Abstract
The aim of this study was to determine the impact of different wheat bran fractions on the gut microbiota and fat binding capacity to explain their differential effects on metabolic and inflammatory disorders induced by a western diet (WD) in mice. Wheat bran derived arabinoxylan oligosaccharides (AXOS), a crude fraction of wheat bran (WB), or the same wheat bran with reduced particle size (WBs) were added to the WD of mice for 8 weeks. AXOS shifted the gut microbiota composition, blunted Clostridium and Turicibacter genera and strongly promoted Bifidobacterium and Butyricicoccus genera, independently of changes in gut antimicrobial peptide expression. AXOS was the most efficient to reduce adiposity. Only WB fraction promoted fat excretion and differed from the other fractions by the capacity to increase the Akkermansia genus and to counteract gut interleukin 1 beta (IL1β) overexpression. Strikingly, WBs promoted steatosis and adipose tissue inflammation, despite its ability -like WB- to increase bacterial diversity. In conclusion, wheat bran fractions differently affect metabolic and inflammatory disorders associated with WD feeding, depending on their particle size, their fat binding capacity and their influence on the gut microbiota. Those results might be useful to take into account in nutritional advices to control obesity.
Similar content being viewed by others
Introduction
In most Western countries, cereals and in particular wheat, represent a major source of dietary fibers. Dietary fibers are one of the most important classes of compounds in cereal grains related to positive health effects, and cereal whole grain products help reaching the recommended overall dietary fiber intake1,2,3. Wheat bran is the outermost layer of the wheat kernel, representing 14 to 19% of the total weight4. Wheat bran is a major source of dietary fiber, such as non-starch polysaccharides and lignin. Arabinoxylan (AX) is the most abundant dietary fiber in wheat bran1, 5 and accounts for 20 to 30% of dry wheat bran mass, or 70% of non-starch polysaccharides4, 6. It is a polymer with a D-xylose backbone linked with L-arabinose. Most AX in wheat bran are water insoluble because of crosslinking with neighboring units of cellulose, lignin, and proteins5. Since it is rich in insoluble fibers, wheat bran is beneficial in producing bulky stool and preventing colon cancer7,8,9,10. Wheat bran is also a low-cost byproduct of conventional wheat milling and a promising starting material for arabinoxylan oligosaccharides (AXOS) production11. AXOS are hydrolysis products of AX and are characterized by their lower average degree of polymerization and their average degree of arabinose substitution5, 12. Because of prehydrolysis, they are highly soluble and rapidly fermentable in the intestine13.
Gut microbiota is considered as an environmental factor involved in the control of body weight, metabolic alterations and inflammatory disorders occurring in obesity14, 15. AXOS derived from wheat bran, have been proposed as prebiotic nutrients prone to modulate the gut microbiota to restore host health1, 16, 17. Different mechanisms have been proposed to link the events occurring in the intestine following carbohydrate fermentation to the control of metabolic energy metabolism. Carbohydrate fermentation may promote the release of gastrointestinal peptides controlling food intake and/or insulin secretion (i.e., glucagon like peptide-1 (GLP-1), peptide YY (PYY), glucose-dependent insulinotropic peptide (GIP)). In addition, it has been shown that bacterial metabolites (short chain fatty acids (SCFA), conjugated linoleic acids) can act as regulators of adiposity (via G-protein-coupled receptors (GPR43) and/or peroxisome proliferator-activated receptor-γ (PPARγ)-dependent mechanisms)18,19,20. Finally, the changes in the activity of peptides/systems involved in the control of gut permeability (e.g., GLP-2, tight junction proteins such as zonula occludens-1 (ZO1) and occludin, endocannabinoid system) also contribute to modulate host metabolic alterations such as inflammation and endotoxemia17, 21.
Particle size influences the physiological effects of wheat bran such as colonic fermentation in humans22. Prehydrolysis of wheat bran AX into AXOS influences the latter even more13. SCFA production and fecal moisture are increased at the expense of other physiological effects such as delayed gastric emptying, increased mean transit time and increased stool weight when particle size decreases23,24,25. In addition, a recent study reported change in postprandial GLP‑1 response after consumption of wheat breads with different particle size in healthy men26. Experimental data show that specific changes in gut microbiota may be related to the improvement of adiposity by some wheat bran derivatives27, 28. However, to date, no study has applied a community-wide approach to evaluate if the changes in the gut microbiota play a role in the impact of wheat bran products differing by their physico-chemical properties -notably their particle size- on obesity and metabolic alterations induced by the western diet. The aim of this study was to determine the impact of different wheat bran fractions on the gut microbiota and fat binding capacity to explain their differential effects on metabolic and inflammatory disorders induced by a WD in mice.
Results
AXOS deeply influenced the gut microbiota composition without changing intestinal SCFA level, whereas WB and WBs induced differential and specific changes in gut microbes
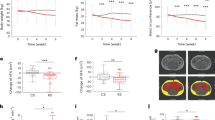
Cecal tissue weight and the cecal content weight were not significantly modified by the western diet. AXOS increased the cecal tissue weight and the cecal content insert (significantly for the cecal tissue weight) versus the WD group (Fig. 1a,b). By contrast, no significant effect was observed for the WB and WBs fractions. SCFA reflecting bacterial fermentation were analysed in the cecal content using gas chromatography coupled to a mass spectrometer (Supplementary Fig. S1). No modification of cecal SCFAs levels was observed whatever the dietary treatment.
Impact of the three cereal fractions on the gut microbiota in the cecal content. Weight of cecal tissue (a), cecal content (b), Principal Coordinate Analysis plot of β-diversity based on Bray-Curtis distance (c) and Principal Coordinate Analysis plot of β-diversity based on Morista-Horn distance (d). Mice were fed a control diet (CT, blue), a western diet (WD, red), a WD supplemented with 5% of wheat bran fraction with large particles (WD+WB, clear green), a WD supplemented with 5% of wheat bran with reduced particle size (WD+WBs, dark green) or a WD supplemented with 5% of AXOS (WD+AXOS, yellow) for 8 weeks. Results are expressed as mean±SEM for a and b (n = 7–9). Data with different superscript letters are significantly different at p < 0.05 (ANOVA). Dietary treatments accounted for 39 and 48% of the variation in the dataset (Bray-Curtis and Morisita-Horn respectively, adonis method, 1000 permutations, p < 0.001).
