Abstract
Formation-free multi-level resistive switching characteristics by using 10 nm-thick polycrystalline GeOx film in a simple W/GeOx/W structure and understanding of switching mechanism through redox reaction in H2O2/sarcosine sensing (or changing Ge°/Ge4+ oxidation states under external bias) have been reported for the first time. Oxidation states of Ge0/Ge4+ are confirmed by both XPS and H2O2 sensing of GeOx membrane in electrolyte-insulator-semiconductor structure. Highly repeatable 1000 dc cycles and stable program/erase (P/E) endurance of >106 cycles at a small pulse width of 100 ns are achieved at a low operation current of 0.1 µA. The thickness of GeOx layer is found to be increased to 12.5 nm with the reduction of polycrystalline grain size of <7 nm after P/E of 106 cycles, which is observed by high-resolution TEM. The switching mechanism is explored through redox reaction in GeOx membrane by sensing 1 nM H2O2, which is owing to the change of oxidation states from Ge0 to Ge4+ because of the enhanced O2− ions migration in memory device under external bias. In addition, sarcosine as a prostate cancer biomarker with low concentration of 50 pM to 10 µM is also detected.
Similar content being viewed by others
Introduction
Recently, resistive switching random access memory (RRAM) has been specified as one of the most progressive next generation nonvolatile memories to replace 3D flash due to its simple metal-insulator-metal structure, complementary metal-oxide-semiconductor (CMOS) compatibility, low power consumption, better uniformity, strong data retention, long endurance, and easier fabrication with low cost1,2,3,4. The RRAM devices with several high-κ materials such as Ta2O5 5, 6, HfOx 7, 8, TiO2 9, BaTiO3 10 have been reported by several groups. Along with these different oxides, the GeOx material has profound potential to execute resistive switching owing to its compatibility with back-end-of-line (BOEL) process in CMOS technology, capability of producing oxygen vacancy at low temperature11, widely spanned dielectric constant values (k~12–15)12, large band gap (Eg~4.3–5.9 eV)13, 14, and good thermal stability15, 16. There are only few studies on GeOx-based RRAM in literature. Applying plasma treatment, the current compliance (CC) is reduced to 600 µA in Ni/GeOx/TiOy/N+ TaN RRAM stack17. Bipolar switching behaviors using GeOx-based different memory structures have been demonstrated at CC of 3.5 µA11. A significant reduction of resistive switching variation has been achieved at a high CC of 50 mA in Au/Zr/GeOx/YSZ/TiN structure18. In addition, multi-level cell (MLC) operation is also very important for high-density data storage RRAM application. There are few reports on MLC operation by using Pt/Ta2O5/TiN19, Ta/TaOx/Pt20, TiN/HfOx/Pt21, Pt/HfO2/TiO2/ITO22 and TiN/Ti/TiO2−x/Pt NC/TiO2−x/Au23 structures. Further, the switching mechanism is not explored clearly. Apart from those different electrode materials, tungsten (W) as a electrode material is also very useful in metal-insulator-metal (MIM) structure because of its CMOS compatibility, easier to complete RESET process, high electron emissivity, thermally and chemically stable24. A simple formation-free W/GeOx/W RRAM device with low operation current of 0.1–100 µA and proper switching mechanism evaluation through H2O2 sensing at a low concentration of 1 nM by oxidation state changing from Ge (Ge0 to Ge4+) in electrolyte-insulator-semiconductor (EIS) structure has not been reported yet. Further, prostate cancer is most common male malignancy in the western world and accountable for second most common male cancer related deaths25. According to Koutros et al., sarcosine is one of the biomarkers of prostate cancer26. Therefore, sarcosine27 has been also detected by using electrolyte-insulator-semiconductor (EIS) structure in this study, which has been not reported yet.
In this study, we have manifested formation-free MLC resistive switching having the range of CCs from 0.1 µA to 100 µA and modulation of negative voltages by using GeOx material in a simple W/GeOx/W structure and the switching mechanism through redox reaction in H2O2 sensing has been explored owing to oxidation states changing of Ge0/Ge4+ under external bias for the first time. High-resolution transmission electron microscope (HRTEM) image has confirmed the layer-by-layer structure of the 200 × 200 nm2 via-hole devices. The polycrystalline GeOx material has Ge0 and Ge4+ oxidation states, which is confirmed by X-ray photo-electron spectroscopy. An excellent dc endurance of 1000 cycles and long P/E endurance of >106 cycles with a small P/E pulse width of 100 ns can be achieved under a low operation current of 0.1 µA. The Fowler-Nordheim (F-N) tunneling conduction dominates at high field in HRS and low field regimes are complied with space-charge limited current conduction (SCLC). This structure has enhanced memory performances with good device-to-device switching uniformity, repeatable multi-level cell (MLC) by varying negative voltage with a high resistance ratio of 600, long P/E endurances of >106 cycles at high Vread of −1V, and robust data retention of >105s at 85 °C after 1000 P/E cycles. The HRTEM images at ‘SET’ condition of the device (after 106 cycles of P/E) show the thickness increment of GeOx layer (12.5 nm vs. 10 nm) along with small nanograins of <7 nm in diameter. The O2− ions migration leads to conducting filament formation/rupture through redox reaction in GeOx material and changes the Ge0/Ge4+ oxidation states. Therefore, this W/GeOx/W RRAM device has very high potential to become the successor of 3D flash non-volatile memory (NVM) in future. The GeOx membrane in EIS structure detects a low concentration of 1 nM H2O2 through oxidation-reduction reaction in GeOx material. Sarcosine as a prostate cancer biomarker has been also detected with a low concentration of 50 pM, which will be useful to diagnosis cancer patient at early stage in future.
