-Oceans 104, 20675–20686 (1999).
Chen, C. T. A., Wang, S. L., Wang, B. J. & Pai, S. C. Nutrient budgets for the South China Sea basin. Mar Chem 75, 281–300 (2001).
Slomp, C. P. & Van Cappellen, P. Nutrient inputs to the coastal ocean through submarine groundwater discharge: controls and potential impact. J Hydrol 295, 64–86 (2004).
Tseng, H. C., Chen, C. T. A., Borges, A. V., DelValls, T. A. & Chang, Y. C. Methane in the South China Sea and the Western Philippine Sea. Cont Shelf Res 135, 23–34 (2017).
Liu, Q. et al. Carbonate system biogeochemistry in a subterranean estuary - Waquoit Bay, USA. Geochim Cosmochim Ac 203, 422–439 (2017).
Chen, C. T. A., Yeh, Y. T., Chen, Y. C. & Huang, T. H. Seasonal and ENSO-related interannual variability of subsurface fronts separating West Philippine Sea waters from South China Sea waters near the Luzon Strait. Deep-Sea Res Pt I 103, 13–23 (2015).
Hong, H. S. et al. Source water of two-pronged northward flow in the southern Taiwan Strait in summer. J Oceanogr 67, 385–393 (2011).
Huang, T. H., Chen, C. T. A., Zhang, W. Z. & Zhuang, X. F. Varying intensity of Kuroshio intrusion into Southeast Taiwan Strait during ENSO events. Cont Shelf Res 103, 79–87 (2015).
Sheu, D. D. et al. Riding over the Kuroshio from the South to the East China Sea: Mixing and transport of DIC. Geophys Res Lett 36(7), L07603 (2009).
Bai, Y. et al. Intrusion of the Pearl River plume into the main channel of the Taiwan Strait in summer. J Sea Res 95, 1–15 (2015).
Chou, W. C. et al. Transport of the South China Sea subsurface water outflow and its influence on carbon chemistry of Kuroshio waters off southeastern Taiwan. J Geophys Res-Oceans 112 (2007).
Pan, Y. W., Fan, W., Huang, T. H., Wang, S. L. & Chen, C. T. A. Evaluation of the sinks and sources of atmospheric CO2 by artificial upwelling. Sci Total Environ 511, 692–702 (2015).
Chen, C. T. A., Lin, C. M., Huang, B. T. & Chang, L. F. Stoichiometry of carbon, hydrogen, nitrogen, sulfur and oxygen in the particulate matter of the western North Pacific marginal seas. Mar Chem 54, 179–190 (1996).
Atkins, M. L., Santos, I. R., Ruiz-Halpern, S. & Maher, D. T. Carbon dioxide dynamics driven by groundwater discharge in a coastal floodplain creek. J Hydrol 493, 30–42 (2013).
Szymczycha, B., Maciejewska, A., Winogradow, A. & Pempkowiak, J. Could submarine groundwater discharge be a significant carbon source to the southern Baltic Sea? Oceanologia 56, 327–347 (2014).
Liu, Q. et al. Estimating dissolved organic carbon inventories in the East China Sea using remote-sensing data. J Geophys Res-Oceans 119, 6557–6574 (2014).
Yang, L. Y., Chen, C. T. A., Lui, H. K., Zhuang, W. E. & Wang, B. J. Effects of microbial transformation on dissolved organic matter in the east Taiwan Strait and implications for carbon and nutrient cycling. Estuar Coast Shelf S 180, 59–68 (2016).
Liu, J. A., Du, J. Z., Wu, Y. & Liu, S. M. Nutrient input through submarine groundwater discharge in two major Chinese estuaries: the Pearl River Estuary and the Changjiang River Estuary. Estuar Coast Shelf S 203, 17–28, https://doi.org/10.1016/j.ecss.2018.02.005 (2018).
Wang, G., Han, A., Chen, L., Tan, E. & Lin, H. Fluxes of dissolved organic carbon and nutrients via submarine groundwater discharge into subtropical Sansha Bay, China. Estuarine, Coastal and Shelf Science 207, 269–282, https://doi.org/10.1016/j.ecss.2018.04.018 (2018).
Hsü, K. J. & Chen, H. H. Geologic Atlas of China: An Application of the Tectonic Facies Concept to the Geology of China. 262 and 23 Atlas Sheets (Elsevier, 1999).
Zhang, J. & Satake, H. In Land and Marine Hydrogeology (eds Taniguchi, M., Wang, k. & Gamo, T.) 45–60 (ELSEVIER B.V., 2003).
Tseng, H. C. et al. Distributions and sea-to-air fluxes of nitrous oxide in the South China Sea and the West Philippines Sea. Deep-Sea Res Pt I 115, 131–144 (2016).
Chen, C. T. In Solubility Data Series Vol. 7 (ed. Battino, R.) 41–55 (Pergamon Press, 1981).
Acknowledgements
This research was supported by the Aim for the Top University Program of the Ministry of Education (06C030203; 07C080102), by the Ocean Affairs Council (OAC-107-A102) and by the Ministry of Science and Technology (MOST 105-2611-M-110-017; 105-2811-M-110-033; 106-2611-M-110-017; 106-2811-M-110-019) of the Republic of China.
Author information
Authors and Affiliations
Contributions
S.L. Wang was in charge of the writing of the manuscript and took part in the sampling and measurements. C.T.A. Chen was in charge of the overall structure of the manuscript as well as field work and laboratory analysis. T.H.H., H.C.T., H.K.L. and T.R.P. took part in the field work, laboratory measurements and data analysis. S.K., J.Z., L.Y.Y., X.L.G., J.Y.L., F.W.K., X.G.C., Y.Y. and Y.J.L. took part in the field work and laboratory measurements. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, SL., Chen, CT.A., Huang, TH. et al. Submarine Groundwater Discharge helps making nearshore waters heterotrophic. Sci Rep 8, 11650 (2018). https://doi.org/10.1038/s41598-018-30056-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-30056-x
This article is cited by
-
Multi-annual variability of pCO2(aq) and air-water CO2 flux in the mangrove-dominated Dhamra Estuary draining into the Bay of Bengal (India)
Environmental Science and Pollution Research (2023)
-
A quarter-century of nutrient load reduction leads to halving river nutrient fluxes and increasing nutrient limitation in coastal waters of central Japan
Environmental Monitoring and Assessment (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.