Abstract
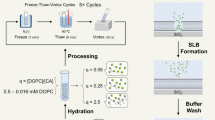
The supported lipid bilayer (SLB) platform is a popular cell membrane mimic that is utilized in the chemical, biological, materials science, and medical fields. To date, SLB preparation has proven challenging because of the need for specialized fabrication equipment, domain-specific knowledge about topics relevant to lipid self-assembly, and extensive training in the interfacial science field. Existing methods, such as vesicle fusion, also work with only a narrow range of lipid compositions and material supports. Here, we describe a recently developed simple and versatile protocol to form SLBs. The protocol is simple because it requires minimal sample preparation and only basic microfluidics, making it technically accessible to researchers across different scientific disciplines. The protocol is versatile because it works on a wide range of material supports, such as silicon oxide, gold, and graphene, and is compatible with diverse lipid compositions, including sterols and signaling lipids. The main stages of the procedure involve dissolving a lipid sample in an organic solvent, depositing the lipid solution on a solid support, and replacing the organic solvent with aqueous buffer. In addition, we provide procedures for characterizing the quality of the prepared SLBs and present examples of biofunctionalization procedures. The protocol takes 1–2 h and is broadly useful in various application contexts, including clinical diagnostics, biosensing, and cellular interfaces.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding authors upon reasonable request.
References
Richter, R. P., Bérat, R. & Brisson, A. R. Formation of solid-supported lipid bilayers: an integrated view. Langmuir 22, 3497–3505 (2006).
Castellana, E. T. & Cremer, P. S. Solid supported lipid bilayers: From biophysical studies to sensor design. Surface Sci. Rep. 61, 429–444 (2006).
Chan, Y.-H. M. & Boxer, S. G. Model membrane systems and their applications. Curr. Opin. Chem. Biol. 11, 581–587 (2007).
Reimhult, E. & Kumar, K. Membrane biosensor platforms using nano-and microporous supports. Trends Biotechnol. 26, 82–89 (2008).
Im, H., Wittenberg, N. J., Lesuffleur, A., Lindquist, N. C. & Oh, S.-H. Membrane protein biosensing with plasmonic nanopore arrays and pore-spanning lipid membranes. Chem. Sci. 1, 688–696 (2010).
Yang, T., Jung, S., Mao, H. & Cremer, P. S. Fabrication of phospholipid bilayer-coated microchannels for on-chip immunoassays. Anal. Chem. 73, 165–169 (2001).
Junesch, J. et al. Location-specific nanoplasmonic sensing of biomolecular binding to lipid membranes with negative curvature. Nanoscale 7, 15080–15085 (2015).
Mingeot-Leclercq, M.-P., Deleu, M., Brasseur, R. & Dufrêne, Y. F. Atomic force microscopy of supported lipid bilayers. Nat. Protoc. 3, 1654 (2008).
Cho, N.-J., Frank, C. W., Kasemo, B. & Höök, F. Quartz crystal microbalance with dissipation monitoring of supported lipid bilayers on various substrates. Nat. Protoc. 5, 1096–1106 (2010).
Lee, T.-H., Hirst, D. J., Kulkarni, K., Del Borgo, M. P. & Aguilar, M.-I. Exploring molecular-biomembrane interactions with surface plasmon resonance and dual polarization interferometry technology: expanding the spotlight onto biomembrane structure. Chem. Rev. 118, 5392–5487 (2018).
Höök, F. et al. Supported lipid bilayers, tethered lipid vesicles, and vesicle fusion investigated using gravimetric, plasmonic, and microscopy techniques. Biointerphases 3, FA108–FA116 (2008).
Mashaghi, A. et al. Label-free characterization of biomembranes: from structure to dynamics. Chem. Soc. Rev. 43, 887–900 (2014).
Limaj, O. et al. Infrared plasmonic biosensor for real-time and label-free monitoring of lipid membranes. Nano Lett. 16, 1502–1508 (2016).
