Abstract
Infiltration of infected leukocytes culminates in establishment of a brain niche for Human Immunodeficiency Virus (HIV) during acute phase of infection, initiating an ongoing cascade of persistent viral replication and inflammation, that causes irreversible neuronal injury and HIV associated neurocognitive disease (HAND). In this study, humanized mice were treated with Smoothened Agonist (SAG), a Sonic Hedgehog (Shh) mimetic in order to fortify blood brain barrier (BBB) and dampen leukocyte extravasation into CNS during AHI. Results indicate that SAG treatment reduced viral burden in the CNS immediately after HIV transmission, but also conferred extended neuroprotection via increased BBB integrity (elevated levels of tight-junction protein, Claudin 5, and reduced S100B levels in periphery). These mice also showed healthier neurons with thick, uniform dendrites and reduced numbers of activated astrocytes. Additional in vitro experiments suggested SAG treatment was not associated with the establishment or reversal of latency in the target cells. Altogether, these findings validate neuroprotective role of Shh signaling and highlight the therapeutic potential of Shh mimetics against CNS complications associated with HIV infection. Further our results strongly demonstrate that pharmacological interventions to reduce leukocyte mobilization during early HIV infection, can provide prolonged neuroprotection, which might significantly delay the onset of HAND.
Similar content being viewed by others
Introduction
Human Immunodeficiency Virus (HIV) invades central nervous system (CNS) within the first few weeks of infection. This phase of infection, also known as acute HIV infection (AHI), is a 3 to 4-week period between HIV acquisition and seroconversion. It is marked by peak viremia, depletion of gastrointestinal CD4+ T cells, seeding of latent reservoirs and initiation and expansion of antiviral immune responses by the host1. A recent clinical study, which evaluated a small subset of high-risk Thai population (RV254/SEARCH 010) further confirmed that invasion of the CNS by HIV occurred as early as 8 days after estimated exposure2. In addition, neuro-invasion was associated with increased markers of inflammation and cellular infiltration, such as elevated cerebrospinal fluid (CSF) neopterin, and Choline/creatinine (tCHO/Cr) in the basal ganglia and occipital gray matter, at a median of 14 days after HIV transmission2,3,4,5. This early burst of inflammatory perturbation is a trigger which initiates the cascade of immune activation in the CNS. It also provides a basis for neurological injury and persistent infection in later stages of the disease, which manifests in HIV associated neurocognitive disease (HAND) in 30–50% of infected individuals despite anti-retroviral therapy (ART)6, 7.
While the virus enters CNS immediately, whether it is in the free virus form or via infected cells is a matter of debate. Multiple studies, support the “Trojan horse” model where virus enters through infected CD4+ T lymphocytes and/or monocytes during routine surveillance and infects CNS resident macrophages and microglia8. Studies in rhesus macaques treated with Natalizumab, which prevents leukocyte migration across endothelial barriers, showed profoundly reduced levels of HIV DNA in brain tissue as compared to untreated animals. These experiments did not utilize ART, hence CNS entry in the form of free virus particles was not affected, indicating that virus trafficking by immune cells, seems to be the preferred route of neuro-invasion9, 10. Further, changes in brain metabolism during the first year of HIV infection were found to correlate with increased numbers of CD16+ inflammatory monocytes indicating that monocyte dynamics in the periphery might affect CNS outcomes3.
Leukocyte transmigration into the CNS is predominantly regulated by the blood brain barrier (BBB), which comprises of specialized endothelial cells (ECs) that are interconnected by tight junctions as well as astrocyte end-feet and pericytes11, 12. Our group has recently shown that administration of Smoothened Agonist (SAG), a small molecule Sonic Hedgehog (Shh) mimetic, to chronic HIV-infected humanized mice resulted in increased BBB integrity and reduced neuro-inflammation, probably by preventing excessive infiltration of activated/infected leukocytes into the CNS13. Shh signaling is activated by binding of Shh to its receptor Patched (PTCH) that causes release of Smoothened (SMO), which then modulates further downstream targets such as tight-junction proteins and adhesion molecules via the transcription factor Gli-114. SAG is a chlorobenzothiophene-containing agonist of SMO, that acts independent of PTCH and is as potent as processed N-terminal fragment of Shh15. It has previously been used to prevent Glucocorticoid induced Neonatal Cerebellar Injury16. We and others have also described a neuroprotective role for Shh signaling in BBB homeostasis13, 17, Our previous report using HIV infected humanized mice underscored the significant connection between Shh signaling and neuro-inflammation, neuronal injury and its subsequent culmination in HAND. Most importantly, these studies brought forward the notion that pharmacologic modulation of Shh signaling can be an interesting avenue of research towards the development of an adjunct therapy for HAND, which is currently unavailable18,19,20.