Results and Discussion
Figure 1a reveals the cross-sectional TEM image with a small via-hole size of 0.2 × 0.2 µm2. Cross-sectional high-resolution TEM image inside the via-hole region quantifies the thickness of GeOx switching material (SM) of approximately 10 nm (Fig. 1b). The Fast Fourier Transformation (FFT) images (inset) of marked region explore that the d-spacing value is 3.9 Å, which lies in between the d- spacing values of Ge (3.27 Å)28 and GeO2 (4.3 Å)29 nanocrystals. It assures the formation of polycrystalline GeOx film with a grain size of approximately 10 nm in pristine device. In order to understand the elemental composition and defects in the GeOx films, the chemical binding states have been investigated by XPS characterization (Fig. 1c,d). Figure 1c shows XPS spectrum of Ge2p core-level electrons, which is fitted by using Shirley background subtraction and Gaussian functions. The corresponding binding energies (BE) of the doublet de-convoluted Ge 2p3/2 spectrum peaks are centered at 1218 and 1220.4 eV. The lower BE peak (1218 eV) is related to the non-oxidized semiconductor Ge (Ge0), while the higher BE peak (1220.4 eV) is assigned to the Ge-O bonding i.e, oxidized Ge4+ state30, 31. Bodlaki et al.30 and Wu et al.31 have reported elemental Ge2p3/2 (Ge0 state) peak at 1217.9 and 1217.8 eV, while the Ge4+ 2p3/2 state exists at 1220.6 eV and 1220.1 eV, respectively. The binding energy peaks are very close to our present observation. The estimated ratio of Ge0/Ge4+ peak is approximately1.6. Therefore, the formation of GeOx (x ≤ 2) film is confirmed. The O1s peak (BE of 530.9 eV) denotes the associated oxygen ions (O2−) in GeOx matrix, as shown in Fig. 1d. Higher BE peak at 531.7 eV addresses to the defects i.e, oxygen deficient (VO) in GeOx layer. Higher BE peak specifies the presence of large amount VO’s in GeOx film, as shown in Fig. 1d. In similar way, it is reported that the position of main O1s peak which remains bonded with GeOx at BE of 531.2 ± 0.2 eV while the rest one at 532.1 ± 0.2 eV indicates the chemisorbed oxygen32. In our study, VO’s plays the most important role to originate the formation-free resistive switching via oxidation state changes of Ge4+/Ge0 in W/GeOx/W structure. Yang et al.33 have also reported the O2− ions migration and oxidation state changes from Mo5+ to Mo6+ in Ag/MoO3−x/FTO resistive switching memory under external bias. Multi-level resistive switching through H2O2 sensing mechanism in W/GeOx/W structure has been discussed later.
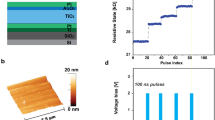
Figure 2a shows the formation-free I–V hysteresis characteristics at current compliances of 0.1, 1, 10, and 100 µA. The voltage sweeping directions are indicated by the arrows: 1 → 2 → 3 → 4. By applying +Ve bias of +3 V on the top electrode (TE), the memory device switches from high resistance state (HRS) to low resistance state (LRS). The SET voltage (VSET) is approximately 2.6 V at a low CC of 100 nA. Initially no extra formation voltage process is needed, which is very necessary to reduce power consumption or reduce the process steps in memory circuit. On the other hand, the RRAM device starts to switch back to HRS at the VRESET/IRESET of −0.9 V/8.8 nA. Therefore, this device can be operated with ultra low SET/RESET current of 0.1 µA/8.8 nA at VSET/VRESET of +2.6 V/−0.9 V, which is very important for low energy memory device application. In similar way, the resistive switching occurs at the CCs of 1, 10 and 100 µA having VSET/VRESET of +3.7/−1.3, +4.8/−3 and +4.5/−4.35 V, respectively. All resistance states at each CC can be repeated more than 1000 cycles following the same track (Supplementary Information; Fig. S1). Both HRS and LRS are decreasing with increasing CCs, which can be controlled by controlling CCs. This suggests that the oxidation-reduction occurs at the W TE/GeOx interface or interface-type34 resistive switching. The HRS current can be decreased with increasing VSTOP, which has been explained later. Good switching reliability is one of the challenging issues to implement RRAM for real application. We have analyzed cumulative probability distribution of randomly chosen 50 devices at a CC of 100 µA to confirm the reliability (Fig. 2b). Average values (σm)/standard deviation (σs) for LRS and HRS are found to be 52.5 kΩ/5.7 kΩ and 2.43 MΩ/2.18 MΩ, respectively at a read voltage (Vread) of 0.2 V. Hence the dispersion (σs/σm) values are very small of 0.1 and 0.9 for LRS and HRS, respectively. Therefore, the device-to-device switching uniformity yield is good (96%) with higher resistance ratio (HRS/LRS) of approximately 46. To ensure the low current operation reproducibility, Fig. 2c exhibits the resistive switching characteristics of 1000 successive dc switching cycles at 0.1 µA. It is noticed that this structure can produce uniform switching with acceptable resistance ratio of approximately 15 even after 1000 dc cycles, which has been presented also in the Supplementary Information (Fig. S2). In addition, this device shows stable operation with more than 106 P/E cycles under a low P/E current of 100 nA and a small P/E pulse width of 100 ns (Fig. 2d). This implies that the GeOx SM in a simple W/GeOx/W structure plays a major role to achieve such a low current operation.
(a) Bipolar I-V resistive switching characteristics having CCs from 0.1 to 100 µA and (b) device-to-device cumulative probability plot at a CC of 100 µA. (c) Consecutive > 1000 dc I-V cycles. (d) The P/E endurance of >106 cycles at a low operation current of 100 nA and a small P/E pulse width of 100 ns is applied.