Tabaei, S. R., Choi, J.-H., Haw Zan, G., Zhdanov, V. P. & Cho, N.-J. Solvent-assisted lipid bilayer formation on silicon dioxide and gold. Langmuir 30, 10363–10373 (2014).
Deshpande, S. & Dekker, C. On-chip microfluidic production of cell-sized liposomes. Nat. Protoc. 13, 856 (2018).
Doktorova, M. et al. Preparation of asymmetric phospholipid vesicles for use as cell membrane models. Nat. Protoc. 13, 2086 (2018).
Szoka, F. & Papahadjopoulos, D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc. Natl Acad. Sci. 75, 4194–4198 (1978).
Hohner, A. O., David, M. P. C. & Rädler, J. O. Controlled solvent-exchange deposition of phospholipid membranes onto solid surfaces. Biointerphases 5, 1–8 (2010).
Tabaei, S. R., Jackman, J. A., Kim, S.-O., Zhdanov, V. P. & Cho, N.-J. Solvent-assisted lipid self-assembly at hydrophilic surfaces: factors influencing the formation of supported membranes. Langmuir 31, 3125–3134 (2015).
Kim, M. C., Gillissen, J. J., Tabaei, S. R., Zhdanov, V. P. & Cho, N.-J. Spatiotemporal dynamics of solvent-assisted lipid bilayer formation. Phys. Chem. Chem. Phys. 17, 31145–31151 (2015).
Gillissen, J. J., Tabaei, S. R. & Cho, N.-J. A phenomenological model of the solvent-assisted lipid bilayer formation method. Phys. Chem. Chem. Phys. 18, 24157–24163 (2016).
Jackman, J. A., Tabaei, S. R., Zhao, Z., Yorulmaz, S. & Cho, N.-J. Self-assembly formation of lipid bilayer coatings on bare aluminum oxide: overcoming the force of interfacial water. ACS Appl. Mater. Interfaces 7, 959–968 (2014).
Tabaei, S. R., Ng, W. B., Cho, S.-J. & Cho, N.-J. Controlling the formation of phospholipid monolayer, bilayer, and intact vesicle layer on graphene. ACS Appl. Mater. Interfaces 8, 11875–11880 (2016).
Losada-Pérez, P., Polat, O., Parikh, A. N., Seker, E. & Renner, F. U. Engineering the interface between lipid membranes and nanoporous gold: a study by quartz crystal microbalance with dissipation monitoring. Biointerphases 13, 011002 (2018).
Tabaei, S. R., Vafaei, S. & Cho, N.-J. Fabrication of charged membranes by the solvent-assisted lipid bilayer (SALB) formation method on SiO2 and Al2O3. Phys. Chem. Chem. Phys. 17, 11546–11552 (2015).
Tabaei, S. R. et al. Multistep compositional remodeling of supported lipid membranes by interfacially active phosphatidylinositol kinases. Anal. Chem. 88, 5042–5045 (2016).
Tabaei, S. R. et al. Formation of cholesterol-rich supported membranes using solvent-assisted lipid self-assembly. Langmuir 30, 13345–13352 (2014).
Tabaei, S. R., Jackman, J. A., Liedberg, B., Parikh, A. N. & Cho, N.-J. Observation of stripe superstructure in the β-two-phase coexistence region of cholesterol–phospholipid mixtures in supported membranes. J. Am. Chem. Soc. 136, 16962–16965 (2014).
Kawakami, L. M. et al. Understanding how sterols regulate membrane remodeling in supported lipid bilayers. Langmuir 33, 14756–14765 (2017).
Sundh, M., Svedhem, S. & Sutherland, D. S. Influence of phase separating lipids on supported lipid bilayer formation at SiO2 surfaces. Phys. Chem. Chem. Phys. 12, 453–460 (2010).
Crane, J. M. & Tamm, L. K. Role of cholesterol in the formation and nature of lipid rafts in planar and spherical model membranes. Biophys. J. 86, 2965–2979 (2004).