It also gave rise to another interesting notion that enhancing BBB integrity during AHI might reduce immune cell infiltration into the CNS, thereby attenuating the establishment of a CNS niche for HIV. Hence, in this report, humanized mice were used as a model to depict CNS related events happening within a few days of HIV infection, i.e. during AHI, marked by active viral replication in absence of adaptive immune responses. The mice were treated with a dose of SAG, before and after HIV infection and were sacrificed either on day 2 or on day 35 post infection. Mice treated with SAG showed significantly reduced leukocyte infiltration into the CNS on day 2, as well as reduced viral load in the brain on day 35. SAG treatment during AHI also resulted in better neuroprotection in terms of increased BBB integrity, reduced astrogliosis and morphologically healthier neurons, as a consequence of reduced leukocyte mobilization early on. Further we did not detect any discernible effect of SAG on establishment or reversal of latency in target cells. Our findings clearly highlight the therapeutic potential of Shh mimetics against CNS complications associated with primary HIV infection.
Results and Discussion
Activation of Shh signaling by SAG significantly reduces leukocyte migration into mouse CNS during acute phase of HIV infection
It is becoming increasingly clear that pathologic processes initiated during acute phase of HIV infection exert a strong influence on disease progression and comorbidities, such as HAND, associated with the chronic phase. The notion that therapeutic interventions during primary HIV infection can be an effective strategy to ameliorate the severity of disease in chronic stage has many precedents. Initiation of ART during early stages of HIV infection has proven beneficial in terms of improved brain metabolite levels and neuropsychological testing as well as preservation of mucosal Th17 function5, 21,22,23. A similar approach to early intervention was used in the current study to dampen the establishment of a CNS niche for HIV by strengthening the BBB with the use of an Shh mimetic, SAG.
A pioneer study by Alvarez et al. conclusively showed the involvement of Shh signaling in BBB homeostasis in wild-type mice17. In accordance, we have previously shown that Shh signaling is down-regulated in chronically infected humanized mice and its induction via SAG decreased neuro-inflammation13. However, the main caveat of this study was that, while SAG administration during chronic infection significantly reduced neuro-inflammation and subsequent neuronal injury, it failed to revert the neuronal damage already caused by almost 9 weeks of infection. With that in mind, the present study aimed to investigate if proactive BBB fortification via SAG can prevent/suppress the infiltration of infected/activated leukocytes into the brain during acute infection and prevent subsequent neuronal injury in later stages of the disease. For this, humanized mice were treated with a dose of SAG (20 μg/g body weight) before and after HIV infection at the interval of 18–20 hours between each administration. The mice were infected with CellMask Red (a membrane dye)-labelled HIV infected PHA blast cells in order to track if the founder cells extravasate into the CNS. On day 4 after the first SAG dose, the mice were sacrificed, perfused and brains were used to isolate infiltrating leukocytes. SAG treated animals had significantly less numbers of p24+CD4+ T cells in the brain (Fig. 1A, N = 5 in each group, p = 0.047). Interestingly we were able to detect CellMask Red+ cells in the brain at day 4, further confirming that HIV does invade the CNS within a few days of transmission (Fig. 1B)2. The ability of SAG to reduce leukocyte migration into the CNS was not specific to HIV infected cells, as there was a decrease in the total numbers of CD4+ T cells (Fig. 1C) and CD14+ monocytes (Fig. 1D) in brains of SAG treated mice. While the difference between CellMask+ BILs, total CD4+ T cells and monocytes among the two treatment groups was not statistically significant by t test, they showed a decreasing trend in the SAG treated group.