The composition of the gut microbiota in the cecal content was analyzed by Illumina sequencing of the 16S rRNA gene and qPCR.
Diversity indexes
Principal Coordinate Analysis (PCoA) of the beta-diversity indexes Bray-Curtis and Morisita-Horn revealed two major findings that were confirmed by further analyses at the taxa and OTU levels. First, WB and WBs fractions exerted a quite similar impact on the gut microbiota composition. Second, AXOS shifted away the composition of the gut microbiota and was the dietary treatment with the strongest impact on the gut microbiota composition (Fig. 1c,d). The impact of the WD on the gut microbiota composition was clear when using Bray-Curtis index, but not when using Morisita-Horn. Dietary treatments accounted for 39 and 48% of the variation in the dataset (Bray-Curtis and Morisita-Horn respectively, adonis method, 1000 permutations, p < 0.001) whereas cage effect accounted for 15% and 19% of the variation, indicating that the effect of the dietary treatment is the most important factor driving gut microbiota composition in our study. When comparing to the WD group, administration of both types of wheat bran fractions increased the alpha-diversity indexes for richness (significant effect of WB and WBs for observed species) and evenness (significant effect of WB and WBs for Shannon index), while administration of AXOS impacted mainly evenness (Supplementary Fig. S2).
Taxonomic analyses
Microbes discriminant for dietary treatment were determined through a pairwise comparison using LEfSe (Fig. 2). For the WB-WBs comparison, bifidobacteria and unclassified Erysipelotrichaceae were identified as biomarkers of the WB group. Several bacteria were found to be discriminant for AXOS when compared to the WD groups, including the phylum Proteobacteria. Using parametric methods, Akkermansia muciniphila (and the higher level taxa from the same lineage) was the only taxon found to be different between WB and WBs at the q-level (Supplementary Fig. S3j and Supplementary Data S1). Akkermansia muciniphila levels were significantly increased by 11-fold in WB vs WD and not significantly increased by WBs (Fig. 3a). We obtained the same tendency with qPCR analysis (Supplementary Fig. S3a). AXOS changed the relative abundance of 25 bacterial taxa (Supplementary Data S1). One of the main changes related to a 57-fold increase for the Bifidobacterium genus and related family and order (Fig. 3b and Supplementary Data S1). This increase translated to a 16-fold increase at the Actinobacteria phylum level. In this set of experiment, bifidobacteria were decreased by the WD and their levels were fully restored by AXOS (Fig. 3b), as confirmed by qPCR (Supplementary Fig. S3b). Of note, a bifidogenic effect versus the WD group was also observed for the WB fraction (significantly only with the qPCR analysis) but it was absent for the WBs fraction (Fig. 3b and Supplementary Fig. S3b). Another important change was observed for Butyricicoccus (13-fold increase) after AXOS supplementation whereas Turicibacter disappeared (Fig. 3c), Clostridium sensu stricto was drastically reduced (113-fold decrease) (Fig. 3d) and Desulfovibrionaceae were lowered by half (Supplementary Data S1).
Discriminant analysis of the cecal microbiota using LEfSe. Mice were fed a control diet (CT), a western diet (WD), a WD supplemented with 5% of wheat bran fraction with large particles (WD+WB), a WD supplemented with 5% of wheat bran with reduced particle size (WD+WBs) or a WD supplemented with 5% of AXOS (WD+AXOS) for 8 weeks. Discriminant analysis between WB and WBs (a). Taxa enriched in the WB group are highlighted in red whereas taxa enriched in the WBs group are highlighted in green. Discriminant analysis between AXOS and WD (b). Taxa enriched in the AXOS group are highlighted in red whereas taxa enriched in the WD group are highlighted in green.
Impact of the three cereal fractions on the gut microbiota composition assessed by Illumina sequencing. Relative abundance of selected genera in cecal content of mice fed a control diet (CT), a western diet (WD), a WD supplemented with 5% of wheat bran fraction with large particles (WD+WB), a WD supplemented with 5% of wheat bran with reduced particle size (WD+WBs) or a WD supplemented with 5% of AXOS (WD+AXOS) for 8 weeks. Individual values are presented with mean. Data with different superscript letters are significantly different at p < 0.05 (ANOVA).
Phylogenetic analyses
Phylogenetic analyses revealed results similar to the taxonomic analyses. Among the 741 OTUs that were detected in the microbial ecosystem, 144 OTUs were significantly affected at the q-level (Supplementary Data S1). Most of these changes related to the impact of AXOS. Indeed, 78 OTUs were modified by the addition of AXOS in the WD. Six OTUs were increased more than a 100 fold, such as OTU 36 (Bifidobacterium sp., 1016-fold increase), OTU 83 (from the Lachnospiraceae family, 931-fold increase), OTU 90 (from the Ruminococcaceae family, 884-fold increase), OTU 100 (from the Clostridiales order, 593-fold increase), OTU 161 (from the Lachnospiraceae family, 181-fold increase) and OTU 35 (from the Clostridiales order, 162-fold increase) (Supplementary Fig. S3). Two OTUs (from the Clostridiales order) were decreased more than a 100-fold in presence of AXOS (OTU 173, 124-fold and OTU 75, 120-fold). When comparing the AXOS and WD groups, 14 OTUs were detected only in the AXOS group whereas 4 OTUs were detected only in the WD group. When comparing the WB and WBs groups, 13 OTUs showed different levels between dietary treatments. The most prominent differences are a 10-fold decrease in OTU 652 (Allobaculum sp. belonging to the Erysipelotrichaceae family) and a 3-fold decrease in OTU 8 (Akkermansia muciniphila) under WBs. The other 11 OTUs belong to the Clostridiales order, and were either increased or decreased by WBs by less than 6-fold. OTU 8 (Akkermansia muciniphila) and OTUs 37 and 138 (from the Clostridiales order) are the only 3 OTUs that were affected by the addition of WB in the WD but not by the addition of WBs to the same diet, if we exclude OTU 659 for which only 5 sequences were found in the all dataset.