To understand the current transport mechanism, double log scale fitting of I-V curves at room temperature in both HRS (Fig. 3a) and LRS (Fig. 3b) is analyzed. There are three regions in HRS current having slope values vary from (i) 0.95 to 1.1 at low bias region then (ii) 1.9 to 2.3 at middle bias region, and finally those values are varied from (iii) 4 to 18.2. On the other side, LRS current consists of two regions having (i) slope values vary from 1 to 1.1 at low bias region and then (ii) 1.7 to 1.8 at higher bias region. Both HRS and LRS currents follow linear relationship, i.e, IαV at low voltage and then quadratic relationship (i.e, IαV2) at high voltage. It signifies that both HRS and LRS currents are Ohmic where thermally generated free electron density becomes much more than the injected electron density from the W electrode. By increasing bias the injected carrier density increases immensely than the thermally generated free carrier density which results current increment rapidly. Thus HRS currents are complied with trap-charge controlled SCLC mechanism. The voltage at which current increases rapidly is reported trap–filled-limited voltage (VTFL). Corresponding trap density (nt) has been calculated using the following equation35,
where n t is trap density, q is the electronic charge, ε (~12) is the dielectric constant of GeOx, ε 0 is free-space permittivity, d (~10 nm) is the thickness of switching layer. The VTFL values of HRS current at CCs of 0.1, 1, 10 and 100 µA are found to be 1.2, 2.3, 2.9, and 3.3 V, while the n t values are found to be 1.59 × 1019, 3.05 × 1019, 3.85 × 1019 and 4.4 × 1019 cm−3, respectively. Similarly, the VTFL/nt values of 2.48 V/6.67 × 1016 cm−3 and 0.46 V/1.22 × 1016 cm−3 are reported for FTO/TiO2 and TiO2/CH3NH3PbI3/Ag structure, respectively35. Higher slope values of HRS current are 9.6 and 18.2 at CC of 10 and 100 µA, respectively, which are owing to F-N tunneling phenomena. Kim et al. have reported analogous F-N tunneling phenomena at higher slope value of 6.6 for the HRS currents above VTFL and SCLC mechanism is reported at LRS current in their ITO/GaZnO/ITO structure36. To ensure the F-N tunneling conduction, experimental I-V curves of the HRS currents in both + Ve and –Ve bias regions are plotted as ln (J/E2) vs. 1/E (Fig. 3c,d), where E is the electric field. The tunneling barrier heights (Φb) have been calculated from the ln (J/E2) vs. 1/E plots using the following F-N tunneling equation below37,
where S is the slope, m ox is the tunneling effective mass of electron (or holes) in the GeOx layer and \(\hbar \) is the reduced Planck’s constant. The linear fitting nature at CCs of 10 and 100 µA confirms the F-N tunneling conduction above critical electric field (EC) of ≥3 MV/cm38. Considering m ox = 0.2m 0 39, the calculated values of Φb are 1.14 eV and 0.71 eV at CC of 100 µA for +Ve and -Ve biases, respectively. The Φb values at the TE/GeOx interface is lower (0.71 eV vs. 1.14 eV) because of more defective than the BE/GeOx interface. Similarly, the Φb value is 1.32 eV at +Ve bias for CC of 10 µA. Therefore, electrons tunnel through the triangular potential barrier at W/GeOx interface into the conduction band of GeOx film. Since F-N tunneling phenomena have been addressed. On the other hand, the F-N tunneling phenomena are eliminated because of lower bias. Therefore, this is suggested that the HRS current at higher bias of ≥3 V is dominated by F-N tunneling.
Figure 4a shows the typical MLC operation by varying VSTOP voltages from −6 to −8V at a CC of 100 µA. The LRS value is independent of the VSTOP. However, both resistance ratio and VSET increase with increasing the VSTOP values (Fig. 4b). This implies that the oxidizing length of the conducting filaments is increased with increasing the VSTOP values. The corresponding Φb values also increases from 0.61 eV to 1.12 eV with increasing VSTOP value from −6 V to −8V, which is owing to gradual generation of Ge4+ ions (explained later). The resistance ratio becomes approximately 600 at a VSTOP of −8 V. Stable MLC operation of successive 200 dc cycles at different VSTOP values are shown in Fig. 4c. Hseih et al.40 have also executed similar MLC by varying VSTOP at higher CC of 1 mA in Ti/MgZnO/Pt structure. Therefore, it can be asserted that the W/GeOx/W structure is worthy of low current multilevel RRAM application under modulation of RESET voltages. From Fig. 4a, the HRS current decreases with increasing VSTOP and the device can be operated at approximately 5 µA after VSTOP of −8 V. From Fig. 2(a), the HRS currents are not overlapped owing to pristine defects related mechanism at low current operation of <1 µA. As long as the device is operated at higher current (>1 µA), it may not be easy to operate at 100 nA further or highly oxygen reservoir is needed at the W TE/GeOx interface to re-oxidize the defects. On the other hand, the device should be operated at low current of <1 µA only. However, further study is needed to operate the device at 100 nA by increasing VSTOP voltage. This memory device can also perform robust data retention at 85 °C, as shown in Fig. 4d. The data retention is measured after 1000 dc cycles. Both LRS and HRS show long data retention time of >105 s without any degradation. In order to describe the bipolar resistive switching process, VO based filamentary-type conduction concept has been established in W/GeOx/W RRAM structure. At first +Ve bias is applied on TE and generated O2− ions are driven towards TE leaving behind the VO’s. These O2− ions are congregated at the TE/GeOx interface having less possibility of WOx layer formation because Gibbs free energy of GeO2, WO3 and WO2 are very close to each other say, −518.5, −510 and −506 kJ/mole at 300 K, respectively11, 24, 41. Thus gradually trap density at the TE/GeOx interface gets lowered and triangular potential barrier is formed which is tunneled by electrons (e−) at the higher electric field. As a result, the TE and BE are connected by VO’s-based filamentary path through which electronic charges flow or the Ge0 based filament is formed under SET operation and the device turns into LRS. Due to semiconductor in nature of Ge0 (or Ge1+, Ge2+, Ge3+), the SCLC conduction is observed in LRS current (Fig. 3a,b). On the reverse bias, the filament is dissolved by the recombination with repelled O2− ions and a dissolution gap is generated by increasing higher oxidation states or Ge0 to Ge4+ states. Eventually device switches back to HRS. Multi-level HRS is observed owing to more generation of Ge4+ oxidation states under RESET, which is also explained through H2O2 sensing later.
