Abstract
The effect of circulating biomarkers in predicting coronary artery disease (CAD) is not fully elucidated. This study aimed to determine the relationship with CAD and the predictive capacity of nine biomarkers of inflammation (TNF-α, IL-10, IL-6, MCP-1, CRP), oxidation (GHS-Px), and metabolism (adiponectin, leptin, and insulin). This was a case-cohort study, within the REGICOR population-cohorts (North-Eastern Spain), of 105 CAD cases and 638 individuals randomly selected from a cohort of 5,404 participants aged 35–74 years (mean follow-up = 6.1 years). Biomarkers’ hazard ratio (HR)/standard deviation was estimated with Cox models adjusted for age, sex, and classical risk factors. Discrimination improvement and reclassification were analyzed with the c-index and the Net reclassification index (NRI). GHS-Px (adjusted HRs = 0.77; 95%CI:0.60–0.99), insulin (1.46; 1.08–1.98), leptin (1.40; 1.03–1.90), IL-6 (1.34; 1.03–1.74), and TNF-α (1.80; 1.26–2.57) were significantly associated with CAD incidence. In the model adjusted for all biomarkers, TNF-α (1.87;1.31–2.66) and insulin (1.59;1.16–2.19) were independently associated with CAD. This final model, compared to a model without biomarkers, showed a c-index difference of 1.3% (−0.7, 3.2) and a continuous NRI of 33.7% (2.6, 61.9). TNF-α and insulin are independently associated with CAD incidence and they improve reclassification when added to a model including classical risk factors.
Similar content being viewed by others
Introduction
More than 30,000 proteins circulate in human plasma, mostly albumin and immunoglobulins. The remaining proteins are present at varying concentrations (e.g., micromolar [10−6 M] such as adiponectin, nanomolar [10−9 M] such as leptin, or picomolar [10−12 M] such as interferon gamma) but most in even lower amounts1. Plasma concentrations of some of these proteins might be good candidates to predict development of coronary artery disease (CAD), particularly inflammatory2,3, metabolic4, and oxidative5 biomarkers closely related to atherosclerosis.
Chronic inflammation in cardiovascular disease (CVD) appears to be associated with the oxidative/anti-oxidative homeostasis, yielding an accumulation of oxidized low-density lipoproteins (LDL) in the arterial wall5. Endogenous antioxidant enzymes such as glutathione peroxidase (GHS-Px)6 play a major role in maintaining oxidative homeostasis, acting as the first-line defense against free radicals. The oxidative process perpetuates an inflammatory response in the subendothelial space, as activated cells secrete pro-inflammatory molecules. Expression of tumor necrosis factor alpha (TNF-α)7 and interleukin (IL)-1 by endothelial cells and monocytes triggers a response involving monocyte chemo-attractant protein-1 (MCP-1), which promotes monocyte recruitment, macrophage activation, and signal expression6,8. Other cytokines, among them IL-10 and IL-6, are released in the process of atherosclerosis development9,10,11, and C-reactive protein (CRP) synthesis is increased by the liver. CRP activates the complement system that promotes phagocytosis which allows clearance of necrotic tissues in the atherosclerotic plaque and perpetuates the inflammatory response3,7.
Within this inflammatory response, metabolism biomarkers–e.g., insulin, leptin, and adiponectin–also play an important role. Adiponectin correlates with lower risk of CAD events, probably through inhibiting TNF-α action in endothelial cells, while increased leptin concentration is associated with CVD4,12,13.
Most of the aforementioned biomarkers have been consistently, but usually separately, found to be associated with future development of CAD events14,15. The measurement of a combination of biomarkers mutually adjusted may offer additive predictive information and improve cardiovascular risk stratification16. However, researchers have explored only some of the potential biomarkers to determine their combined effect on the CAD predictive capacity17.
The objectives of the present study were: (1) to determine the individual and mutually adjusted relationship between the development of CAD events and systemic levels of a set of biomarkers of inflammation, oxidation, and metabolism and (2) to test the biomarkers’ incremental predictive capacity for CAD, beyond that of classical cardiovascular risk factors.