Stottrup, B. L., Veatch, S. L. & Keller, S. L. Nonequilibrium behavior in supported lipid membranes containing cholesterol. Biophys. J. 86, 2942–2950 (2004).
Ziblat, R., Kjaer, K., Leiserowitz, L. & Addadi, L. Structure of cholesterol/lipid ordered domains in monolayers and single hydrated bilayers. Angew. Chem. Int. Ed. Engl. 48, 8958–8961 (2009).
Lee, T.-H., Hirst, D. J. & Aguilar, M.-I. New insights into the molecular mechanisms of biomembrane structural changes and interactions by optical biosensor technology. Biochim. Biophys. Acta 1848, 1868–1885 (2015).
Czogalla, A., Grzybek, M., Jones, W. & Coskun, Ü. Validity and applicability of membrane model systems for studying interactions of peripheral membrane proteins with lipids. Biochim. Biophys. Acta 1841, 1049–1059 (2014).
Wittenberg, N. J. et al. High-affinity binding of remyelinating natural autoantibodies to myelin-mimicking lipid bilayers revealed by nanohole surface plasmon resonance. Anal. Chem. 84, 6031–6039 (2012).
Rascol, E., Devoisselle, J.-M. & Chopineau, J. The relevance of membrane models to understand nanoparticles–cell membrane interactions. Nanoscale 8, 4780–4798 (2016).
Costello, D. A., Villareal, V. A. & Yang, P. L. Desmosterol increases lipid bilayer fluidity during hepatitis C virus infection. ACS Infect. Dis. 2, 852–862 (2016).
Naumann, R. et al. Incorporation of membrane proteins in solid-supported lipid layers. Angew. Chem. Int. Ed. Engl. 34, 2056–2058 (1995).
Robelek, R. et al. Incorporation of in vitro synthesized GPCR into a tethered artificial lipid membrane system. Angew. Chem. Int. Ed. Engl. 46, 605–608 (2007).
Chadli, M. et al. New tethered phospholipid bilayers integrating functional G-protein-coupled receptor membrane proteins. Langmuir 33, 10385–10401 (2017).
Chadli, M. et al. A new functional membrane protein microarray based on tethered phospholipid bilayers. Analyst 143, 2165–2173 (2018).
Boxer, S. G. Molecular transport and organization in supported lipid membranes. Curr. Opin. Chem. Biol. 4, 704–709 (2000).
Su, X. et al. Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 352, 595–599 (2016).
Van Oudenaarden, A. & Boxer, S. G. Brownian ratchets: molecular separations in lipid bilayers supported on patterned arrays. Science 285, 1046–1048 (1999).
Bally, M. et al. Liposome and lipid bilayer arrays towards biosensing applications. Small 6, 2481–2497 (2010).
Yusko, E. C. et al. Controlling protein translocation through nanopores with bio-inspired fluid walls. Nat. Nanotechnol. 6, 253 (2011).
Schiller, S. M., Naumann, R., Lovejoy, K., Kunz, H. & Knoll, W. Archaea analogue thiolipids for tethered bilayer lipid membranes on ultrasmooth gold surfaces. Angew. Chem. Int. Ed. Engl. 42, 208–211 (2003).
Naumann, R. et al. Tethered lipid bilayers on ultraflat gold surfaces. Langmuir 19, 5435–5443 (2003).
Keizer, H. M. et al. Functional ion channels in tethered bilayer membranes—implications for biosensors. ChemBioChem 8, 1246–1250 (2007).
Wiegand, G., Arribas-Layton, N., Hillebrandt, H., Sackmann, E. & Wagner, P. Electrical properties of supported lipid bilayer membranes. J. Phys. Chem. B 106, 4245–4254 (2002).
Jackman, A. J., Knoll, W. & Cho, N.-J. Biotechnology applications of tethered lipid bilayer membranes. Materials 5, 2637–2657 (2012).