To investigate the resistive switching mechanism inside of the polycrystalline GeOx layer, the TEM images have been taken at ‘SET’ condition of the memory device after a long P/E endurance of 106 cycles at a high Vread of −1V without any series transistor (Fig. 5a). This long endurance is obtained due to our novel W/GeOx/W structure design. The P/E current, voltage, and pulse width are 100 µA, +6/−6.5 V, and 0.5 ms, respectively. A little variation of HRS is observed than the LRS value because no resistance verification circuit is used. However, the large Vread of −1V is used to read during P/E cycles which will be easier for circuit design. There is no report of the switching material and investigation of mechanism after randomly 106 P/E cycles operation, as we have reported in Fig. 5b,c. Basically, a structural change is observed due to combined effect of O2− ions movement and thermal agitation, as shown in Fig. 5c (inset). Isolated small nanograins or nanocrystals (pointed by P1 and P2) with diameters of approximately 2 to 7 nm are observed clearly (density of 2 × 1012/cm2) at ‘SET’ state. The FFT image (Fig. 5d) and plane spacing image (Fig. 5e) of Ge nanograins show that the d-spacing value is 3.33 Å at the point P1. This is alike to the reported d-spacing values of 3.27 Å and 3.3 Å for Ge nanocrystal in (111) plane28, 42. Along with that, the d-spacing value of 4.28 Å is obtained from the FFT images (Fig. 5f,g) at the point P2 which indicates the formation of hexagonal GeO2 quartz like nanocrystals at the TE/GeOx interface. Liu et al. have demonstrated the formation of hexagonal quartz like GeO2 nanocrystals with the d-spacing value of 4.3 Å in (01\(\bar{1}\)0) plane29. Those d-spacing values are quite different as compared to the as-deposited polycrystalline GeOx SM at pristine state (d = 3.9 Å), as shown in Fig. 1b. Even the grain size is very small after 106 P/E cycles as compared to pristine one (<5 nm vs. 10 nm). This is due to migration of O2− ions under electrical thrust during SET operation towards the TE/GeOx interface. Consequently, thickness augmentation of the GeOx layer is also observed (Fig. 5c) at the SET state than the pristine state (~12.5 nm vs. 10 nm). In addition, sufficient localize joule heating factor during random P/E operation also trigger to construct nanograins in the GeOx layer. Qian et al.43 have shown crystallization from TEM images due to joule heating effect in ITO/WO3/ITO RRAM structure. To understand the switching mechanism, further study has been explained below.
(a) P/E endurance of >106 cycles at high Vread of −1 V. (b) TEM and (c) HRTEM images are obtained after P/E endurance of 106 cycles which shows the increment of thickness of GeOx switching layer than the pristine one (12.5 nm vs. 10 nm). Corresponding FFT images show the formation of (d,e) Ge and (f,g) GeO2 nanograins or nanocrystals with a small size of 2–7 nm in diameter.
Figure 6 shows the H2O2 and sarcosine sensing by using GeOx membrane in EIS structure. Figure 7 shows measurement set up for GeOx-based sensing membrane in EIS structure. The as-deposited GeOx membrane shows the pH sensitivity of approximately 33 mV/pH with excellent linearity of 99.8% (Supplementary Information; Fig. S3). Adding different pH buffer solutions, the surface charge on membrane is changing. By increasing pH value, the OH− ions are adsorbed on the sensing surface or reduction of H+ ions. On the other hand, de-protonation44 occurs on the sensing membrane surface as well as amount of surface potential is reduced. The Si band bending is also decreased as compare to lower pH value. This needs less potential to have a flat-band condition. As a result, the flat-band voltage or reference voltage is shifted towards positive direction. Therefore, the protonation/de-protonation (i.e., H+/OH−) of the sensing membrane in contact of different pH values changes the reference voltage, which is measured by C-V curves. A similar pH sensitivity of approximately 35 mV/pH is observed for bare SiO2 membrane45. In addition, the H2O2 is an active oxidizing agent which has more application in bio-medical research and will be used here to identify the oxidation states as well as the switching mechanism has been investigated. Figure 6a shows the reference voltage shift with a low concentration of 1 nM H2O2. The reference voltage shift is approximately 11 mV in presence H2O2 with PBS (pH 7) solution (inset of Fig. 6a). Our low concentration H2O2 sensing (1 nM) is comparable with other reported values of 5 nM–3.1 µM by cyclic voltametry measurement46,47,48,49,50. Voltage shift occurs due to oxidation of GeOx membrane using following oxidation-reduction equation below.