SAG mediated reduction in CNS leukocyte infiltration during acute HIV infection. Humanized CD34+-NSG mice were pretreated with SAG (20 μg/g body weight) (N = 5) or vehicle control (PEG 400 in PBS; N = 5) via i.p followed by injection with CellMask Red labeled HIV-infected PHA blasts (2 × 106 cells/mouse) i.p. 24 hours later. Mice were given another dose of SAG on the next day and were sacrificed on day 4. Brain infiltrating leukocytes (BILs) were isolated and analyzed using flow cytometry to measure (A) p24+ BILs (B) CellMask Red+ BILs (C) CD4+ T cells and (D) CD14+ Monocytes. There was a significant decrease in p24+ BILs in SAG treated mice and similar trend was shown by all other cell types. Detection of CellMask Red+ cells in brain indicated that the founder cells were able to cross BBB. *Indicates p < 0.05 by unpaired t test.
Reduced leukocyte infiltration into CNS during primary HIV infection confers extended neuroprotection in SAG treated mice
In order to examine if decrease in migration of leukocytes into the brain during early HIV infection had any long-lasting effects on the BBB and CNS, parallel groups of mice were terminated on day 35 (i.e. almost 5 weeks, a midpoint to our previous chronic SAG study)13. Whole blood obtained from these mice was analyzed by flow cytometry to measure the percentage of circulating CD4+ T cells and p24+ CD4+ T cells. There was a significant decrease in CD4+ T cell percentages in mice comparing day 4 and 35 in mice treated with or without SAG (Fig. 2A, N = 3 per group, p = 0.003), indicating that the disease progression in periphery was not different among the groups. The decrease in p24 + CD4+ T cells on day 35 was probably reflective of the severe decrease in the total number on CD4+ T cells (Fig. 2B, N = 3 per group, p = 0.043). While the viral RNA copy levels in plasma showed an increasing trend in both the vehicle and SAG treated mice (N = 3–5 per group, p = 0.0534), there was a significant decrease in the viral RNA levels in SAG treated brains (Fig. 2C and D, N = 3 per group, p = 0.03). Further in vitro experiments were performed to rule out a possibility whether the observed reduction in CNS viral burden was due to diminished infiltration of infected leukocytes into the CNS early on during the infection and not because of any un-anticipated effect of SAG on establishment of viral latency. In vitro cultured TCM (Central Memory CD4+ T) cells were infected with HIV and allowed to become latent in presence or absence of SAG. The cells were analyzed every alternate day (Day 2 being 48 hours post infection), for intracellular p24 expression and no effect of SAG was observed on establishment of latency (gradual decrease in intracellular p24 levels, Fig. 2E, N = 3 per group per time point). On Day 8, 1 million cells were reactivated with CD3/CD28 beads in present of SAG. As seen in data point 10 R of Fig. 2E no discernible effect of SAG was observed on reversal of latency in these cells. This evidence conclusively indicates that SAG-mediated reduction in CNS viral burden during later stages of the infection is indeed due to reduced migration of infected leukocytes in acute stage of the disease.
SAG treatment in primary HIV infection causes prolonged decrease in CNS viral burden. Whole blood obtained from HIV infected mice with or without SAG treatment was analyzed by flow cytometry to enumerate (A) total CD4+ T cells and (B) p24+ CD4+ T cells at Day 4 and Day 35 post-SAG pretreatment (N = 3 per group per time point). (C) Plasma samples and (D) total brain RNA from a few representative mice were analyzed by Cobas Ampliprep to measure viral RNA copies (N = 3–5 per group per time point). *Indicates p < 0.05 by unpaired t test (E) % p24+ cells in in vitro cultured TCM cells infected with HIV.