Several markers involved in gut homeostasis were not modified by the three cereal fractions
Several markers involved in gut immune function and gut permeability were analysed both in the ileum (Fig. 4) and the colon. IL-1β and IL6 expression was slightly upregulated in the ileum by the WD as compared to the control mice without reaching significant p value. In addition, neither occludin expression, nor zonula occludens-1 (ZO1) expression were altered by the WD, suggesting that WD did not alter gut permeability (Fig. 4). WB fraction decreased the expression of IL1β in the ileum as compared to WD group and it is the sole significant effect of wheat bran materials observed on those gut inflammatory/permeability markers. We measured the expression of secreting antimicrobial peptides produced by epithelial cells in the ileum and the colon: phospholipase A2 group-II (PlA2g2) and C-type lectin, primarily the regenerating islet-derived 3-gamma (RegIIIγ) (Fig. 4). Although we observed significantly lower expression of RegIIIγ in the ileum segment due to the WD, none of the analysed antimicrobial peptides were affected by the supplementation with the cereal fractions (versus WD group) whatever the gut segments.
Impact of the three cereal fractions on gut barrier function. Expression of inflammatory markers (a), expression of junction proteins (b) and expression of secreting antimicrobial peptides (c) in the ileum of mice fed a control diet (CT), a western diet (WD), a WD supplemented with 5% of wheat bran (WD+WB), a WD supplemented with 5% of wheat bran with reduced particle size (WD+WBs) or a WD supplemented with 5% of AXOS (WD+AXOS) for 8 weeks. Results are expressed as mean±SEM (n = 7–9). Data with different superscript letters are significantly different at p < 0.05 (ANOVA).
AXOS, WB and WBs differently impact obesity and white adipose tissue
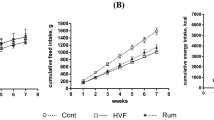
The WD consumption increased the body weight gain and the adiposity compared to the control diet (Fig. 5a,c). Fat mass development due to the WD was confirmed by an increase of the visceral, subcutaneous and epididymal adipose tissues as compared with the control group (Fig. 5d–f). AXOS fraction supplementation reduced body weight gain and fat mass expansion without reaching significant p value except for the epididymal adipose tissue. Although wheat bran fractions WB and WBs had no significant effect on body weight gain and fat mass expansion due to WD, it is important to note that WBs group exhibited the higher values of body weight gain, fat mass gain and adiposity whereas wheat bran fraction with large particle (WB) tended to decrease body weight and adiposity whatever the type of adipose tissues. The differences between WB and WBs groups reached significant p value for all the parameters. However, the three cereal fractions did not modify significantly the total caloric intake (Fig. 5b). In accordance with the data relating the expansion of white adipose tissue, the portal levels of leptin, an adipokine produced proportionally to fat mass, were markedly increased in WD and WD+WBs groups as compared to the control group (Supplementary Fig. S4a). In contrast, the plasma levels of several gastrointestinal peptides regulating appetite that were secreted by the stomach (ghrelin), by the intestinal K-cells (GIP) or by the intestinal L-cells (GLP-1) were not significantly affected by the dietary treatments (Supplementary Fig. S4b–d).
Impact of the three cereal fractions on obesity, dietary intake and fat mass development. Body weight gain (a), total caloric intake (b), fat mass gain measured by NMR at days 14, 28, 35 and 49 (c), weight of visceral adipose tissue (d), weight of subcutaneous adipose tissue (e), weight of epididymal adipose tissue (f). Mice were fed a control diet (CT), a western diet (WD), a WD supplemented with 5% of wheat bran fraction with large particles (WD+WB), a WD supplemented with 5% of wheat bran with reduced particle size (WD+WBs) or a WD supplemented with 5% of AXOS (WD+AXOS) for 8 weeks. Results are expressed as mean±SEM (n = 7–9 for a, c–f and n = 2–3 for b). Data with different superscript letters are significantly different at p < 0.05 (ANOVA).
Key inflammatory markers were analysed by qPCR in the visceral adipose tissue (Fig. 6). WD did not affect the mRNA expression of inflammatory markers. By contrast and in accordance with the fat mass expansion, WD+WBs was the sole dietary treatment able to upregulate some of these markers (MCP-1 and F4/80) as compared to the WD group or CT group but also as compared to the WB group; this effect being significant for MCP-1 expression. The AXOS fraction and the crude WB fraction did not change significantly any of the four inflammatory markers analysed as compared to WD group.
Inflammatory markers in visceral adipose tissue. Mice were fed a control diet (CT), a western diet (WD), a WD supplemented with 5% of wheat bran (WD+WB), a WD supplemented with 5% of wheat bran with reduced particle size (WD+WBs) or a WD supplemented with 5% of AXOS (WD+AXOS) for 8 weeks. Results are expressed as mean±SEM (n = 6–9). Data with different superscript letters are significantly different at p < 0.05 (ANOVA).
Wheat bran with reduced particle size (WBs) induced lipid accumulation in the liver
Histological analysis after oil red O staining did not reveal steatosis in WD group as compared to CT group (Fig. 7a,b). Importantly, the wheat bran with reduced particle size (WBs) induced lipid accumulation as compared to control group or WD group whereas wheat bran with large particle size (WB) or AXOS did not exert any effect. Biochemical analysis of lipid content of the liver confirmed this result (Fig. 7c). Interestingly, in contrast to WBs fraction, WB supplementation leads to the higher excretion of triglycerides in the feces among all groups(Fig. 8a). This effect is probably linked to its higher fat binding capacity compared to the WBs or AXOS fractions (Fig. 8b).
Impact of the three cereal fractions on lipid accumulation in the liver. Histochemical detection of neutral lipids of the liver sections (a), lipid area (% versus total area) (b) and lipid content of the liver (c) of mice fed a control diet (CT), a western diet (WD), a WD supplemented with 5% of wheat bran (WD+WB), a WD supplemented with 5% of wheat bran with reduced particle size (WD+WBs) or a WD supplemented with 5% of AXOS (WD+AXOS) for 8 weeks (scale bar = 100 μm). Results are expressed as mean±SEM (n = 7–9). Data with different superscript letters are significantly different at p < 0.05 (ANOVA).
Impact of the three wheat bran fractions on triglyceride excretion in feces and fat binding capacity. Triglyceride content in feces (a) of mice fed a control diet (CT), a western diet (WD), a WD supplemented with 5% of wheat bran (WD+WB), a WD supplemented with 5% of wheat bran with reduced particle size (WD+WBs) or a WD supplemented with 5% of AXOS (WD+AXOS) for 8 weeks. In vitro fat binding capacity of starch and cereal fractions for soybean oil (b). Results are expressed as mean+SEM (n = 3 for a and n = 5 for b). Data with different superscript letters are significantly different at p < 0.05 (ANOVA).