From equation (3), the oxidation state of Ge changes from Ge0 to Ge4+ via Ge1+, Ge2+, Ge3+ states51. First, H2O2 in PBS buffer solution will receive electron (e−) from the GeOx surface (Fig. 7e). This will produce OH− ions and OH* radicals in solution (H2O2+e− ↔ OH− + OH*; OH* + e− ↔ OH−). Finally, the OH− ions will receive H+ ions from the PBS buffer solution and will produce H2O. In this exchange reaction, the pH value of 7 will be unchanged. Basically, the GeOx membrane will be oxidized and the reference voltage will be changed. The work function of Ge is 4.67 eV52 and it is increased to 4.94 eV53 for GeO2. By considering the doping (5 × 1015 cm−3) of p-Si, the work function is 4.94 eV. Therefore, the work function of GeOx membrane increases with increasing H2O2 concentration as well as Si band bending is reduced. As a result, the reference voltage is shifted towards positive direction. Previously, we have also reported the oxidation states changed of Zn to ZnO in contact of H2O2 45. Figure 6b shows time-dependent H2O2 response with a concentration of 500 nM. The C-V curve is measured at a regular time interval of 1 minute and the reference voltage is plotted with time. It is clear that the GeOx-based sensor retains to its original response in PBS buffer solution after 5 minutes of H2O2 sensing. Therefore, this sensor can be used again or the H2O2 sensing is reversible. Similarly, the resistive switching is also reversible under SET and RESET operation because of redox reaction in GeOx material. The reference voltage shift gradually increases with successive addition of H2O2 from 1 nM to 500 nM, where as bare SiO2 based membrane is unable to execute any voltage shift (Fig. 6c). Therefore, the GeOx membrane has redox properties. We have fitted the reference voltage shift curve of GeOx sensing membrane and generated mathematical equation is below,
where, β H2O2 is the concentration of H2O2, ∆V is the corresponding reference voltage shift, ‘b’ and ‘a’ are the slope and intercept of the fitted curve having valued 5.7 and 11.4, respectively. From the above equation (4), a required H2O2 concentration can be calculated at any arbitrary voltage shift. Figure 6d shows the reference voltage shift with increasing sarcosine concentration from 50 pM to 10 µM. Sarcosine prostate cancer biomarker reacts with enzyme sarcosine oxidase and produces H2O2 as shown equation (5) below.
From fitting curve, the values of ‘b’ and ‘a’ are found to be 6.1 and 28, respectively. A minimum concentration of 50 pM sarcosine is detected. Both H2O2 and sarcosine have similar relationship. The pH7 value does not change during successive addition of sarcosine in pBS buffer with enzyme. On the other hand, pure sarcosine with PBS buffer or enzyme with PBS buffer solution does not show reasonable sensing. Therefore, sarcosine concentration (enzyme with PBS buffer solution) will be detected from the reference voltage shift as well as the sensor will be useful to detect early stage of prostate cancer patient in future or prostate cancer can be monitored. Basically, the H2O2/sarcosine sensing by using GeOx membrane or switching material proves that the O2− ions migration lead to the oxidation-reduction through changing of Ge0/Ge4+ oxidation states under external bias. This is responsible the switching mechanism and the multi-level occurs owing to more generation of Ge0 under SET or Ge4+ under RESET. Therefore, the GeOx material is not only attractive for multi-level resistive switching memory in a simple W/GeOx/W structure but also could be potentially useful for H2O2/prostate cancer detection in near future.
(a) C-V curve by adding 1 nM H2O2 in pH7. The reference voltage shift is 11.3 mV, as shown in inset. (b) Time-dependent reference voltage shift with and without H2O2 in PBS buffer solution. This sensor shows reversible phenomena because the GeOx sensing membrane has redox characteristics. It implies that the sensor can be re-used. This reversible phenomenon makes us understand the resistive switching mechanism through changing Ge0/Ge4+ oxidation states. (c) Comparison of H2O2 detection characteristics between GeOx and bare SiO2 sensing membranes. The GeOx membrane can sense H2O2 with concentration ranging from 1 nM to 500 nM, which is good for real application. (d) The reference voltage shift versus sarcosine concentration from 50 pM to 10 µM.
(a) The sensor chip and Ag/AgCl reference electrode are immersed in PBS buffer solution. (b) Sensor chip mounted on PCB and epoxy was used to isolate in between Cu line and sensor. (c) Schematic diagram of GeOx-based sensor in electrolyte-insulator-semiconductor structure and oxidation-reduction is shown. This measurement system sets up in our lab.
Conclusion
In conclusion, forming-free MLC bipolar resistive switching characteristics and switching mechanism using a simple W/GeOx/W structure, and evidence of redox reaction in GeOx material through H2O2 sensing by changing oxidation state of Ge0/Ge4+ have been reported for the first time. The oxidation states are confirmed by XPS. Via-hole device with a size of 200 × 200 nm2 and polycrystalline grain in GeOx material are investigated by HRTEM. The W/GeOx/W memory device is able to execute a stable dc endurance of 1000 cycles at ultra low CC of 0.1 µA. The SCLC conduction leads in LRS and low field regime of HRS. But, F-N tunneling dominates in high field regime (>3MV/cm) of HRS. This stack also produces good uniformity, long P/E endurances of >106 at high Vread of −1V, and excellent data retention of >105 s at 85 °C after few dc cycles at a low operation current of 100 µA. The HRTEM at SET state of the device (after 106 P/E cycles) shows the Ge and GeO2 nanograins with small size of <7 nm as well as the thickness is expanded than the pristine one (12.5 nm vs. 10 nm) due to O2− ions movement and joule heating effect or the conducting filament formation is due to O2− ions migration towards TE/GeOx interface as well as Ge0 formation through reduction of GeOx. Generation of Ge0 by increasing CC and generation of Ge4+ ions (via Ge1+, Ge2+, Ge3+) by increasing VSTOP values as well as barrier heights controls the LRS and HRS currents. The redox reaction and understanding of switching mechanism in GeOx material confirm by measuring H2O2 sensing with concentration ranging from 1 nM to 500 nM in PBS buffer solution. In addition, sarcosine prostate cancer biomarker with a low concentration of 50 pM is detected successfully. Thus eventually it can be concluded that GeOx-based W/GeOx/W memory device as well as H2O2/sarcosine sensing will be very propitious for the forthcoming RRAM and bio-sensor for diagnosis of human diseases as well as early detection of prostate cancer in near future.