Andersson, J., Köper, I. & Knoll, W. Tethered membrane architectures—design and applications. Front. Mater. 5, 55 (2018).
De Leo, V. et al. Liposome-modified titanium surface: a strategy to locally deliver bioactive molecules. Colloids Surf. B Biointerfaces 158, 387–396 (2017).
Glasmästar, K., Larsson, C., Höök, F. & Kasemo, B. Protein adsorption on supported phospholipid bilayers. J. Colloid Interface Sci. 246, 40–47 (2002).
Andersson, A. S., Glasmästar, K., Sutherland, D., Lidberg, U. & Kasemo, B. Cell adhesion on supported lipid bilayers. J. Biomed. Mater. Res. A 64, 622–629 (2003).
van Weerd, J., Karperien, M. & Jonkheijm, P. Supported lipid bilayers for the generation of dynamic cell–material interfaces. Adv. Healthc. Mater. 4, 2743–2779 (2015).
Koçer, G. & Jonkheijm, P. Guiding hMSC adhesion and differentiation on supported lipid bilayers. Adv. Healthc. Mater. 6, 1600862 (2017).
Nair, P. M., Salaita, K., Petit, R. S. & Groves, J. T. Using patterned supported lipid membranes to investigate the role of receptor organization in intercellular signaling. Nat. Protoc. 6, 523 (2011).
Glazier, R. & Salaita, K. Supported lipid bilayer platforms to probe cell mechanobiology. Biochim. Biophys. Acta 1859, 1465–1482 (2017).
Koçer, G. & Jonkheijm, P. About chemical strategies to fabricate cell‐instructive biointerfaces with static and dynamic complexity. Adv. Healthc. Mater. 7, 1701192 (2018).
Svedhem, S. et al. In situ peptide-modified supported lipid bilayers for controlled cell attachment. Langmuir 19, 6730–6736 (2003).
Huang, C.-J. et al. Type I collagen-functionalized supported lipid bilayer as a cell culture platform. Biomacromolecules 11, 1231–1240 (2010).
Yorulmaz Avsar, S. et al. Immobilization strategies for functional complement convertase assembly at lipid membrane interfaces. Langmuir 33, 7332–7342 (2017).
Wu, J.-C. et al. Antibody conjugated supported lipid bilayer for capturing and purification of viable tumor cells in blood for subsequent cell culture. Biomaterials 34, 5191–5199 (2013).
Chen, J.-Y. et al. Sensitive and specific biomimetic lipid coated microfluidics to isolate viable circulating tumor cells and microemboli for cancer detection. PLoS One 11, e0149633 (2016).
Vafaei, S., Tabaei, S. R., Biswas, K. H., Groves, J. T. & Cho, N. J. Dynamic cellular interactions with extracellular matrix triggered by biomechanical tuning of low‐rigidity, supported lipid membranes. Adv. Healthc. Mater. 6, 1700243 (2017).
Kubalek, E. W., Le Grice, S. F. J. & Brown, P. O. Two-dimensional crystallization of histidine-tagged, HIV-1 reverse transcriptase promoted by a novel nickel-chelating lipid. J. Struct. Biol. 113, 117–123 (1994).
Chikh, G. G., Li, W. M., Schutze-Redelmeier, M.-P., Meunier, J.-C. & Bally, M. B. Attaching histidine-tagged peptides and proteins to lipid-based carriers through use of metal-ion-chelating lipids. Biochim. Biophys. Acta 1567, 204–212 (2002).
Lata, S., Gavutis, M. & Piehler, J. Monitoring the dynamics of ligand–receptor complexes on model membranes. J. Am. Chem. Soc. 128, 6–7 (2006).
Nye, J. A. & Groves, J. T. Kinetic control of histidine-tagged protein surface density on supported lipid bilayers. Langmuir 24, 4145–4149 (2008).
Galush, W. J. et al. A nanocube plasmonic sensor for molecular binding on membrane surfaces. Nano Lett. 9, 2077–2082 (2009).