To analyze these objectives we used the REGICOR population cohorts18 from the Girona province in North-Eastern Spain. The individuals from these cohorts were recruited in 1995–2000–2005 and have been extensively characterized regarding cardiovascular and lifestyle risk factors and incidence of cardiovascular events.
Results
Baseline characteristics
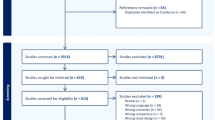
The randomly selected subcohort and the cases included 667 and 117 individuals, respectively. There were 13 individuals that were in both groups. We finally included the 105 cases and 638 subcohort members that had sufficient biological sample to complete the laboratory tests (Supplementary Fig. S1).
Cases were older and more frequently men than individuals of the subcohort (Table 1). Cases had a worse profile of CV risk factors except for estimated glomerular filtration rate (eGFR). Biomarkers of inflammation, oxidation, and metabolism differed between cases and subcohort participants except for leptin, IL-10, and MCP-1 (Table 2). Compared to the subcohort, cases showed a higher concentration of hs-CRP, insulin, IL-6, and TNF-α, and a lower concentration of GHS-Px, and adiponectin.
Correlation of biomarkers and association with CAD events
The Pearson correlation and the variation inflation factor (VIF) are shown in Supplementary Table S1. None had a VIF > 2.5, which indicates that no excessive multiple linear correlation existed between any single biomarker and the rest.
The adjusted effect of one standard deviation of each biomarker on CAD incidence is presented in Table 3. GHS-Px, insulin, leptin, IL-6, and TNF-α, were associated with CAD events even after adjustment for classical CV risk factors and statin use. GHS-Px showed a protective effect, while insulin, leptin, IL-6, and TNF-α, increased the risk of CAD events. With minimal adjustment for age and sex adiponectin had a protective effect for CAD events. Hs-CRP, IL-10, and MCP-1 were not associated with CAD events in any of the models.
Effect of biomarkers on CAD prediction
After a sequential elimination of the non-significant biomarkers, insulin, and TNF-α were significantly and independently associated with CAD events in all models (Table 4). IL-6 was associated with CAD events only in the model adjusted for age and sex. In this model, the inclusion of IL-6, insulin, and TNF-α significantly improved discrimination and reclassification (both continuous and categorical NRI)(Table 4, column 1). In the models adjusted for the rest of CV risk factors and statin use, the inclusion of insulin, and TNF-α did not improve discrimination significantly but increased reclassification when measured with the continuous NRI (Table 4, columns 2 and 3).
Discussion
Baseline values of systemic GHS-Px, IL-6, insulin, leptin, and TNF-α were associated with 6-year incidence of CAD, independently of classical risk factors; this was not true for other well-known biomarkers (hs-CRP, IL-10, adiponectin, and MCP-1). Only insulin and TNF-α were independently associated with CAD incidence when considered together in fully adjusted models. These two biomarkers improved reclassification measured with the continuous NRI in the model adjusted for classical risk factors and statin use.
Among all biomarkers, inflammatory ones seem the most promising for the assessment of CVD risk in the general population19. After adjustment for CV risk factors and statin use we found a significant association between CAD events and systemic levels of GHS-Px, IL-6, insulin, leptin, and TNF-α. Our study confirms, in a south European population, the association of CAD events with GHS-Px, IL-6, and TNF-α described in previous studies20,21,22,23. A recent case-cohort and meta-analysis yielded similar individual HR per standard deviation in adjusted models for IL-6 (1.26 vs 1.34) but lower for TNF-α (1.14 vs 1.80) compared with our results14. These difference could be due to the longer median follow-up and the older participants included in their study. We also found a direct association between leptin levels and CAD events while recent meta-analyses have shown significant associations in minimal adjusted models but non-significant associations in models adjusted for CV risk factors24,25. This discrepancy is probably due to the lower number of cases included in our study. In our study insulin was directly associated with CAD events. Recent analyses have shown that insulin markers may be strongly associated with CAD, particularly proinsulin levels26.