Methods
Memory device fabrication
Resistive switching memory device with a small via-hole size of 0.2 × 0.2 µm2 was fabricated on 200 nm-thick SiO2 layer. The 8-inch p-type Si wafers were used. A 100 nm-thick W bottom electrode (BE) was deposited by RF magnetron sputtering. To define an active area, a 150 nm-thick SiO2 layer was deposited on the BE. Standard lithography and etching processes were used to expose the active area. Photo-resist was used to open active area and the top electrode (TE) regions. Then, a GeOx switching material (SM) with a thickness of approximately 10 nm was deposited by RF sputtering process by using Ge target (purity: 99.99%) at room temperature. The base pressure of the chamber was 2 × 10−5 Torr. Argon (Ar) gas with a flow rate of 25 sccm had been kept fix during deposition and the deposition power was 50 W. The residual oxygen (O2), inside the chamber had been deliberately allowed to react during the deposition of Ge, which created GeOx layer. Then, a W top electrode (TE) with a thickness of approximately 200 nm was deposited by the same RF sputtering process using W target (purity: 99.9%). Ar flow rate was 25 sccm and deposition power was 150 W. The deposition time was 30 min. Finally, lift-off process was done to get W/GeOx/W RRAM device with a size of 0.2 × 0.2 µm2. The thickness and microscopic structure of GeOx layer in W/GeOx/W RRAM device were analyzed by transmission electron microscopy (TEM-JEOL 2100F) with 200 keV energy. Deposition repeatability had been checked by preparing samples three times under invariant conditions along with their electrical measurements. All the electrical characteristics have been measured by Agilent 4156C precision semiconductor analyzer in our lab. Throughout the measurement, sweeping bias is applied on the TE, keeping BE as grounded.
Sensor fabrication
A 4-inch p-type Si wafer was cleaned by standard Radio Corporation of America (RCA) process. Then, a 40 nm-thick SiO2 layer was grown on Si by dry oxidation process with a temperature 950 °C in presence of 2.5 sccm O2 gas flow for 70 min. Then, a 10 nm-thick GeOx film using Ge target was deposited by RF sputtering process. Back side SiO2 layer from the wafers was removed by buffer oxide etchant (BOE) solution. Then, a 300 nm-thick Al back contact was grown by thermal evaporation process. The sensing area of 3.24 mm² was defined by SU-8 negative photo resist using photo-lithography technique. Finally, the sensor was placed on copper coated printed circuit board (PCB) through Ag paste and it was encapsulated by epoxy to isolate solution from the Si and PCB. Both PCB chip and an Ag/AgCl reference electrode were placed inside a beaker. A 5 ml solution kept in a small glass beaker. To reduce outside noise during capacitance-voltage (C-V) measurement, the glass beaker kept inside black box, which one was purchased from Super Solutions & Services Co., Ltd, Hsinchu, Taiwan. The sensor and reference electrode were connected to HP 4284 A LCR semiconductor precision analyzer with computer interfacing. The measurement was controlled the computer and collected all data. The reference electrode and PCB chip are shown in Fig. 7a. The optical microscope image of the PCB chip is shown in Fig. 7b. A schematic view of the sensor in electrolyte-insulator-semiconductor (EIS) structure is shown in Fig. 7c. The oxidation mechanism in contact of H2O2 solution on GeOx surface is also demonstrated.
pH, H2O2 and sarcosine solution preparation
In order to check the pH sensitivity and H2O2 sensing in EIS structure, the pH solutions from 2 to 10 were prepared in the following process. At first, 30 ml of each pH2 to pH10 solution was taken directly into different containers. All the pH solutions were bought from Alfa Aesar Company. Then, the Ag/AgCl reference electrode along with the GeOx based sensor had been dipped into each solution. The corresponding C-V characteristics were measured at 100 Hz frequency by applying electrode bias in the voltage range from −1.5 volt to 0.5 volt. By calculating the reference voltage shifts at 60% maximum capacitance from pH2 to pH10, the pH sensitivity value was calculated accordingly. For H2O2 measurement firstly a 1 µM H2O2 stock was prepared by diluting 10 M H2O2 concentration with DI water. Later on the PBS (Phosphate Buffer Silane) solution has been prepared by mixing sodium phosphate (Na2HPO4), sodium diphosphate (NaH2PO4), sodium chloride (NaCl) and DI water in proper way. The mentioned solutions were purchased from J. T. Baker, Avantor Performance Materials Incorporation. Then, the C-V characteristics are measured by taking 5 ml of 5 mM pH7 PBS solution in a beaker. Then, the reference voltage shifts at 60% of maximum capacitance were measured and the reference voltage versus H2O2 concentration from 1 nM to 500 nM in PBS solution was plotted.
First, 100 ml of TRIS buffer (purchased from UniRegion Bio-Tech) was diluted in DI water and the pH value was adjusted to pH 7 by monitoring pH meter. Then a stock solution of sarcosine-oxidase with 300 units was diluted in 500 µl TRIS. From the enzyme stock solution, 25 units of enzyme was added in 5 ml of pH7 PBS buffer solution. Sarcosine oxidase was purchased from TOYOBO CO. LTD, Osaka, Japan and sarcosine was purchased from Sigma-Aldrich, China. Now, a 10 mM of sarcosine stock was prepared in DI water. By diluting this stock solution, variation of sarcosine concentration ranging from 50 pM to 10 µM was successively added in the pH7 PBS buffer-enzyme solution and corresponding reference voltage shifts were plotted.
References
Waser, R. & Aono, M. Nanoionics-based resistive switching memories. Nat. Mater. 6, 833–840 (2007).
Kumar, S. et al. Direct observation of localized radial oxygen migration in functioning tantalum oxide memristors. Adv. Mater. 28, 2772–2776 (2016).
Nakamura, H. & Asai, Y. Competitive effects of oxygen vacancy formation and interfacial oxidation on an ultra-thin HfO2-based resistive switching memory: beyond filament and charge hopping models. Phys. Chem. Chem. Phys. 18, 8820–8826 (2016).
Zhu, Y. B., Zheng, K., Wu, X. & Ang, L. K. Enhancement stability of filament type resistive switching by interface engineering. Sci. Rep. 7, 43664 (2017).
Wang, Q. et al. Dynamic moderation of an electric field using a SiO2 switching layer in TaO x -based ReRAM. Phys. Status Solidi RRL. 9, 166–170 (2015).