Busch, D. J. et al. Intrinsically disordered proteins drive membrane curvature. Nat. Commun. 6, 7875 (2015).
van der Meulen, S. A. J., Dubacheva, G. V., Dogterom, M., Richter, R. P. & Leunissen, M. E. Quartz crystal microbalance with dissipation monitoring and spectroscopic ellipsometry measurements of the phospholipid bilayer anchoring stability and kinetics of hydrophobically modified DNA oligonucleotides. Langmuir 30, 6525–6533 (2014).
Dubacheva, G. V. et al. Controlling multivalent binding through surface chemistry: model study on streptavidin. J. Am. Chem. Soc. 139, 4157–4167 (2017).
Tamm, L. K. & McConnell, H. M. Supported phospholipid bilayers. Biophys. J. 47, 105–113 (1985).
Girard-Egrot, A. P. & Blum, L. J. Langmuir-Blodgett technique for synthesis of biomimetic lipid membranes. in Nanobiotechnology of Biomimetic Membranes (ed. Martin, D. K.) 23–74 (Springer, 2007).
Hardy, G. J., Nayak, R. & Zauscher, S. Model cell membranes: techniques to form complex biomimetic supported lipid bilayers via vesicle fusion. Curr. Opin. Colloid Interface Sci. 18, 448–458 (2013).
Keller, C. & Kasemo, B. Surface specific kinetics of lipid vesicle adsorption measured with a quartz crystal microbalance. Biophys. J. 75, 1397–1402 (1998).
Keller, C., Glasmästar, K., Zhdanov, V. & Kasemo, B. Formation of supported membranes from vesicles. Phys. Rev. Lett. 84, 5443 (2000).
Cremer, P. S. & Boxer, S. G. Formation and spreading of lipid bilayers on planar glass supports. J. Phys. Chem. B 103, 2554–2559 (1999).
Biswas, K. H., Jackman, J. A., Park, J. H., Groves, J. T. & Cho, N.-J. Interfacial forces dictate the pathway of phospholipid vesicle adsorption onto silicon dioxide surfaces. Langmuir 34, 1775–1782 (2018).
Sackmann, E. Supported membranes: scientific and practical applications. Science 271, 43–48 (1996).
Zwang, T. J., Fletcher, W. R., Lane, T. J. & Johal, M. S. Quantification of the layer of hydration of a supported lipid bilayer. Langmuir 26, 4598–4601 (2010).
Reimhult, E., Höök, F. & Kasemo, B. Intact vesicle adsorption and supported biomembrane formation from vesicles in solution: influence of surface chemistry, vesicle size, temperature, and osmotic pressure. Langmuir 19, 1681–1691 (2003).
Gracià, R. S., Bezlyepkina, N., Knorr, R. L., Lipowsky, R. & Dimova, R. Effect of cholesterol on the rigidity of saturated and unsaturated membranes: fluctuation and electrodeformation analysis of giant vesicles. Soft Matter 6, 1472–1482 (2010).
McLaughlin, S. & Murray, D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature 438, 605 (2005).
Mager, M. D., Almquist, B. & Melosh, N. A. Formation and characterization of fluid lipid bilayers on alumina. Langmuir 24, 12734–12737 (2008).
Mager, M. D. & Melosh, N. A. Lipid bilayer deposition and patterning via air bubble collapse. Langmuir 23, 9369–9377 (2007).
Hirtz, M., Oikonomou, A., Georgiou, T., Fuchs, H. & Vijayaraghavan, A. Multiplexed biomimetic lipid membranes on graphene by dip-pen nanolithography. Nat. Commun. 4, 2591 (2013).
Lenhert, S., Sun, P., Wang, Y., Fuchs, H. & Mirkin, C. A. Massively parallel dip-pen nanolithography of heterogeneous supported phospholipid multilayer patterns. Small 3, 71–75 (2007).