Changes in the gut microbiota composition induced by wheat bran fractions correlated with host parameters
Multiple correlation analyses were performed to evaluate the potential links between gut microbial taxa and host metabolism and immunity among the five experimental groups (Fig. 9). Such analyses revealed a clear negative correlation between adipose tissue weights and two families from the Actinobacteria phylum, the Coriobacteriaceae and the Bifidobacteriaceae. The Bifidobacterium genus and its parent taxa were associated with a reduced body weight gain, reduced fat mass as assessed by NMR and reduced plasma leptin levels. In contrast, increased Bilophila levels were associated with increased fat mass, adipose tissue weights and plasma leptin levels. Finally, mRNA expression of PlA2g2 in the ileum was strongly positively correlated with the Bifidobacteriaceae, Coriobacteriaceae and Sutterellaceae families (and related parent taxa).
Correlations between bacterial taxa and host parameters. Pearson correlations were computed for all bacterial taxa with a relative abundance above 0.01% in at least one animal, and all measured host parameters. P values were adjusted for multiple testing according to the Bonferroni and Hochberg procedure. The color at each intersection refers to the value of the r coefficient; an asterisk indicates a significance correlation between these two parameters (adjusted p < 0.05). Only the bacterial taxa for which at least one significant correlation with a host parameter was detected, are displayed. Bacterial taxonomic level is indicated at the end of the name.
Discussion
In the present study, we confirmed the prebiotic effect of AXOS produced from WB in a model of WD-induced obesity. Indeed, AXOS feeding increased bifidobacteria (demonstrated by qPCR and for the first time by sequencing of the 16S rRNA gene) in the cecal content of WD fed mice. This effect was associated with a reduction of epididymal fat expansion due to WD feeding confirming some of the results obtained in our previous study27. The lack of significant effect of AXOS on body weight gain may be related to the lower proportion of fat (45%) in the WD compared to the high fat (60%) diet used in previous study in which the body weight change due to HF diet was more drastic. Moreover, use of next-generation sequencing revealed other important microbial changes induced by AXOS. Sixty-one bacterial taxa were significantly affected by the dietary treatments despite the high inter-individual variation. No clear-cut separation between responders and non-responders were observed in our dataset, as it has been described with other fibers29, 30.Taxa related to bacteria associated with colitis and inflammatory disorders (Turicibacter, ClostridiumProteobacteria) or related to endotoxin-producing opportunistic pathogens (Desulfovibrionaceae) were reduced significantly, while those related to gut barrier protection (Butyricicoccus) increased31, 32. Correlation analysis revealed that higher abundancy of bifidobacteria was strongly associated with a reduced body weight gain, reduced fat mass as assessed by NMR and reduced plasma leptin levels. Although dietary treatments did not significantly modify its abundancy, it is worth to note that Bilophila genus proportion was negatively correlated to the fat mass, adipose tissue weights and plasma leptin levels in accordance with previous studies showing a link between Bilophila wadsworthia, the unique species described to date within this genus, and high fat diet-induced altered metabolism, inflammation and weight gain33,34,35,36.
One of the main objectives of our current work was to assess the potential relevance for host health of wheat bran and the importance of their particle size in relation to the modulation of the gut microbial composition in obese mice. Although AXOS and WB exhibited different fat binding capacities, those fractions exerted similar impacts on obesity and adiposity; a phenomenon that could be related to their influence on the gut microbiota1, 14. Indeed, a bifidogenic effect was observed upon supplementation with both the crude fraction of WB and the AXOS fraction. Importantly and in contrast to the AXOS fraction or the crude wheat bran (WB), wheat bran with reduced particle size (WBs) led to a highest body weight gain, fat mass gain and adiposity among the five groups. Accordingly, WBs increased the levels of adipo(cyto)kines in mice fed a WD as evidenced by the higher expression of inflammatory markers in visceral adipose tissue and higher leptinemia. Although the composition of the wheat bran fractions was not modified after particle size reduction, we observed important differences in metabolic effects after WB and WBs supplementation. This discrepancy may be the result of their different fat binding capacity (as shown in vitro) leading to fat leakage in fecal matter (as shown in vivo by a higher proportion of fecal triglycerides) as already mentioned for two distinct fermentable carbohydrates targeting the gut microbiota (long chain arabinoxylans and inulin-type fructans)37. The different fat binding capacity between the fractions could be also one of the mechanisms explaining the difference in ectopic lipid accumulation in the liver tissue, which could result from a decrease in the pool of dietary non esterified fatty acids redistributed from the adipose tissue to the liver. However, we may not exclude that different effects observed with the WB fractions on adiposity resulted from their intrinsic capacity to modulate gut microbiota. For example, when comparing the WB and WBs groups, one of the most prominent differences is an increase of Akkermansia muciniphila under WB. Our recent data obtained in high fat diet fed mice have shown that Akkermansia muciniphila administrated through daily gavage is able to increase the fecal energy excretion and could therefore, participate to the control of adiposity38. Furthermore, a bifidogenic effect was observed only with the crude fraction, as already shown in a previous study only after 3 weeks of supplementation39. In fact, both taxa were identified as key players in the cross-talk between gut microbiota and host metabolism particularly in the context of obesity40. Gut microbiota may contribute to energy metabolism through the production of SCFA by colonic fermentation and in vitro studies reported changes in their proportions between large particle wheat bran and small particle wheat bran41, 42. It is generally admitted that the fermentation of AXOS is able to promote butyrate production43. However, in our study, cecal contents of butyrate, propionate and acetate were not significantly modified whatever the dietary treatments. We may not rule out that an increased production of butyrate (or other SCFA) could occur, but the fact that the SCFA produced may be absorbed, or excreted, which would not allow to point out changes in their mean level in the intestinal content. Probably, in our context, difference in SCFA cannot explain the difference in phenotype.