Our results also showed non-significant associations of hs-CRP, IL-10, adiponectin, and MCP-1 with CAD in fully adjusted models. Hs-CRP has consistently predicted CVD in large prospective studies16,27,28, and a HR per standard deviation of 1.23 has been reported16, very similar to the 1.26 found in the present study. However, due to evidence of reporting bias the true association of Hs-CRP with future CAD events is not clear and, in accordance with our results, its contribution to CVD risk assessment is assumed to be small29. IL-10 has been associated with CVD events in elderly individuals30 but there are no consistent data of its association with CAD events in general population. Neither adiponectin nor MCP-1 were strong predictors of coronary events in healthy individuals at intermediate risk in addition to CV risk factors31.
Despite promising results in some studies16,21,32,33, efforts to systematically analyze risk prediction improvements using a set of atherosclerosis-related biomarkers have been scarce. The relationship between some of the tested biomarkers and CVD or CAD has been reported in a limited number of articles. These studies analyzed a large set of biomarkers21,22,34, a subset of the assayed biomarkers35 or a biomarker score16,25,34,36. The studies analyzing a large set or a subset of a biomarker set found an improvement in discrimination and reclassification. Such as the work by Herder et al., in which the addition of 13 inflammatory biomarkers, including CRP, IL-6, MCP-1, adiponectin, and leptin, among others, yielded a modest but significant improvement of coronary risk prediction in adjusted models21. On the other hand, the effect of a biomarker score differed between studies. Blankenberg and collaborators showed no improvement of risk estimation by single biomarkers but significant improvement of discrimination and reclassification by a biomarker score including CRP, N-terminal pro-B-type natriuretic peptide, and troponin I16. The inclusion of these independent biomarkers also improved 10-year CVD risk prediction in a larger cohort32. In another study, the addition of a biomarker score including CRP and IL-6 among others, did not improve reclassification35. In our study, the addition of insulin and TNF-α showed an improvement in reclassification but not in discrimination as in the study by Würtz et al.36.
While IL-6, insulin, and TNF-α were independently associated with CAD incidence when their effect was adjusted for age and sex, a significant independent effect persisted only for insulin and TNF-α in the fully adjusted model. These results suggest that high levels of insulin, and TNF-α may indicate the presence of coronary artery atherosclerosis that translates into CAD events during a 6-year follow-up. In addition, insulin levels were associated with 6-year CAD incidence independently of adiponectin and leptin, and of inflammatory cytokines.
Our results are in line with recent European guidelines for CVD prevention, which state that the known circulating biomarkers have limited value for CVD risk assessment29. However, the lack of discrimination improvement and the limited change in reclassification could also be due to the absence of recently identified biomarkers which would be more strongly associated with CAD, such as kallikrein, lipoprotein a, and matrix metalloproteinase 933. It is also possible that a multimodal strategy combining circulating biomarkers and vascular markers such as coronary artery calcium could yield larger improvements in CVD risk assessment34,37.
The present study has several strengths. First, we included a large number of biomarkers of inflammation, oxidation, and metabolism –three different but complementary pathways of atherosclerotic disease-. Second, we used the well characterized REGICOR population cohorts which have high quality data and have previously been used to examine CAD risk prediction. These cohorts are representative of a population of approximately 600,000 individuals supporting the external validity of the results. Third, samples were analyzed in a unique laboratory to ensure reliable determinations of biomarkers18. Fourth, we used a robust design that allowed the calculation of discrimination and reclassification statistics.
Our study has also limitations that should be considered. First, no basal biological sample was available for 12 of 117 cases and for 29 of 667 individuals of the subcohort. These losses represent an approximately similar proportion of cases and subcohort candidates, indicating that sample availability was not associated with case status. Second, the cost of assessing the full set of biomarkers under assay precluded testing in the full cohort. Therefore, the statistical power is limited to HR >1.58. Finally, it is possible that longer follow-up could slightly modify our results.
In conclusion, our study showed an association of systemic levels of GHS-Px, IL-6, TNF-α, insulin, and leptin, with incidence of CAD after adjustment for CV risk factors and statin use. The combination of TNF-α and insulin was associated with CAD incidence independently of risk factors and biomarkers. However, in fully adjusted models, the inclusion of TNF-α and insulin achieved only marginal improvement in reclassification and no improvement in discrimination compared to classical risk factors.