Gao, S. et al. Tuning the switching behavior of binary oxide based resistive memory devices by inserting an ultra-thin chemically active metal nanolayer: a case study on the Ta2O5–Ta system. Phys. Chem. Chem. Phys. 17, 12849–12856 (2015).
Yu, S., Chen, H. Y., Gao, B., Kang, J. F. & Wong, H. S. HfOx-based vertical resistive switching random access memory suitable for bit-cost-effective three-dimensional cross-point architecture. ACS Nano. 7, 2320–2325 (2013).
Niu, G. et al. Material insights of HfO2 based integrated 1-transistor -1-resistor resistive random access memory devices processed by batch atomic layer deposition. Sci. Rep. 6, 28155 (2016).
Regoutz, A. et al. Role and optimization of the active oxide layer in TiO2 -based RRAM. Adv. Funct. Mater. 26, 507–513 (2016).
Wei, J. L. et al. Bipolar resistive switching with negative differential resistance effect in a Cu/BaTiO3/Ag device. Phys. Chem. Chem. Phys. 19, 11864–11868 (2017).
Cheng, C. H., Chin, A. & Yeh, F. S. Novel ultra-low power RRAM with good endurance and retention. IEEE VLSI Symp. Tech. Dig. 978, 85–86 (2010).
Kahan, A., Goodrum, J. W., Singh, R. S. & Mitra, S. S. Polarized reflictivity spectra of tetragonal GeO2. J. Appl. Phys. 42, 4444 (1971).
Afanasev, V. V. et al. Electronic structure of GeO2-passivated interfaces of (100) Ge with Al2O3 and HfO2. Appl. Phys. Lett. 92, 022109 (2008).
Ghosh, A. et al. Growth of Au capped GeO2 nanowires for visible-light photodetection. Appl. Phys. Lett. 109, 123105 (2016).
Ray, S. K., Katiyar, K. A. & Raychaudhuri, K. A. One-dimensional Si/Ge nanowires and their heterostructures for multifunctional applications — a review. Nanotechnology. 28, 092001 (2017).
Ray, S. K., Maikap, S., Banerjee, W. & Das, S. Nanocrystals for silicon – based light – emitting and memory devices. J. Phys. D: Appl. Phys. 46, 153001 (2013).
Zheng, Z. W., Hsu, H. S. & Cheng, C. H. Interface-engineered resistive memory using plasma-modified electrode on polyimide substrate. Phys. Status Solidi RRL 8, 100–104 (2014).
Gorshkov, O. N., Antonov, I. N., Belov, A. I., Kasatkin, A. P. & Mikhaylov, A. N. Resistive switching in metal–insulator–metal structures based on germanium oxide and stabilized zirconia. Tech. Phys. Lett. 40, 101–103 (2014).
Hu, W., Zou, L., Gao, C., Guo, Y. & Bao, D. High speed and multi-level resistive switching capability of Ta2O5 thin films for nonvolatile memory application. J. Alloy. Comp. 676, 356–360 (2016).
Chen, Y. C., Chung, Y. L., Chen, B. T., Chen, W. C. & Chen, J. S. Revelation on the interrelated mechanism of polarity-dependent and multilevel resistive switching in TaOx-based memory devices. J. Phys. Chem. C 117, 5758–5764 (2013).
Li, H. K. et al. Study of multilevel high-resistance states in HfO x -based resistive switching random access memory by impedance spectroscopy. IEEE Trans. Electron Devices 62, 2684–2688 (2015).
Ye, C. et al. Enhanced resistive switching performance for bilayer HfO2/TiO2 resistive random access memory. Semicond. Sci. Technol. 31, 105005 (2016).
Bousoulas, P., Stathopoulos, S., Tsialoukis, D. & Tsoukalas, D. Low-power and highly uniform 3-bit multilevel switching in forming free TiO2−x -based RRAM with embedded Pt nanocrystals. IEEE Electron Device Lett. 37, 874–877 (2016).
Kim, W., Menzel, S., Wouters, D. J., Waser, R. & Rana, V. 3-Bit multilevel switching by deep reset phenomenon in Pt/W/TaOx/Pt-ReRAM devices. IEEE Electron Device Lett. 37, 564–567 (2016).
Sigel, R. L., Miller, K. D. & Jemal, A. Cancer statistic 2015. CA Canc. J. Clin. 65, 5 (2015).
Koutros, S. et al. Prospective evaluation of serum sarcosine and risk of prostate cancer in the prostate, lung, colorectal and ovarian cancer screening trial. Carcinogen 34, 2281 (2013).
Cernei, N. et al. Sarcosine as a Potential Prostate Cancer Biomarker—A Review. Int. J. Mol. Sci. 14, 13893–13908 (2013).
Bar, R. et al. Multilayer Ge nanocrystals embedded within Al2O3 matrix for high performance floating gate memory devices. Appl. Phys. Lett. 107, 093102 (2015).
Liu, P., Wang, C. X., Chen, X. Y. & Yang, G. W. Controllable Fabrication and cathodoluminescence performance of high-index facets GeO2 micro- and nanocubes and spindles upon electrical-field-assisted laser ablation in liquid. J. Phys. Chem. C 112, 13450–13456 (2008).
Bodlaki, D., Yamamoto, H., Waldeck, D. H. & Borguet, E. Ambient stability of chemically passivated germanium interfaces. Surf. Sci. 543, 63–74 (2003).
Wu, N. et al. Effect of surface NH3 anneal on the physical and electrical properties of HfO2 films on Ge substrate. Appl. Phys. Lett. 84, 3741–3743 (2004).
Mukherjee, B. et al. Direct laser micropatterning of GeSe2 nanostructures film with controlled optoelectrical properties. RSC Adv. 4, 10013–10021 (2014).
Yang, C. S. et al. Moisture effects on the electrochemical reaction and resistance switching at Ag/molybdenum oxide interfaces. Phys. Chem. Chem. Phys. 18, 12466–12475 (2016).