We were unable to highlight inflammatory disorders in the visceral adipose tissue or the ileum in WD mice as compared to control mice. The lack of significant inflammatory disorders between WD group versus CT group -together with the lack of effect on parameters related to gut permeability (expression of 2 key tight junction proteins)- may be related to the lower proportion of fat (45%) in the WD compared to the high fat (60%) diet used in previous studies (for review see ref. 44). We have analyzed antimicrobial peptides that are produced by the host and contribute to shape the composition of the gut microbiota. These peptides participate to the gut barrier function and are an attractive mechanism for the modulation of the gut ecosystem by nutrients45, 46. Among them, the expression of RegIIIγ, a key host factor controlling microbiota composition and involved in gut immunity, was lower in the ileum of WD mice as compared to control mice as previously shown in a diet induced obesity model using high fat diet (60%)40, 46. Interestingly, an anti-inflammatory effect of wheat bran was evidenced by a significantly lower expression of IL1β in the ileum only for the unmodified crude fraction (WB). This effect seemed to be independent of the gut barrier functions as the expression of tight junction proteins (ZO1 and occludin) and of the antimicrobial peptide RegIIIγ was not affected in contrast to what is observed for fructan-type prebiotics40. Correlation analysis revealed that the ileal expression of Il1β was positively correlated to the Bacteroides genus although this genus was not significantly affected by the dietary treatments. The usual limitations linked to individual experimental animals also apply to this study47. Only one gender and one genetic background were tested. In our case, we controlled for cage effects.
In conclusion, wheat bran fractions differently affect metabolic and inflammatory disorders associated with WD feeding. Particle size can determine health effects of wheat bran in the context of obesity. In addition, differences in metabolic response of the different fractions also depend on their fat binding capacity and gut microbiota modulating properties (namely Akkermansia and bifidobacteria). Indeed, only the crude fraction of wheat bran promoted fat excretion and distinguished itself from the other fractions by the capacity to increase the Akkermansia genus and to counteract gut IL1β overexpression. Moreover, our findings confirmed the prebiotic potential of AXOS. Although AXOS and WB exhibited different fat binding capacities, those fractions exerted similar impacts on adiposity. This phenomenon could be related to their influence on the gut microbiota.
Materials and Methods
Ethics Statement
All experiments were performed in strict accordance with relevant guidelines and regulations for the care and use of animals. All mouse experiments were approved by and performed in accordance with the guidelines of the local ethics committee for animal care of the Health Sector of the Université catholique de Louvain under the supervision of Prof. F. Lemaigre and Prof. JP Dehoux and under the specific agreement numbers 2014/UCL/MD/022. Housing conditions were as specified by the Belgian Law of 29 May 2013, on the protection of laboratory animals (Agreement LA 1230314). Every effort was made to minimize animal pain, suffering, and distress and to reduce the number of animals used.
Animals, diets and experimental setup
Forty-five male C57BL6 mice (9 weeks old at the beginning of the experiment, Janvier laboratories, France) were housed in groups of 3 per cage in a controlled environment (12-hour daylight cycle) with free access to food and water. After one week of acclimatisation, mice were divided in 5 groups (n = 9/group): a control group (CT), fed with a control diet (D12450K, Research Diet®, 10% fat, 70% carbohydrates in kcal/g), a group fed with a WD (D12451, Researcher Diet®, 45% fat, 35% carbohydrates in kcal/g), a group fed the WD, supplemented with 5% unmodified wheat bran (1690 µm, WD+WB group), a group fed the WD, supplemented with 5% wheat bran with reduced particle size (150 µm, WD+WBs group) and a group fed the WD, supplemented with 5% wheat bran derived arabinoxylan oligosaccharides (WD+AXOS group). The composition of the wheat bran materials has been detailed in Supplementary Table S1. Food intake and water intake were recorded twice a week. The total caloric intake was obtained by multiplying total food intake (g) for 3 mice per cage (n = 3) by the caloric value of the diets, i.e. 3.85 kcal/g, 4.73 kcal/g for CT and WD diets respectively. The caloric value of the WD supplemented with cereal fractions was calculated taking in to account that these diets were composed of 95% WD diet and 5% fractions considering that those fractions were completely processed by the gut microbiota into SCFA available to the host and that the maximal caloric intake through this process would be 2 kcal/g on average (value established by the commission directive 2008/100/EC). The total caloric intake results are presented for the whole study and represent the sum of the diet consumed during 8 weeks for three mice. Total fat mass was determined using a 7.5 MHz Time domain-Nuclear magnetic resonance (LF50 minispec, Bruker, Germany). After 8 weeks of dietary treatment and a 6-hour period of fasting, mice were anesthetised with isoflurane (Forene®, Abbott, Queenborough, Kent, England) before exsanguination and tissue sampling. Mice were killed by cervical dislocation. Portal blood was taken in <30 sec and directly flushed within tubes containing dipeptidyl peptidase IV (DPPIV) inhibitor (Millipore, St Charles, MO, USA) and a cocktail of general protease inibitors containing EDTA, bestatin, leupeptin, aprotinin, E-64 and 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride (P2714-Sigma protease cocktail, Sigma, Saint Louis, MO, USA and Roche Pefabloc SC, Roche Diagnostics, Vilvoorde, Belgium). Plasma was immediately collected after centrifugation and stored at −80 °C in tubes containing the same protease inhibitors as those used for gut peptide determination. Liver, white adipose tissues (visceral, epididymal and inguinal subcutaneous), and gut segments (from ileum, proximal colon and caecum) were carefully dissected, weighed and immersed in liquid nitrogen before storage at −80 °C.
Biochemical analysis
Portal concentrations of GLP-1, GIP, ghrelin and leptin were determined in 2 × 15 µl of plasma using a multiplex immunoassay kit (Bioplex, Bio-Rad) and measured using Luminex technology (Bioplex, Bio-Rad). Lipid content was measured in the liver tissue after extraction with chloroform–methanol according to the Folch method48. Triglycerides were measured in feces after extraction with chloroform-methanol. Briefly, 100 mg of dried powder-reduced feces were homogenised in 2.6 ml of chloroform: methanol (2:1). The homogenate was filtered using a Whatman filter placed at the end of a syringe, and recovered in a 15 ml falcon tube. The filtrate was washed up three times with phosphate buffer (pH 7.4). The chloroform phase was evaporated under nitrogen flux and the dried residue was solubilised in 800 µl of isopropanol. Triglycerides concentration was measured using a kit coupling an enzymatic reaction and spectrophotometric detection of the final product (Diasys Diagnostic and System, Holzheim, Germany). All samples were run in duplicate.