Methods
This study was approved by the Parc de Salut Mar Ethics Committee (#2011/4309/I) and was performed in accordance with the Declaration of Helsinki. All participants signed a written informed consent.
Participants and design
We included individuals from the 2005 REGICOR (Registre Gironí del Cor) population survey of 6,352 participants, from the Girona area in Spain18. Inclusion and follow-up was carried out in 2004–2006 and in 2009–2011, respectively. For this study, participants aged 35–74 years and with no cardiovascular disease (CVD) at baseline were included (n = 5,404). A case-cohort study was designed with all cases during follow-up (n = 117) and a random subsample of the cohort (subcohort) (n = 667). Only participants with sufficient basal samples were included (105 and 638, respectively).
Power calculation
With a sample size of 743, an outcome incidence of 2.7% in the participants without previous CVD, and assuming a 5% type I error, we had 80% statistical power to identify a hazard ratio (HR) ≥ 1.58 per standard deviation of a normally distributed biomarker. Power calculation was based on “ccsize” function from the “gap” R package.
Follow-up and composite endpoint
Participants were followed-up by re-examination and a structured telephone interview. The composite endpoint included fatal or nonfatal first occurrence of myocardial infarction or angina (International Classification of Diseases (ICD)-9 codes: 410, 411.0, 411.1, 412, 414, 429; and ICD-10 codes: I21-I25, including subtypes). Nonfatal events were validated with medical records. The participant database was linked with the Catalan Death Registry and the Mortality Log of the Spanish Ministry of Health to identify fatal cases (ICD-9 codes: 410–414; and ICD-10 codes: I20-I22, I24, I25). Diagnoses were collected from autopsies if performed and from medical records. All events were classified by an expert committee according to standardized criteria: myocardial infarction was defined according to the American Heart Association definition for epidemiology and clinical research studies; angina was defined according to the presence of symptoms and objective demonstration of ischemia or presence of coronary stenosis; death due to CAD was determined by the reported ICD codes.
Laboratory determinations
Blood samples were obtained in the morning after 10–14 hours fasting, centrifuged, aliquoted, and frozen at −80 °C until assayed. Basal serum glucose, total cholesterol, and triglycerides, were determined by enzymatic methods, and high-density lipoprotein cholesterol (HDL) by a direct methodology (Roche Diagnostics, Basel, Switzerland), in a Cobas Mira Plus autoanalyzer (Roche Diagnostics, Basel, Switzerland). High-sensitivity CRP (hs-CRP) and creatinine were determined in plasma by immunoturbidimetry (ABX Diagnostic, Montpellier, France). eGFR was obtained using the Modification of Diet in Renal Disease equation38. LDL cholesterol was calculated by the Friedewald equation when triglycerides were lower than 300 mg/dL.
Plasma GSH-Px activity was measured by a modification of the Paglia and Valentine method, using cumene hydroperoxide to oxidize glutathione (Ransel RS 505, Randox Laboratories, Crumlin, UK).
The simultaneous determination of adiponectin, leptin, and insulin was performed in plasma with bead-based multiplexing technology, using a XMAG-Luminex assay (Biorad, Hercules, California, USA). Standards, blanks, controls, and patients samples were applied in duplicate. The fluorescence signal was read on a BioPlex 200 equipment (Biorad). A single-plex Luminex assay was performed to determine serum MCP-1 (R&D Systems, Minneapolis, USA). Finally, plasma concentrations of TNF-α, IL-10, and IL-6 were analyzed by high-sensitivity ELISAs (R&D Systems, Minneapolis, USA). External quality assessment was performed with Quality Control BIORAD (Bio-Rad, Hercules, California, USA) and Assessment-SEQC (Sociedad Española Química Clínica, Barcelona, Spain).
The coefficient of variation range was 1.60%-3.29% for the general biochemistry (glucose, total and HDL cholesterol, triglycerides, and creatinine), 3.39%-7.07% for the GSH-Px activity, 1.75%-6.35% for the immunoturbidimetric assay, 10.89%-15.00% for the ELISAs, and 7.30%-14.00% for the Luminex assays.
Other basal measurements
Systolic and diastolic blood pressure (mmHg), body mass index (BMI, kg/m2), smoking status (current, former, never smoker), diabetes (yes/no), hypertension history (yes/no), and hypertension and dyslipidemia treatment (yes/no) were obtained by standardized and validated methods18.