Ielmini, D. & Waser, R. Resistive Switching: From Fundamentals of Nanoionic Redox Processes to Memristive Device Applications. John Wiley & Sons (2016).
Yang, D. et al. Surface optimization to eliminate hysteresis for record efficiency planar perovskite solar cells. Energy Environ. Sci. 9, 3071 (2016).
Kim, A., Song, K., Kim, Y. & Moon, J. All Solution-Processed, Fully Transparent Resistive Memory Devices. ACS Appl. Mater. Interfaces. 3, 4525–4530 (2011).
Sze, S. M. Physics of Semiconductor Devices, Hoboken, NJ, USA: Wiley (1981).
Mehonic, A. et al. Resistive Switching in Silicon Suboxide Films. J. Appl. Phys. 111, 074507 (2012).
Shaposhnikov, A. V., Perevalov, T. V., Gritsenko, V. A. & Cheng, C. H., Chin, A. Mechanism of GeO2 resistive switching based on the multi-phonon assisted tunneling between traps. A. Appl. Phys. Lett. 100, 243506 (2012).
Hsieh, W. K., Chuang, R. W. & Chang, S. J. Two-bit-per-cell resistive switching memory device with a Ti/MgZnO/Pt structure. RSC Adv. 5, 88166–88170 (2015).
Weast (ed.) R. C. CRC Handbook of Chemistry and Physics, 64 ed., CRC Press, Taylor and Francis Group, Newyork (1984).
Cho, Y. J. et al. Tetragonal Phase Germanium Nanocrystals in Lithium Ion Batteries. ACS Nano 7, 9075–9084 (2013).
Qian, K., Cai, G., Nguyen, V. C., Chen, T. & Lee, P. S. Direct observation of conducting filaments in tungsten oxide based transparent resistive switching memory. ACS Appl. Mater. Interfaces. 8, 27885–27891 (2016).
Zheng, G., Patolsky, F., Cui, Y., Wang, U. W. & Lieber, M. C. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat. Biotech 23, 1294–1301 (2005).
Kumar, P. et al. Highly reliable label-free detection of urea/glucose and sensing mechanism using SiO2 and CdSe-ZnS nanoparticles in electrolyte-insulator-semiconductor structure. J. Electrochem. Soc. 163, B580–B587 (2016).
Sun, X., Guo, S., Liu, Y. & Sun, S. Dumbbell-like Pt-Pd-Fe3O4 nanoparticles for enhanced electrochemical detection of H2O2. Nano Lett. 12, 4859–4863 (2012).
Sun, Y. et al. Ultra sonic electrodeposition of Pt-Pd alloy nanoparticles on ionic liquid-functionalized graphene paper: towards a flexible and versatile nanohybrid electrode. Nanoscale 8, 1523–1534 (2016).
Zhang, Y., Li, Y., Jiang, Y., Li, Y. & Li, S. The synthesis of Au/C/Pt coredouble shell nanocomposite and its application in enzyme-free hydrogen peroxide sensing. Appl. Surf. Sci. 378, 375–383 (2016).
Huang, J., Zhu, Y., Zhong, H., Yang, X. & Li, C. Dispersed CuO nanoparticles on a silicon nanowire for improved performance of nonenzymatic H2O2 detection. ACS Appl. Mater. Interfaces. 6, 7055–7062 (2014).
Li, Y., Zhang, J. J., Xuan, J., Jiang, L. P. & Zhu, J. J. Fabrication of a novel nonenzymatic hydrogen peroxide sensor based on Se/Pt nanocomposites. Electrochem. Commun. 12, 777 (2010).
Kim, G. S. et al. Effective Schottky barrier height lowering of metal/n-Ge with a TiO2/GeO2 interlayer stack. ACS Appl. Mater. Interfaces 8, 35419–35425 (2016).
Hellberg, P. E., Zhang, S. L. & Petersson, C. S. Work function of boron-doped polycrystalline SixGe1−x films. IEEE Electron Device Lett. 18, 456–458 (1997).
Xu, M. F. et al. Aqueous solution-processed GeO2: An anode interfacial layer for high performance and air-stable organic solar cells. ACS Appl. Mater. Interfaces 5, 10866–10873 (2013).
Acknowledgements
This work was supported by Ministry of Science and Technology (MOST), Taiwan under contract numbers: MOST-104-2221-E-182-075, MOST-105-2221-E-182-002, and Chang Gung Memorial Hospital (CGMH), Linkou under contract number: CMRPD2E0091. The authors are grateful to MSSCORPS CO., LTD., Hsinchu, Taiwan for their TEM analysis. The authors are also grateful to Electronics and Opto-electronics Laboratories (EOL), Industrial Technology Research Institute (ITRI), Hsinchu, Taiwan for their partial experimental support.
Author information
Authors and Affiliations
Contributions
S.S. wrote the first draft and analyzed data. S.Z.R. fabricated the RRAM device, helped to measured memory characteristics, and modify the manuscript. A.R. fabricated EIS structure and measured C.-V. characteristics. S.J. helped for measurement of the sensors. Both AR and SJ analyzed the sensor characteristics. S.C., R.P., S.R., M.D., and S.G. analyzed memory characteristics. A.P. helped to analyze data and developed software for the memory/sensor measurement set up. S.R. helped also to set up measurement system. H.M.C. and L.N.T. measured XPS of the GeOx film. They analyzed the spectra. J.T.Q. explained the sensors. S.K.R. helped to explain memory/sensor characteristics and modify the manuscript. This research work was carried out under the instruction of SM. All the authors contributed to the revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Samanta, S., Rahaman, S.Z., Roy, A. et al. Understanding of multi-level resistive switching mechanism in GeOx through redox reaction in H2O2/sarcosine prostate cancer biomarker detection. Sci Rep 7, 11240 (2017). https://doi.org/10.1038/s41598-017-11657-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-11657-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.