Fat binding assay
Fat binding capacity (FBC) of wheat fractions was initially carried out by weighing a centrifuge tube containing 0.5 g of sample, adding 10 ml of soybean oil, and mixing on a vortex mixer for 1 min to disperse the sample. The contents were left at ambient temperature for 30 min with shaking for 5 seconds every 10 min and then centrifuged at 2500 g for 25 minutes. After the supernatant was removed, the tube was weighed again. FBC was calculated as follows: FBC (%) = [fat bound (g)/sample weight (g)] × 10049.
Fat histochemical detection
A fraction of the main liver lobe was fixed-frozen in Tissue-tek in liquid nitrogen-cold isopentane. For the detection of neutral lipids, frozen sections were sliced and stained with the oil red O, using 0.5% oil red O dissolved in propylene glycol for 10 min at 60 °C. The sliced sections were then counterstained with a Hemalum solution. The lipid area was analysed as previously described in Neyrinck et al.50.
Real-time quantitative PCR
Total RNA was isolated from tissues using the TriPure isolation reagent kit (Roche Diagnostics, Penzberg, Germany). For adipose tissue, RNA integrity number (RIN) was calculated using a Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA); samples were selected only when their RIN value was above 6. Complementary DNA was prepared by reverse transcription of 1 μg total RNA using the Kit Reverse Transcription System (Promega,Madison,WI). Real-time polymerase chain reaction (PCR) was performed with a CFX96 Touch Real-Time PCR Detection System and software (Biorad Laboratories Ltd, UK) using SYBR Green (Applied Biosystems and Eurogentec, Verviers, Belgium) for detection. All samples were run in duplicate in a single 96-well reaction plate, and data were analyzed according to the 2-ΔΔCT method. The purity of the amplified product was verified by analyzing the melting curve performed at the end of amplification. The ribosomal protein L19 (RPL19) gene was chosen as a reference gene. Primer sequences are presented in Supplementary Table S2.
Short chain fatty acids analysis
SCFA levels in cecal content were analyzed using gas chromatography coupled to a mass spectrometer (GC-MS). Cecal aliquots of 60 mg were suspended in 1 ml saturated NaCl and 2-ethylbutyrate (50 µL; 1.25 mg/mL) was added as internal standard. Samples were shortly vortexed after which 3 mL of diethylether (Sigma) and 150 µL of sulfuric acid were added to the sample. After centrifugation, the ether layer was separated and dried over anhydrous Na2SO4. The short-chain fatty acids (SCFAs) were analyzed on a GC-MS system (Trace GC, Thermoquest, Rodano, Italy and DSQ II, Thermo Electron, San Jose, USA) equipped with a Stabilwax DA column (30 m × 0.25 mm ID, 0.25 µm film thickness; Interscience, Belgium). The initial oven temperature of 40 °C was held for 3 min and ramped with 4 °C/min to 140 °C and with 16 °C/min to 240 °C. Acetate, propionate and butyrate were quantified with appropriate calibration curves obtained using internal standard quantitation.
Analysis of the gut microbiota
Genomic DNA was extracted from the cecal content using a QIAamp DNA Stool Mini Kit (Qiagen, Germany), including a bead-beating step. The composition of the gut microbiota was analysed by Illumina sequencing of the 16S rRNA gene and qPCR. Absolute quantification of Bifidobacterium spp. and Akkermansia muciniphila was performed by quantitative real-time PCR (qPCR) (primers presented in Supplementary Table S2) as previously described51. The V5-V6 region of the 16S rRNA gene was amplified by PCR with modified primers52. The amplicons were purified, quantified and sequenced using an Illumina Miseq to produce 2 × 300-bp sequencing products at the University of Minnesota Genomics Center. Initial quality-filtering of the reads was conducted with Illumina Software, yielding an average of 67765 pass-filter reads per sample. Quality scores were visualized, and reads were trimmed to 220 bp (R1) and 200 bp (R2). The reads were merged with the merge-Illumina-pairs application53. For all samples but one, a subset of 25000 reads was randomly selected using Mothur v.1.25.054 to avoid large disparities in the number of sequences. Subsequently, the UPARSE pipeline implemented in USEARCH v7.0.100155 was used to further process the sequences. Putative chimaeras were identified against the Gold reference database and removed. Clustering was performed with a 98% similarity cut-off to designate operational taxonomic units (OTUs). Non-chimeric sequences were also subjected to taxonomic classification using the RDP MultiClassifier 1.1 from the Ribosomal Database Project56 for phylum to genus characterization of the cecal microbiome. The phylotypes were computed as percent proportions based on the total number of sequences in each sample. Full protocol, detailed statistical analysis and accession numbers are provided in the Supplemental materials.
Statistical analysis
Data are presented as mean ± SEM. Statistical significance between groups was assessed by one-way ANOVA. Dixon’s Q-test was performed to remove outliers (95% confidence level). The statistically significant ANOVA tests were followed by post hoc Tukey’s multiple comparison tests using GraphPad Prism software (version 5.00, GraphPad Software, San Diego, California, USA). Data with different superscript letters were significantly different (p ≤ 0.05) according to the post hoc ANOVA statistical analysis. Multiple correlation analyses were computed and visualized in R using Pearson correlation method in a personal script, with p values being adjusted for multiple testing according to the Benjamini and Hochberg procedure57; p ≤ 0.05 was considered as statistically significant.
References
Neyrinck, A. M. & Delzenne, N. M. Potential interest of gut microbial changes induced by non-digestible carbohydrates of wheat in the management of obesity and related disorders. Curr Opin Clin Nutr Metab Care 13, 722–728, doi:10.1097/MCO.0b013e32833ec3fb (2010).
Deehan, E. C. & Walter, J. The Fiber Gap and the Disappearing Gut Microbiome: Implications for Human Nutrition. Trends Endocrinol Metab 27, 239–242, doi:10.1016/j.tem.2016.03.001 (2016).
Delcour, J. A., Aman, P., Courtin, C. M., Hamaker, B. R. & Verbeke, K. Prebiotics, Fermentable Dietary Fiber, and Health Claims. Adv Nutr 7, 1–4, doi:10.3945/an.115.010546 (2016).
Maes, C. & Delcour, J. A. Structural Characterisation of Water-extractable and Water-unextractable Arabinoxylans in Wheat Bran. Journal of Cereal Science 35, 315–326, doi:10.1006/jcrs.2001.0439 (2002).
Broekaert, W. F. et al. Prebiotic and other health-related effects of cereal-derived arabinoxylans, arabinoxylan-oligosaccharides, and xylooligosaccharides. Crit Rev Food Sci Nutr 51, 178–194, doi:10.1080/10408390903044768 (2011).