Statistical analyses
Baseline characteristics were summarized as mean and standard deviation or as median and first and third quartiles if they were continuous and normally or non-normally distributed, respectively. Categorical variables were summarized by proportions.
Correlation between biomarkers was analyzed with the Pearson correlation coefficient and with the VIF, which measured multiple linear correlations between each biomarker and the rest.
The Lin-Ying weighted estimate for random sample case-cohort design was used to estimate differences in demographic, risk factors, and biomarkers, between individuals in the case and subcohort groups39. Cox proportional hazard regression, weighted by the Lin-Ying method, was used to model time to CAD event and estimate the HR for the effect of one standard deviation increase in biomarker levels. An incremental adjustment strategy was used to test each biomarker effect: the first model was adjusted for age and sex; the second added systolic and diastolic blood pressure, HDL and total cholesterol as continuous variables, and diabetes and smoking as categorical variables; and the third incorporated statin use as a dichotomous variable to take into account the pleiotropic effect of these drugs. In addition, for each biomarker, the effect of one standard deviation was mutually adjusted for the other biomarkers in three backward-elimination models with the same adjustment strategy as above. When necessary, the biomarkers were log-transformed to normalize their distribution.
Contribution to predictive capacity of biomarkers over classical risk factors was assessed by the discrimination improvement, computing the increment of C-statistic as described for case-cohort studies40,41. Reclassification was assessed with the Net Reclassification Index (NRI) both categorical and continuous. For the categorical NRI, 5% and 10% were used as cutoff points, as these correspond to the cutoff points of CHD risk at 10 years in Spain. Confidence intervals were obtained by bootstrapping.
Statistical analyses were performed using R version 3.4.042. The “cch” function from the “survival” R package was used to obtain the Lin-Ying weighted estimates.
Data Availability
The dataset analyzed during the current study is available from the corresponding authors on reasonable request.
References
Anderson, N. L. et al. The human plasma proteome: a nonredundant list developed by combination of four separate sources. Mol. Cell Proteomics 3, 311–326 (2004).
Ross, R. Atherosclerosis-an inflammatory disease. N. Engl. J. Med. 340, 115–126 (1999).
Zakynthinos, E. & Pappa, N. Inflammatory biomarkers in coronary artery disease. J. Cardiol. 53, 317–333 (2009).
Northcott, J. M., Yeganeh, A., Taylor, C. G., Zahradka, P. & Wigle, J. T. Adipokines and the cardiovascular system: mechanisms mediating health and disease. Can. J. Physiol. Pharmacol. 90, 1029–1059 (2012).
Steinberg, D., Parthasarathy, S., Carew, T. E., Khoo, J. C. & Witztum, J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med. 320, 915–924 (1989).
Lubrano, V. & Balzan, S. Enzymatic antioxidant system in vascular inflammation and coronary artery disease. World J. Exp. Med. 5, 218–224 (2015).
Kampoli, A. M., Tousoulis, D., Antoniades, C., Siasos, G. & Stefanadis, C. Biomarkers of premature atherosclerosis. Trends Mol. Med. 15, 323–332 (2009).
Niu, J. & Kolattukudy, P. E. Role of MCP-1 in cardiovascular disease: molecular mechanisms and clinical implications. Clin. Sci. (Lond) 117, 95–109 (2009).
Han, X. & Boisvert, W. A. Interleukin-10 protects against atherosclerosis by modulating multiple atherogenic macrophage function. Thromb. Haemost. 113, 505–512 (2015).
Ridker, P. M., Rifai, N., Stampfer, M. J. & Hennekens, C. H. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 101, 1767–1772 (2000).
Sprague, A. H. & Khalil, R. A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharm. 78, 539–552 (2009).
Zeng, R., Xu, C. H., Xu, Y. N., Wang, Y. L. & Wang, M. Association of leptin levels with pathogenetic risk of coronary heart disease and stroke: a meta-analysis. Arq. Bras. Endocrinol. Metabol. 58, 817–823 (2014).