Preemptive Shh activation via SAG is beneficial in maintaining BBB integrity as measured by tight junction protein expression and S100B levels despite progressive HIV infection
Astrocytes and neurons secrete Shh in adult brain, which then binds to its receptor Patched (PTCH) on microvasculature endothelial cells. Shh-PTCH interaction causes Smoothened (SMO) activation, which in turn leads to increased expression of tight junction proteins (e.g. Claudin 5), through Gli-1 transcription factor24. SAG bypasses Shh to directly activate SMO and the down-stream proteins Gli-1 and Claudin 514. Therefore, we assessed the expression level of Gli-1 in mouse brains, 35 days post infection. There was a significant increase in Gli-1 expression in the endothelial cells in SAG treated brains (Fig. 3A and B, N = 2–3 per group, p = 0.028). In order to test if this effect of SAG on Gli-1 expression could be recapitulated in human cells, primary brain microvasculature endothelial cells (HBMECs) were treated with increasing doses of SAG (0.5–2 μM) for 24 hours and Gli-1 mRNA levels were measured by RT-QPCR. Maximum Gli-1 mRNA expression was observed in cells treated with 0.5 μM SAG, while cells treated with 2 μM SAG expressed Gli-1 levels comparable to non-treated cells (Fig. 3C, N = 3 per group, p = 0.0287). These results corroborate an earlier report by Chen et al.15. Next we measured RNA transcript levels of Claudin 5, a downstream target of Gli-1 in SAG treated brains and found that it was significantly elevated in treated brains (Fig. 3D, N = 3 per group, p = 0.042). These results indicate that SAG mediated induction of Shh signaling in AHI was sustained as the infection progressed and resulted in elevated levels of Tight junction protein expression. Next we determined if up-regulated Shh signaling was associated with a more functional, tighter BBB by measuring the levels of S100B, an astrocyte origin protein in peripheral plasma. Increased S100B levels in plasma are known to correlate positively with BBB leakage25. Indeed, while the S100B levels were increased significantly in vehicle treated mice from day 4 to day 35, they were unchanged in the SAG treated mice (Fig. 3E, N = 3–5 per group, p = 0.006), indicating that SAG treated BBB was impermeable to leukocytes.
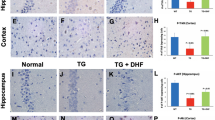
BBB fortification by SAG treatment during primary HIV infection is sustained in later stages of the disease. (A) Paraffin sections of representative brains obtained on day 35 post SAG pretreatment, were labeled with anti-Gli-1 (red) and Dapi (Blue). Images were taken from cortical region at 40X zoom 3. (B) Relative Gli-1 intensity in the outlined regions of interests (in green) from three representative images per group (C). Relative Gli-1 expression levels in SAG treated HBMECs by RT-QPCR (N = 3 per group) (D). Claudin 5 RNA levels by RT-QPCR (N = 3 per group) on day 35. (E) S100B levels in plasma as measured by ELISA (N = 3–5 per group per time point). *Indicates p < 0.05 and **Indicates p < 0.01 by unpaired t test or 1-way ANOVA and 2-way ANOVA respectively.
SAG treatment during AHI prevents astrogliosis and neuronal injury
Next we sought to determine if the enhanced BBB integrity established a more neuroprotective environment in SAG treated brains. For this, we performed immune-histochemical analysis of brain cortex to evaluate astrocyte activation i.e. GFAP expression levels (a marker of neuro-inflammation) as well as neuronal morphology as seen by MAP2 expression. MAP2 is a microtubule assembly protein that plays a crucial role in determining and stabilizing dendritic shape in post-mitotic neurons26. As expected, SAG treatment lead to significantly reduced levels of GFAP expressing astrocytes (Fig. 4A and B). Although we do not see significant difference in the intensity of MAP2 expression (data not shown), SAG treated mouse brains showed thicker and uniformly shaped neuronal dendrites as compared to thin and beaded dendrites in vehicle treated mouse brains (Fig. 4C and D). Overall our findings show that Shh induction by SAG treatment reduced leukocyte infiltration into the CNS during AHI and also conferred extended neuroprotection for up to 35 days, with respect to CNS viral burden, BBB integrity, neuro-inflammation and neuronal injury.
Increased Shh signaling in primary HIV infection results in decreased neuro-inflammation and neuronal damage Paraffin sections of representative brains obtained on day 35 post SAG pretreatment, were labeled with (A). anti- GFAP (red), an astrocyte activation marker and (B). GFAP+ cells were enumerated using Particle Analysis tool from ImageJ software from three random fields of view. (C) anti-MAP2 (red), a neuronal marker and Dapi (blue). Images were taken from cortical region at 40X and (D). regions enclosed in a white box in Fig. 4C were zoomed 3 times. White arrows indicate beaded, thin dendrites in brains without SAG treatment.