Mennicke, U. & Salditt, T. Preparation of solid-supported lipid bilayers by spin-coating. Langmuir 18, 8172–8177 (2002).
Dols-Perez, A., Fumagalli, L., Simonsen, A. C. & Gomila, G. Ultrathin spin-coated dioleoylphosphatidylcholine lipid layers in dry conditions: a combined atomic force microscopy and nanomechanical study. Langmuir 27, 13165–13172 (2011).
Dols-Perez, A., Fumagalli, L. & Gomila, G. Structural and nanomechanical effects of cholesterol in binary and ternary spin-coated single lipid bilayers in dry conditions. Colloids Surf. B Biointerfaces 116, 295–302 (2014).
Raedler, J., Strey, H. & Sackmann, E. Phenomenology and kinetics of lipid bilayer spreading on hydrophilic surfaces. Langmuir 11, 4539–4548 (1995).
Nissen, J., Gritsch, S., Wiegand, G. & Rädler, J. O. Wetting of phospholipid membranes on hydrophilic surfaces—concepts towards self-healing membranes. Eur. Phys. J. B 10, 335–344 (1999).
Ohlsson, G. et al. A miniaturized flow reaction chamber for use in combination with QCM-D sensing. Microfluid. Nanofluid. 9, 705–716 (2010).
Sauerbrey, G. Use of quartz vibration for weighing thin films on a microbalance. J. Phys. 155, 206–222 (1959).
Dodd, C. E. et al. Native E. coli inner membrane incorporation in solid-supported lipid bilayer membranes. Biointerphases 3, FA59–FA67 (2008).
Hsia, C.-Y., Chen, L., Singh, R. R., DeLisa, M. P. & Daniel, S. A molecularly complete planar bacterial outer membrane platform. Scientific Rep. 6, 32715 (2016).
Liu, H.-Y. et al. Supported planar mammalian membranes as models of in vivo cell surface architectures. ACS Appl. Mater. Interfaces 9, 35526–35538 (2017).
Pace, H. et al. Preserved transmembrane protein mobility in polymer-supported lipid bilayers derived from cell membranes. Anal. Chem. 87, 9194–9203 (2015).
Pace, H. P. et al. Structure and composition of native membrane derived polymer-supported lipid bilayers. Anal. Chem. 90, 13065–13072 (2018).
Peerboom, N. et al. Cell membrane derived platform to study virus binding kinetics and diffusion with single particle sensitivity. ACS Infect. Dis. 4, 944–953 (2018).
Richards, M. J. et al. Membrane protein mobility and orientation preserved in supported bilayers created directly from cell plasma membrane blebs. Langmuir 32, 2963–2974 (2016).
Simonsson, L., Gunnarsson, A., Wallin, P., Jönsson, P. & Höök, F. Continuous lipid bilayers derived from cell membranes for spatial molecular manipulation. J. Am. Chem. Soc. 133, 14027–14032 (2011).
Griebenow, K. & Klibanov, A. M. On protein denaturation in aqueous–organic mixtures but not in pure organic solvents. J. Am. Chem. Soc. 118, 11695–11700 (1996).
Acknowledgements
This work was supported by the National Research Foundation of Singapore through a Proof-of-Concept grant (NRF2015NRF-POC0001-19) to N.-J.C. Additional support was provided by the Creative Materials Discovery Program through the National Research Foundation of Korea, funded by the Ministry of Science, ICT and Future Planning (NRF-2016M3D1A1024098) to N.-J.C.