Kizer, J. R. Adiponectin, cardiovascular disease, and mortality: Parsing the dual prognostic implications of a complex adipokine. Metabolism 63, 1079–1083 (2014).
Kaptoge, S. et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur. Heart J. 35, 578–589 (2014).
Danesh, J. et al. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: Two new prospective studies and a systematic review. PLoS Med. 5, e78, https://doi.org/10.1371/journal.pmed.0050078 (2008).
Blankenberg, S. et al. MORGAM Project. Contribution of 30 Biomarkers to 10-Year Cardiovascular Risk Estimation in 2 Population Cohorts. The MONICA, Risk, Genetics, Archiving, and Monograph (MORGAM) Biomarker Project. Circulation 121, 2388–2397 (2010).
Folsom, A. R. et al. An assessment of incremental coronary risk prediction using C-reactive protein and other novel risk markers: the atherosclerosis risk in communities study. Arch. Intern. Med. 166, 1368–1373 (2006).
Grau, M. et al. Trends in cardiovascular risk factor prevalence (1995–2000–2005) in northeastern Spain. Eur. J. Cardiovasc. Prev. Rehabil. 14, 653–659 (2007).
Kablak-Ziembicka, A., Przewlocki, T., Sokołowski, A., Tracz, W. & Podolec, P. Carotid intima-media thickness, hs-CRP and TNF-α are independently associated with cardiovascular event risk in patients with atherosclerotic occlusive disease. Atherosclerosis 214, 185–190 (2011).
Flores-Mateo, G. et al. Antioxidant enzyme activity and coronary heart disease: meta-analysis of observational studies. Am. J. Epidemiol. 170, 135–147 (2009).
Herder, C. et al. Immunological and cardiometabolic risk factors in the prediction of type 2 diabetes and coronary events: MONICA/KORA Augsburg case-cohort study. PLoS One 6, e19852, https://doi.org/10.1371/journal.pone.0019852 (2011).
Wennberg, P. et al. Haemostatic and inflammatory markers are independently associated with myocardial infarction in men and women. Thromb. Res. 129, 68–73 (2012).
Tuomisto, K., Jousilahti, P., Sundvall, J., Pajunen, P. & Salomaa, V. C-reactive protein, interleukin-6 and tumor necrosis factor alpha as predictors of incident coronary and cardiovascular events and total mortality. A population-based, prospective study. Thromb. Haemost. 95, 511–518 (2006).
Chai, S. B., Sun, F., Nie, X. L. & Wang, J. Leptin and coronary heart disease: a systematic review and meta-analysis. Atherosclerosis 233, 3–10 (2014).
Yang, H. et al. Leptin concentration and risk of coronary heart disease and stroke: a systematic review and meta-analysis. PLoS One. 12, e0166360, https://doi.org/10.1371/journal.pone.0166360 (2017).
Sarwar, N., Sattar, N., Gudnason, V. & Danesh, J. Circulating concentrations of insulin markers and coronary heart disease: a quantitative review of 19 Western prospective studies. Eur. Heart J. 28, 2491–2497 (2007).
Emerging Risk Factors Collaboration, Kaptoge, S. et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N. Engl. J. Med. 367, 1310–1320 (2012).
Ahmadi-Abhari, S., Luben, R. N., Wareham, N. J. & Khaw, K. T. Seventeen year risk of all-cause and cause-specific mortality associated with C-reactive protein, fibrinogen and leukocyte count in men and women: the EPIC-Norfolk study. Eur. J. Epidemiol. 28, 541–550 (2013).
Piepoli, M. F. et al. Authors/Task Force Members. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 37, 2315–2381 (2016).
Welsh, P. et al & PROSPER Study Group. Circulating interleukin-10 and risk of cardiovascular events: a prospective study in the elderly at risk. Atherioscler. Thromb. Vasc. Biol. 31, 2338-2344 (2011).
Rana, J. S. et al. Inflammatory biomarkers and the prediction of coronary events among people at intermediate risk: the EPIC-Norfolk prospective population study. Heart 95, 1682–1687 (2009).
Hughes, M. F. et al. MORGAM project. A multiple biomarker risk score for guiding clinical decisions using a decision curve approach. Eur. J. Prev. Cardiol. 19, 874–884 (2012).