This report adds significant new information complimentary to our previous report13 in which we showed neuroprotective effect of Shh signaling during chronic HIV infection. However, as stated before, SAG treatment during chronic infection failed to reverse the already incurred neuronal injury. We addressed this limitation by proactively inducing Shh activation via SAG before HIV infection in the present study. Secondly, in the earlier report, we were not able to provide direct evidence linking BBB tightening by SAG with reduction in the CNS infiltration by leukocytes, which was achieved here by measuring brain infiltrating leukocytes. Most importantly, since HIV-infected PHA blasts labeled with CellMask Red membrane dye were used as the mode of HIV infection, we were able to track the entry of founder cells injected intraperitoneally, into the CNS just within 48 hours of injection and substantiate that HIV indeed infiltrates the CNS very early during infection.
Further these findings are very significant because, as of now there are no (even partially successful) treatment options for HAND18,19,20. There is an ever increasing need for these therapeutics in order to improve the life-quality of HIV infected individuals, as they are living near-normal life spans with the advent of ART. Even the less severe forms of HAND, which are more prevalent in the post-ART era, have substantial effect on day-to day functions27. Treatments like SAG that delay the onset of neurocognitive impairment could potentially serve as functional cure for HAND. The second important conclusion from this study is the unequivocal merit to early-intervention especially with respect to neuronal complications. While ART buffers from the key pathologic indices i.e. sufficiently elevated CD4+ T cell numbers and undetectable viral loads, certain disease processes are irreversible such as the loss of mucosal CD4+ T cells28, neuronal injury29 and establishment of latent reservoirs30, especially in the CNS. It needs to be further investigated if combination treatment with ART and SAG protects from neuronal injury and prevents the establishment of the CNS niche. In addition, in-depth characterization of SAG, or similar Shh agonists like Purmorphamine, as therapeutic candidates for HIV associated complications of the CNS is also warranted.
Materials and Methods
Ethics statement
Mouse experiments were carried out in accordance with the Animal Welfare Act and the National Institute of Health (NIH) guidelines, and the University Committee on Animal Resources of the University of Rochester Medical Center approved the animal protocol (protocol # 2005-161). The Research Subjects Review Board at the University of Rochester Medical Center approved studies involving human samples. All the study participants were adults and blood samples were obtained after written informed consent, in accordance with the Declaration of Helsinki.
Preparation of HIV-infected PHA blasts
PHA blasts were generated as per the ACTG Laboratory manual version 1.0 and were infected with HIV-1 BAL (Zeptomatrix Corp. NY) at the MOI (multiplicity of infection) of 1. A fraction of cells (0.1 × 106) were fixed using 4% paraformaldehyde (PFA) and permeabilized using IC Perm (Invitrogen Inc, USA), followed by staining with antibody against p24 FITC (Beckman Coulter Inc. USA) and anti-human CD4 PE on a daily basis. The cells were analyzed using Accuri C6 flow cytometer to measure the percentage of p24 + CD4+ T cells. Once the p24 + CD4+ T cells reached to 60% of all viable cells, blasts were labelled with CellMask Red membrane dye (Invitrogen Inc. USA) as per the manufacturer’s instructions and used for infecting mice.
SAG treatment and HIV infection of humanized mice
Humanized CD34+-NSG mice were generated as reported earlier31. 20–22 week old mice were administered SAG (20 μg/g body weight, Cayman Chemicals, USA) or vehicle control (PEG-400 in PBS, Sigma Aldrich, USA) intraperitoneally (i.p.). The mice were injected i.p. with HIV-infected PHA blasts (2 × 106 cells/mouse) 18–20 hours later. 24 hours post-infection, the mice were given another dose of SAG and were sacrificed on the next day (day 4), or on day 35. Whole blood was collected by cardiac exsanguination. The mice were then perfused and brains were harvested. Blood was either used to isolate plasma or to perform flow cytometry to estimate cell counts for CD4+ T cells and p24 expression as described above (N = 3 per group). Plasma was used to perform S100B ELISA (as per manufacturer’s instructions, #SEA567Mu, Clone-Cloud Corp; N = 3–5 per group) or for viral RNA measurements (N = 3–5 per group). Levels of viral RNA copies/ml were analyzed with the automated COBAS Ampliprep system V2 (Roche Molecular Diagnostics, Mannheim, Germany) as described31. Brains were used for RNA extraction (N = 3 per group), immune-histochemical analyses (N = 2–3 per group) or isolation of brain infiltrating leukocytes (BILs; N = 5 per group).