Hemdane, S. et al. Wheat (Triticum aestivum L.) Bran in Bread Making: A Critical Review. Comprehensive Reviews in Food Science and Food Safety 15, 28–42, doi:10.1111/1541-4337.12176 (2016).
Zoran, D. L., Turner, N. D., Taddeo, S. S., Chapkin, R. S. & Lupton, J. R. Wheat bran diet reduces tumor incidence in a rat model of colon cancer independent of effects on distal luminal butyrate concentrations. J Nutr 127, 2217–2225 (1997).
Kritchevsky, D. Protective role of wheat bran fiber: preclinical data. Am J Med 106, 28S–31S (1999).
Pesarini, J. R. et al. Antimutagenic and anticarcinogenic effects of wheat bran in vivo. Genet Mol Res 12, 1646–1659, doi:10.4238/2013.May.14.5 (2013).
Ferguson, L. R. & Harris, P. J. Protection against cancer by wheat bran: role of dietary fibre and phytochemicals. Eur J Cancer Prev 8, 17–25 (1999).
Pollet, A. et al. In vitro fermentation of arabinoxylan oligosaccharides and low molecular mass arabinoxylans with different structural properties from wheat (Triticum aestivum L.) bran and psyllium (Plantago ovata Forsk) seed husk. J Agric Food Chem 60, 946–954, doi:10.1021/jf203820j (2012).
Van Craeyveld, V. et al. Structurally different wheat-derived arabinoxylooligosaccharides have different prebiotic and fermentation properties in rats. J Nutr 138, 2348–2355, doi:10.3945/jn.108.094367 (2008).
Sanchez, J. I. et al. Arabinoxylan-oligosaccharides (AXOS) affect the protein/carbohydrate fermentation balance and microbial population dynamics of the Simulator of Human Intestinal Microbial Ecosystem. Microb Biotechnol 2, 101–113, doi:10.1111/j.1751-7915.2008.00064.x (2009).
Delzenne, N. M. & Cani, P. D. Interaction between obesity and the gut microbiota: relevance in nutrition. Annu Rev Nutr 31, 15–31, doi:10.1146/annurev-nutr-072610-145146 (2011).
Sonnenburg, J. L. & Backhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 535, 56–64, doi:10.1038/nature18846 (2016).
Verspreet, J. et al. A Critical Look at Prebiotics Within the Dietary Fiber Concept. Annu Rev Food Sci Technol 7, 167–190, doi:10.1146/annurev-food-081315-032749 (2016).
Geurts, L., Neyrinck, A. M., Delzenne, N. M., Knauf, C. & Cani, P. D. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: novel insights into molecular targets and interventions using prebiotics. Benef Microbes 5, 3–17, doi:10.3920/BM2012.0065 (2014).
Druart, C. et al. Gut microbial metabolites of polyunsaturated fatty acids correlate with specific fecal bacteria and serum markers of metabolic syndrome in obese women. Lipids 49, 397–402, doi:10.1007/s11745-014-3881-z (2014).
Bindels, L. B., Dewulf, E. M. & Delzenne, N. M. GPR43/FFA2: physiopathological relevance and therapeutic prospects. Trends Pharmacol Sci 34, 226–232, doi:10.1016/j.tips.2013.02.002 (2013).
Canfora, E. E., Jocken, J. W. & Blaak, E. E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 11, 577–591, doi:10.1038/nrendo.2015.128 (2015).
Cani, P. D. et al. Endocannabinoids–at the crossroads between the gut microbiota and host metabolism. Nat Rev Endocrinol 12, 133–143, doi:10.1038/nrendo.2015.211 (2016).
Jenkins, D. J. et al. The effect of wheat bran particle size on laxation and colonic fermentation. J Am Coll Nutr 18, 339–345 (1999).
Heller, S. N. et al. Dietary fiber: the effect of particle size of wheat bran on colonic function in young adult men. Am J Clin Nutr 33, 1734–1744 (1980).
Vincent, R. et al. Effect of bran particle size on gastric emptying and small bowel transit in humans: a scintigraphic study. Gut 37, 216–219 (1995).
Brodribb, A. J. & Groves, C. Effect of bran particle size on stool weight. Gut 19, 60–63 (1978).
Eelderink, C. et al. Difference in postprandial GLP-1 response despite similar glucose kinetics after consumption of wheat breads with different particle size in healthy men. Eur J Nutr, doi:10.1007/s00394-016-1156-6 (2016).
Neyrinck, A. M. et al. Wheat-derived arabinoxylan oligosaccharides with prebiotic effect increase satietogenic gut peptides and reduce metabolic endotoxemia in diet-induced obese mice. Nutr Diabetes 2, e28, doi:10.1038/nutd.2011.24 (2012).
Neyrinck, A. M. et al. Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS One 6, e20944, doi:10.1371/journal.pone.0020944 (2011).
Salonen, A. et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J 8, 2218–2230, doi:10.1038/ismej.2014.63 (2014).
Yang, J. et al. Disparate Metabolic Responses in Mice Fed a High-Fat Diet Supplemented with Maize-Derived Non-Digestible Feruloylated Oligo- and Polysaccharides Are Linked to Changes in the Gut Microbiota. PLoS One 11, e0146144, doi:10.1371/journal.pone.0146144 (2016).
Xiao, S. et al. A gut microbiota-targeted dietary intervention for amelioration of chronic inflammation underlying metabolic syndrome. FEMS Microbiol Ecol 87, 357–367, doi:10.1111/1574-6941.12228 (2014).
Devriese, S. et al. Reduced Mucosa-associated Butyricicoccus Activity in Patients with Ulcerative Colitis Correlates with Aberrant Claudin-1 Expression. J Crohns Colitis, doi:10.1093/ecco-jcc/jjw142 (2016).
Devkota, S. et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 487, 104–108, doi:10.1038/nature11225 (2012).
Barlow, G. M., Yu, A. & Mathur, R. Role of the Gut Microbiome in Obesity and Diabetes Mellitus. Nutr Clin Pract 30, 787–797, doi:10.1177/0884533615609896 (2015).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563, doi:10.1038/nature12820 (2014).
Schneeberger, M. et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep 5, 16643, doi:10.1038/srep16643 (2015).