Author information
Authors and Affiliations
Contributions
A.R.F., J.A.J., and N.-J.C. designed the study. A.R.F., J.A.J., and N.-J.C. wrote the initial draft of the manuscript. B.K.Y., T.N.S., S.P., H.C., and J.H.P. contributed to protocol development. A.R.F., B.K.Y., T.N.S., S.P., H.C. J.H.P., and J.A.J. interpreted the results. N.-J.C. obtained funding. All authors reviewed, edited, and approved the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Journal peer review information: Nature Protocols thanks Ling Chao and other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Tabaei, S. R., Jackman, J. A., Liedberg, B., Parikh, A. N. & Cho, N.-J. J. Am. Chem. Soc. 136, 16962–16965 (2014): https://doi.org/10.1021/ja5082537
Tabaei, S. R., Choi, J.-H., Haw Zan, G., Zhdanov, V. P. & Cho, N.-J. Langmuir 30, 10363–10373 (2014): https://doi.org/10.1021/la501534f
Tabaei, S. R., Jackman, J. A., Kim, S.-O., Zhdanov, V. P. & Cho, N.-J. Langmuir 31, 3125–3134 (2015): https://doi.org/10.1021/la5048497
Vafaei, S., Tabaei, S. R., Biswas, K. H., Groves, J. T. & Cho, N. J. Adv. Healthc. Mater. 6, 1700243 (2017): https://doi.org/10.1002/adhm.201700243
Integrated supplementary information
Supplementary Fig. 1 Direct comparison of the gradual solvent-exchange and SALB methods for SLB formation on a gold surface.
QCM-D measurements were conducted in order to track SLB formation on a gold surface by the (a) gradual solvent-exchange method and (b) SALB method. Briefly, a gold surface was first (i) incubated with 0.5 mg ml−1 DOPC phospholipids below the critical micelle-to-vesicle transition (80/20 isopropanol-water by volume percentage). Following the gradual solvent-exchange approach, we increased the aqueous content gradually in 10% increments, from (ii) 30% to (ix) 100%. Following the SALB method, we quickly performed (ii) the solvent-exchange with 100% aqueous buffer in a single exchange step. The results verify that the SALB method can be applied to form SLBs on gold surfaces (as indicated by Δf and ΔD shifts of around −27 Hz and 1 × 10−6, respectively) and further show that the gradual solvent-exchange method was not able to form SLBs on gold surfaces (minimal lipid adsorption, as indicated by Δf and ΔD shifts of around −3 Hz and 0.4 × 10−6, respectively).
Supplementary Fig. 2 QCM-D responses for pH-dependent lipid adsorption onto a silicon oxide surface after the SALB procedure was performed.
Final QCM-D (a) frequency and (b) energy dissipation shifts are reported after the SALB procedure was completed using 0.5 mg ml−1 DOPC lipid on a silicon oxide surface at two different pH conditions. At pH 7, typical values for SLB formation were obtained while smaller measurement responses were recorded at pH 12. The inability to form SLBs on silicon oxide at pH 12 is consistent with what is known about weaker lipid-substrate interactions under such conditions due to a strongly coupled, “ice-like” hydration layer on the silicon oxide surface. Results are expressed as mean ± standard deviation (n = 3) and all individual data points are plotted.
Supplementary information
Supplementary Information
Supplementary Figures 1 and 2
Supplementary Video 1
Lipid solubilization and sample preparation.
Supplementary Video 2
Microfluidic chamber assembly and attachment in microscope setup.
Rights and permissions
About this article
Cite this article
Ferhan, A.R., Yoon, B.K., Park, S. et al. Solvent-assisted preparation of supported lipid bilayers. Nat Protoc 14, 2091–2118 (2019). https://doi.org/10.1038/s41596-019-0174-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-019-0174-2
This article is cited by
-
Structural and functional insights into the delivery of a bacterial Rhs pore-forming toxin to the membrane
Nature Communications (2023)
-
Nanoarchitectured air-stable supported lipid bilayer incorporating sucrose–bicelle complex system
Nano Convergence (2022)
-
Membrane curvature regulates the spatial distribution of bulky glycoproteins
Nature Communications (2022)
-
Spatiotemporal stop-and-go dynamics of the mitochondrial TOM core complex correlates with channel activity
Communications Biology (2022)
-
Ligand functionalization of titanium nanopattern enables the analysis of cell–ligand interactions by super-resolution microscopy
Nature Protocols (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.