Isolation and quantitation of brain infiltrating leukocytes
BILs were isolated as previously described32 and resuspended in 100 µl PBS and fixed as described above. The cells were stained with antibodies against human CD4-PE and CD14 PE Cy 7 (a monocyte marker). The cells were then permeabilized using IC Perm and stained with anti-p24 FITC. The number of BILs were measured based on forward and side scatter and the expression of specific surface markers using flow cytometry (Accuri Cytometers, Ann Arbor, MI). CellMask Red+ cells are detected in channel 4 (FL4) and their percentage in BILs was enumerated to estimate the number of injected cells (i.e. founder cells) that had transmigrated into the CNS.
In vitro culture of Central Memory CD4+ T cells (TCM) and HIV infection
TCM cells were infected with single cycle VSV-G pseudotyped HIV-1 isolate and were cultured to establish latency as described previously33, 34. Briefly, naïve CD4+ T cells isolated from HIV uninfected healthy donors were cultured with a cytokine cocktail containing 10 ng/ml TGF-β, 2 μg/ml IL-12, 1μg/ml IL-4 and CD3/CD38 Dynabeads for three days. The cytokine cocktail and the dynabeads were then removed and replaced with 30U/ml of IL-2. After three more days, the cells were infected with HIV (0.5μg p24/million cells) by spinoculation. Cells were treated with 500ng/ml SAG one day before and 1 day after HIV infection (as done in the mouse experiments) or were left untreated. Cells were harvested 48 hours later (labelled as Day 2 in Fig. 2E) and fixed/permeabilized using BD Cytofix/Cytoperm reagent followed by staining with 5μl/million cells of anti-p24 FITC antibody. Stained cells were acquired on Accuri C6 Flow Cytometer. The staining was repeated every alternate day for 6 more days. By this time the cells acquire a latent phenotype (i.e. p24 negative). On Day 8, one million non-treated and SAG treated cells were reactivated to reverse latency using CD3/CD28 Dynabeads in presence of 500ng/ml SAG. The cells were analyzed for p24 expression on Day 10. All the cytokines were purchased from R&D systems.
SAG treatment of Primary human brain microvasculature endothelial cells (HBMECs)
Cells were treated with treated with 0.5 μM, 1 μM and 2 μM SAG for 24 hours. Gli-1 RNA levels were measured by RT-QPCR as described previously13. The primers used for this experiment are from, Biorad prime PCR assay (Catalog # 10025636, Gli-1: qHsaCED0043346, GAPDH: qHsaCED0038674).
Immunohistochemistry
Brains from representative mice in each group (N = 2–3 per group) were post-fixed overnight followed by paraffin embedding. 5 μm thick sections from cortex were immuno-stained with MAP2 (catalog# AB5622; Millipore, Darmstadt, Germany; 1:500), GFAP (catalog# ab7260; Abcam, 1:5000), and Gli1 (sc-20687; Santacruz, 1:100) and processed as described previously13. ImageJ software was used to quantify IHC data in Fig. 3 and 4 as described previously13.
Statistical analysis
Graphpad Prism version 7 was used to perform all statistical analyses. Whole blood cell percentages, S100B levels and % p24+ TCM cells were analyzed using 2-way ANOVA and Tukey post-test. Gli-1 RNA levels with different SAG doses was analyzed using 1-way ANOVA. All other comparisons were done using unpaired t test to compare group means or group variance respectively. Statistical significance by t test or 2-way ANOVA is indicated in the figures as *p < 0.05, **p < 0.01, and ***p < 0.001.
References
Fuller, R. A. et al. A prospective longitudinal in vivo 1H MR spectroscopy study of the SIV/macaque model of neuroAIDS. BMC Neurosci 5, 10, doi:10.1186/1471-2202-5-10 (2004).