Druart, C. et al. Implication of fermentable carbohydrates targeting the gut microbiota on conjugated linoleic acid production in high-fat-fed mice. Br J Nutr 110, 998–1011, doi:10.1017/S0007114513000123 (2013).
Plovier, H. et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med, doi:10.1038/nm.4236 (2016).
Neyrinck, A. M. et al. Immunomodulatory properties of two wheat bran fractions - aleurone-enriched and crude fractions - in obese mice fed a high fat diet. Int Immunopharmacol 8, 1423–1432, doi:10.1016/j.intimp.2008.05.015 (2008).
Everard, A. et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA 110, 9066–9071, doi:10.1073/pnas.1219451110 (2013).
Stewart, M. L. & Slavin, J. L. Particle size and fraction of wheat bran influence short-chain fatty acid production in vitro. Br J Nutr 102, 1404–1407, doi:10.1017/S0007114509990663 (2009).
Dziedzic, K. et al. Effect of Wheat Dietary Fiber Particle Size during Digestion In Vitro on Bile Acid, Faecal Bacteria and Short-Chain Fatty Acid Content. Plant Foods Hum Nutr 71, 151–157, doi:10.1007/s11130-016-0537-6 (2016).
Riviere, A., Selak, M., Lantin, D., Leroy, F. & De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front Microbiol 7, 979, doi:10.3389/fmicb.2016.00979 (2016).
Neyrinck, A. M., Schüppel, V. L., Lockett, T., Haller, D. & Delzenne, N. M. Microbiome and metabolic disorders related to obesity: Which lessons to learn from experimental models? Trends in Food Science & Technology 57, 256–264, doi:10.1016/j.tifs.2016.08.012 (2016).
Mukherjee, S. & Hooper, L. V. Antimicrobial defense of the intestine. Immunity 42, 28–39, doi:10.1016/j.immuni.2014.12.028 (2015).
Everard, A. et al. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J 8, 2116–2130, doi:10.1038/ismej.2014.45 (2014).
Hormannsperger, G., Schaubeck, M. & Haller, D. Intestinal Microbiota in Animal Models of Inflammatory Diseases. ILAR J 56, 179–191, doi:10.1093/ilar/ilv019 (2015).
Folch, J., Lees, M. & Sloane Stanley, G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226, 497–509 (1957).
No, H. K., Lee, S. H., Park, N. Y. & Meyers, S. P. Comparison of physicochemical, binding, and antibacterial properties of chitosans prepared without and with deproteinization process. J Agric Food Chem 51, 7659–7663, doi:10.1021/jf030226w (2003).
Neyrinck, A. M. et al. Rhubarb extract prevents hepatic inflammation induced by acute alcohol intake, an effect related to the modulation of the gut microbiota. Mol Nutr Food Res 61, doi:10.1002/mnfr.201500899 (2017).
Bindels, L. B. et al. Synbiotic approach restores intestinal homeostasis and prolongs survival in leukaemic mice with cachexia. ISME J 10, 1456–1470, doi:10.1038/ismej.2015.209 (2016).
Claesson, M. J. et al. Comparison of two next-generation sequencing technologies for resolving highly complex microbiota composition using tandem variable 16S rRNA gene regions. Nucleic Acids Res 38, e200, doi:10.1093/nar/gkq873 (2010).
Eren, A. M., Vineis, J. H., Morrison, H. G. & Sogin, M. L. A filtering method to generate high quality short reads using illumina paired-end technology. PLoS One 8, e66643, doi:10.1371/journal.pone.0066643 (2013).
Schloss, P. D. et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75, 7537–7541, doi:10.1128/AEM.01541-09 (2009).
Edgar, R. C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10, 996–998, doi:10.1038/nmeth.2604 (2013).
Cole, J. R. et al. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res 42, D633–642, doi:10.1093/nar/gkt1244 (2014).
Benjamini, Y. & Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological) 57, 289–300 (1995).
Acknowledgements
We thank Véronique Allaeys, Remi Selleslagh and Bouazza Es Saadi for skillful technical assistance. LBB is a recipient of FSR subsidies (UCL, Belgium) and was a Postdoctoral Researcher from the FRS-FNRS (Fond National de la Recherche Scientifique, Belgium) when the study started. NMD is a recipient of FRS-FNRS grants. We thank the Flanders Innovation & Entrepreneurship (Vlaio or”Agentschap Innoveren & Ondernemen”, former IWT) for the financial support of the SBO BRANDING project. This work is also supported by grant from the European Union’s Seventh Framework Program (MYNEWGUT project). PDC, a research associate at the FRS-FNRS (Belgium), is a recipient of an ERC Starting Grant 2013 (European Research Council, Starting Grant 336452-ENIGMO), Baillet Latour grant for medical research 2015 and is supported by the FRS-FNRS via the FRFS-WELBIO under Grant number WELBIO-CR-2012S-02R.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: L.B.B. A.M.N. N.M.D. Conducted the experiments: F.S., L.B.B., A.M.N. Analyzed the chemical composition of wheat bran materials: J.V., C.M.C. Analyzed of short chain fatty acids: K.V. Analyzed the data: F.S., L.B.B., A.M.N. Wrote the paper: F.S., A.M.N., L.B.B., N.M.D. Provided intellectual input on the paper and reviewed the paper: J.V., C.M.C., K.V., P.D.C. Planned and supervised all experiments and manuscript preparation: N.M.D. All authors reviewed the manuscript
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suriano, F., Bindels, L.B., Verspreet, J. et al. Fat binding capacity and modulation of the gut microbiota both determine the effect of wheat bran fractions on adiposity. Sci Rep 7, 5621 (2017). https://doi.org/10.1038/s41598-017-05698-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-05698-y
This article is cited by
-
A Review: Cereals on Modulating the Microbiota/Metabolome for Metabolic Health
Current Nutrition Reports (2022)
-
Chitin–glucan and pomegranate polyphenols improve endothelial dysfunction
Scientific Reports (2019)
-
Dietary fibers inhibit obesity in mice, but host responses in the cecum and liver appear unrelated to fiber-specific changes in cecal bacterial taxonomic composition
Scientific Reports (2018)
-
Divergent short-chain fatty acid production and succession of colonic microbiota arise in fermentation of variously-sized wheat bran fractions
Scientific Reports (2018)
-
Wheat-derived arabinoxylan oligosaccharides with bifidogenic properties abolishes metabolic disorders induced by western diet in mice
Nutrition & Diabetes (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.