Wilsgaard, T. et al. Clinically significant novel biomarkers for prediction of first ever myocardial infarction: the Tromsø Study. Circ. Cardiovasc. Genet. 8, 363–371 (2015).
de Lemos, J. A. et al. Multimodality strategy for cardiovascular risk assessment: performance in two population-based cohorts. Circulation 135, 2119–2132 (2017).
Rana, J. S. et al. Comparative value of coronary artery calcium and multiple blood biomarkers for prognostication of cardiovascular events. Am. J. Cardiol. 109, 1449–1453 (2012).
Würtz, P. et al. Metabolite profiling and cardiovascular event risk: a prospective study of 3 population-based cohorts. Circulation. 131, 774–785 (2015).
Ikonomidis, I., Stamatelopoulos, K., Lekakis, J., Vamvakou, G. D. & Kremastinos, D. T. Inflammatory and non-invasive vascular markers: the multimarker approach for risk stratification in coronary artery disease. Atherosclerosis. 199, 3–11 (2008).
Levey, A. S. et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 130, 461–470 (1999).
Lin, D. & Ying, Z. Cox regression with incomplete covariate measurements. J. Am. Stat. Assoc. 88, 1341–1349 (1993).
Sanderson, J., Thompson, S. G., White, I. A., Aspelund, T. & Pennels, L. Derivation and assessment of risk prediction models using case-cohort data. BMC Med. Res. Methodol. 13, 113, https://doi.org/10.1186/1471-2288-13-113 (2013).
Ganna, A. et al. Risk prediction measures for case-cohort and nested case-control designs: an application to cardiovascular disease. Am. J. Epidemiol. 175, 715–724 (2012).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available from: https://www.R-project-org/ (2017).
Acknowledgements
This project was funded by Grant 081630 from Marató TV3; Health Research Fund [FIS 94/0540, FIS 99/0013-01, FIS 99/9342, FIS PI020471, INTRASALUD PI11/01801, FIS PI08/1327, CP 06/00100, CES12/025, CP12/03287 and ECA07/060]; Spain’s Ministry of Science and Innovation through the Carlos III Health Institute [HERACLES Program RD12/0042/0010/0011/0013/0061], CIBERESP, CIBEROBN, and CIBERCV [CB06/02/0029, CB06/03/0028 and CB16/11/00229], European Regional Development Funds (ERDF-FEDER); Beca SEC-FEC-Fuente Liviana 2011; and the Government of Catalonia through the Agency for Management of University and Research Grants [2009SGR1195 and 2014SGR240]. IRD was funded by the RECERCAIXA Program, Obra Social “LaCaixa” [RE087465]. The authors thank Elaine Lillly, Ph.D., for editing our manuscript.
Author information
Authors and Affiliations
Contributions
J.M. designed the study. M.F., O.D., R.E., and D.M.A. obtained the data. J.M., M.F., and R.E. supervised the analysis. I.S. and J.V. did the statistical analysis. A.F., E.D., J.S., R.E., I.R.D., and J.M. analyzed the results. I.S., J.M., and I.R.D. drafted the manuscript. All authors revised the manuscript for important intellectual content and approved the final version.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Subirana, I., Fitó, M., Diaz, O. et al. Prediction of coronary disease incidence by biomarkers of inflammation, oxidation, and metabolism. Sci Rep 8, 3191 (2018). https://doi.org/10.1038/s41598-018-21482-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-21482-y
This article is cited by
-
Dyslipidemia and associated factors among adult cardiac patients: a hospital-based comparative cross-sectional study
European Journal of Medical Research (2024)
-
Introducing Circulating Vasculature-Related Transcripts as Biomarkers in Coronary Artery Disease
Molecular Diagnosis & Therapy (2023)
-
Lipid biomarkers in statin users with coronary artery disease annotated by coronary computed tomography angiography
Scientific Reports (2021)
-
Plaque erosion and acute coronary syndromes: phenotype, molecular characteristics and future directions
Nature Reviews Cardiology (2021)
-
From CT to artificial intelligence for complex assessment of plaque-associated risk
The International Journal of Cardiovascular Imaging (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.