Valcour, V. et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis 206, 275–282, doi:10.1093/infdis/jis326 (2012).
Lentz, M. R. et al. Alterations in brain metabolism during the first year of HIV infection. J Neurovirol 17, 220–229, doi:10.1007/s13365-011-0030-9 (2011).
Lentz, M. R. et al. Changes in MRS neuronal markers and T cell phenotypes observed during early HIV infection. Neurology 72, 1465–1472, doi:10.1212/WNL.0b013e3181a2e90a (2009).
Sailasuta, N. et al. Change in brain magnetic resonance spectroscopy after treatment during acute HIV infection. PLoS One 7, e49272, doi:10.1371/journal.pone.0049272 (2012).
Heaton, R. K. et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 17, 3–16, doi:10.1007/s13365-010-0006-1 (2011).
McArthur, J. C. et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology 43, 2245–2252 (1993).
Kim, W. K., Corey, S., Alvarez, X. & Williams, K. Monocyte/macrophage traffic in HIV and SIV encephalitis. J Leukoc Biol 74, 650–656, doi:10.1189/jlb.0503207 (2003).
Campbell, J. H. et al. Anti-alpha4 antibody treatment blocks virus traffic to the brain and gut early, and stabilizes CNS injury late in infection. PLoS Pathog 10, e1004533, doi:10.1371/journal.ppat.1004533 (2014).
Zayyad, Z. & Spudich, S. Neuropathogenesis of HIV: from initial neuroinvasion to HIV-associated neurocognitive disorder (HAND). Curr HIV/AIDS Rep 12, 16–24, doi:10.1007/s11904-014-0255-3 (2015).
Atluri, V. S. et al. Effect of human immunodeficiency virus on blood-brain barrier integrity and function: an update. Frontiers in cellular neuroscience 9, 212, doi:10.3389/fncel.2015.00212 (2015).
Strazza, M., Pirrone, V., Wigdahl, B. & Nonnemacher, M. R. Breaking down the barrier: the effects of HIV-1 on the blood-brain barrier. Brain research 1399, 96–115, doi:10.1016/j.brainres.2011.05.015 (2011).
Singh, V. B., Singh, M. V., Gorantla, S., Poluektova, L. Y. & Maggirwar, S. B. Smoothened Agonist Reduces Human Immunodeficiency Virus Type-1-Induced Blood-Brain Barrier Breakdown in Humanized Mice. Sci Rep 6, 26876, doi:10.1038/srep26876 (2016).
Bambakidis, N. C. et al. Intravenous hedgehog agonist induces proliferation of neural and oligodendrocyte precursors in rodent spinal cord injury. Neurosurgery 67, 1709–1715; discussion 1715, doi:10.1227/NEU.0b013e3181f9b0a5 (2010).
Chen, J. K., Taipale, J., Young, K. E., Maiti, T. & Beachy, P. A. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci USA 99, 14071–14076, doi:10.1073/pnas.182542899 (2002).
Heine, V. M. et al. A small-molecule smoothened agonist prevents glucocorticoid-induced neonatal cerebellar injury. Sci Transl Med 3, 105ra104, doi:10.1126/scitranslmed.3002731 (2011).
Alvarez, J. I. et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 334, 1727–1731, doi:10.1126/science.1206936 (2011).
Clifford, D. B. et al. A randomized clinical trial of CPI-1189 for HIV-associated cognitive-motor impairment. Neurology 59, 1568–1573 (2002).
Schifitto, G. et al. Selegiline and oxidative stress in HIV-associated cognitive impairment. Neurology 73, 1975–1981, doi:10.1212/WNL.0b013e3181c51a48 (2009).
Zhao, Y. et al. Memantine for AIDS dementia complex: open-label report of ACTG 301. HIV clinical trials 11, 59–67, doi:10.1310/hct1101-59 (2010).
Hellmuth, J. et al. Neurologic signs and symptoms frequently manifest in acute HIV infection. Neurology 87, 148–154, doi:10.1212/WNL.0000000000002837 (2016).
Schuetz, A. et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog 10, e1004543, doi:10.1371/journal.ppat.1004543 (2014).
Valcour, V. G. et al. Neurological Response to cART vs. cART plus Integrase Inhibitor and CCR5 Antagonist Initiated during Acute HIV. PLoS One 10, e0142600, doi:10.1371/journal.pone.0142600 (2015).
Bambakidis, N. C., Wang, R. Z., Franic, L. & Miller, R. H. Sonic hedgehog-induced neural precursor proliferation after adult rodent spinal cord injury. Journal of neurosurgery 99, 70–75 (2003).
Woertgen, C., Rothoerl, R. D., Metz, C. & Brawanski, A. Comparison of clinical, radiologic, and serum marker as prognostic factors after severe head injury. The Journal of trauma 47, 1126–1130 (1999).
Kosik, K. S. & Finch, E. A. MAP2 and tau segregate into dendritic and axonal domains after the elaboration of morphologically distinct neurites: an immunocytochemical study of cultured rat cerebrum. The Journal of neuroscience: the official journal of the Society for Neuroscience 7, 3142–3153 (1987).
Doyle, K. L. et al. Real-world impact of neurocognitive deficits in acute and early HIV infection. J Neurovirol 19, 565–573, doi:10.1007/s13365-013-0218-2 (2013).
Guadalupe, M. et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol 77, 11708–11717 (2003).
Gray, F. et al. Neuropathology and neurodegeneration in human immunodeficiency virus infection. Pathogenesis of HIV-induced lesions of the brain, correlations with HIV-associated disorders and modifications according to treatments. Clin Neuropathol 20, 146–155 (2001).
Chun, T. W. et al. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci USA 95, 8869–8873 (1998).
Dash, P. K. et al. Loss of neuronal integrity during progressive HIV-1 infection of humanized mice. The Journal of neuroscience: the official journal of the Society for Neuroscience 31, 3148–3157, doi:10.1523/JNEUROSCI.5473-10.2011 (2011).
Moller, K., Stahl, T., Boltze, J. & Wagner, D. C. Isolation of inflammatory cells from rat brain tissue after stroke. Experimental & translational stroke medicine 4, 20, doi:10.1186/2040-7378-4-20 (2012).
Bosque, A. et al. Benzotriazoles Reactivate Latent HIV-1 through Inactivation of STAT5 SUMOylation. Cell Rep 18, 1324–1334, doi:10.1016/j.celrep.2017.01.022 (2017).
Piekna-Przybylska, D., Sharma, G., Maggirwar, S. B. & Bambara, R. A. Deficiency in DNA damage response, a new characteristic of cells infected with latent HIV-1. Cell Cycle 16, 968–978, doi:10.1080/15384101.2017.1312225 (2017).
Acknowledgements
This work was supported by the University of Rochester Center for AIDS Research (NIH P30AI078498) and NIH grants RO1 NS054578, RO1 HL123346 and RO1 HL128155 to SBM, R24 OD018546 to LYP and SG. We would like to thank Dr. Paivi Jordan from the confocal core for her help with imaging. We would like to thank Daria Krenitsky for her help with tissue sectioning. We would like to thank Rochester Victory Alliance and Ann Casey for helping us with blood draws from healthy individuals. We would also like to thank Emily Weber for editing the manuscript and for valuable comments.
Author information
Authors and Affiliations
Contributions
V.B.S., M.V.S. and S.B.M. conceived/designed experiments, analyzed data and wrote the manuscript. V.B.S., M.V.S. and D.P.P. performed experiments. S.G. and L.Y.P. provided reconstituted humanized mice. All authors have reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Singh, V.B., Singh, M.V., Piekna-Przybylska, D. et al. Sonic Hedgehog mimetic prevents leukocyte infiltration into the CNS during acute HIV infection. Sci Rep 7, 9578 (2017). https://doi.org/10.1038/s41598-017-10241-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-10241-0
This article is cited by
-
Human immunodeficiency virus-1 Tat exerts its neurotoxic effects by downregulating Sonic hedgehog signaling
Journal of NeuroVirology (2022)
-
Meningitic Escherichia coli α-hemolysin aggravates blood–brain barrier disruption via targeting TGFβ1-triggered hedgehog signaling
Molecular Brain (2021)
-
Modeling blood–brain barrier pathology in cerebrovascular disease in vitro: current and future paradigms
Fluids and Barriers of the CNS (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.