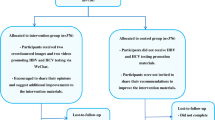
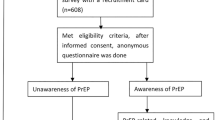
Abstract
Pay-it-forward incentives involve having a person receive a free test with community-generated messages and then asking if those who received a free test would like to donate money to support others to receive free testing. Here we undertook a two-arm cluster-randomized trial to evaluate pay-it-forward incentives with active community participation to promote hepatitis B virus (HBV) and hepatitis C virus (HCV) testing among men who have sex with men in China. Men randomized to the pay-it-forward arm received free HBV and HCV testing and were offered a chance to pay-it-forward by donating money to support the testing of another anonymous person. Each participant paid for their HCV and HBV test at 7.7 USD per test in the standard-of-care arm. The primary outcome was the proportion of men who tested for HBV and HCV. Between 28 March and 6 November 2021, 32 groups (10 men per group) of men were randomized to the pay-it-forward (n = 160, 16 clusters) and standard-of-care (n = 162, 16 clusters) arms, respectively. HBV and HCV rapid testing were higher in the pay-it-forward arm (59.4%) than in the standard-of-care arm (25.3%) (proportion difference 35.2%, 95% confidence interval 24.1–46.3%). No adverse events were reported. The community-led pay-it-forward incentives improved HBV and HCV testing among men who have sex with men. Clinical Trial registration: ChiCTR 2100046140.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data are not publicly available for everyone because making the data publicly available would require additional consent. If other investigators are interested in performing additional analysis, data requests can be submitted to the corresponding author, explaining the analyses planned. Access to data will be provided upon application, with a timeline of within 1 month determined in accordance with the request.
Code availability
All codes are available on GitHub. The code is freely accessible at https://github.com/PIFHepstudy/code.git.
References
Chen, S., Mao, W., Guo, L., Zhang, J. & Tang, S. Combating hepatitis B and C by 2030: achievements, gaps, and options for actions in China. BMJ Glob. Health 5, e002306 (2020).
Polaris Observatory, C. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol. Hepatol. 3, 383–403 (2018).
Liu, C. R. et al. Prevalence of hepatitis C virus infection among key populations in China: a systematic review. Int J. Infect. Dis. 80, 16–27 (2019).
Foreman, K. J. et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 392, 2052–2090 (2018).
Combating Hepatitis B and C to Reach Elimination by 2030: Advocacy Brief (World Health Organization, 2016).
Chen, S. et al. The hepatitis B epidemic in China should receive more attention. Lancet 391, 1572 (2018).
The Status of Notifiable Infectious Diseases in China in 2020. Vol. 2022 (Chinese Center for Disease Control and Prevention, 2021).
Hepatitis in China. Vol. 2022 (World Health Organization, 2022).
Su, S. et al. Cost-effectiveness of universal screening for chronic hepatitis B virus infection in China: an economic evaluation. Lancet Glob. Health 10, e278–e287 (2022).
Adee, M. et al. A tool to inform hepatitis C elimination: a case for hepatitis C elimination in China. Clin. Liver Dis. 17, 99–106 (2021).
Mei, X. & Lu, H. Prevalence, diagnosis, and treatment of hepatitis C in Mainland China. Glob. Health Med. 3, 270–275 (2021).
Roberts, H., Jiles, R., Harris, A. M., Gupta, N. & Teshale, E. Incidence and prevalence of sexually transmitted hepatitis B, United States, 2013–2018. Sex. Transm. Dis. 48, 305–309 (2021).
Inoue, T. & Tanaka, Y. Hepatitis B virus and its sexually transmitted infection—an update. Microb. Cell 3, 420–437 (2016).
Hou, J., Liu, Z. & Gu, F. Epidemiology and prevention of hepatitis B virus infection. Int J. Med Sci. 2, 50–57 (2005).
Li, M. et al. Evaluating the independent influence of sexual transmission on HBV infection in China: a modeling study. BMC Public Health 21, 388 (2021).
Hui, Z. et al. Progress towards elimination of mother-to-child transmission of hepatitis B virus infection in China: a modelling analysis. Bull. World Health Organ. 99, 10–18 (2021).
Hepatitis B Virus Infection—Global Drug Forecast and Market Analysis to 2029 (GlobalData, 2021).
Tang, W. et al. How kindness can be contagious in healthcare. Nat. Med. 27, 1142–1144 (2021).
Marley, G. et al. What facilitates hepatitis B and hepatitis C testing and the role of stigma among primary care patients in China? J. Viral Hepat. 29, 637–645 (2022).
Wang, R., Cui, N., Long, M., Mu, L. & Zeng, H. Barriers to uptake of hepatitis C virus (HCV) health intervention among men who have sex with men in Southwest China: a qualitative study. Health Soc. Care Community 29, 445–452 (2021).
Shen, K. et al. A crowdsourced intervention to decrease hepatitis B stigma in men who have sex with men in China: a cohort study. J. Viral Hepat. 27, 135–142 (2020).
Wei, C. et al. Accessing HIV testing and treatment among men who have sex with men in China: a qualitative study. AIDS Care 26, 372–378 (2014).
Fitzpatrick, T. et al. A crowdsourced intervention to promote hepatitis B and C testing among men who have sex with men in China: a nationwide online randomized controlled trial. EClinicalMedicine 16, 64–73 (2019).
Feng, Y., Wu, Z. & Detels, R. Evolution of men who have sex with men community and experienced stigma among men who have sex with men in Chengdu, China. J. Acquir. Immune Defic. Syndr. 53, S98–S103 (2010). Suppl 1.
Liu, Y. et al. Qualitative assessment of barriers and facilitators of access to HIV testing among men who have sex with men in China. AIDS Patient Care STDS 29, 481–489 (2015).
Duan, Z. et al. Current challenges and the management of chronic hepatitis C in mainland China. J. Clin. Gastroenterol. 48, 679–686 (2014).
Sun, J., Cheng, H., Hassan, M. R. A., Chan, H. K. & Piedagnel, J. M. What China can learn from Malaysia to achieve the goal of ‘eliminate hepatitis C as a public health threat’ by 2030—a narrative review. Lancet Reg. Health West Pac. 16, 100261 (2021).
WHO Guidelines on Hepatitis B and C testing (World Health Organization, 2017).
Tang, W. et al. How kindness can be contagious in healthcare. Nat. Med. 27, 1142–1144 (2021).
Li, K. T. et al. Pay-it-forward strategy to enhance uptake of dual gonorrhea and chlamydia testing among men who have sex with men in China: a pragmatic, quasi-experimental study. Lancet Infect. Dis. 19, 76–82 (2019).
Yang, F. et al. Pay-it-forward gonorrhoea and chlamydia testing among men who have sex with men in China: a randomised controlled trial. Lancet Infect. Dis. 20, 976–982 (2020).
Oru, E., Trickey, A., Shirali, R., Kanters, S. & Easterbrook, P. Decentralisation, integration, and task-shifting in hepatitis C virus infection testing and treatment: a global systematic review and meta-analysis. Lancet Glob. Health 9, e431–e445 (2021).
Kpokiri, E. E. et al. Diagnostic infectious diseases testing outside clinics: a global systematic review and meta-analysis. Open Forum Infect. Dis. 7, ofaa360 (2020).
Knight, V. et al. Implementation and operational research: convenient HIV testing service models are attracting previously untested gay and bisexual men: a cross-sectional study. J. Acquir. Immune Defic. Syndr. 69, e147–e155 (2015).
Knight, V. et al. A novel time-limited pop-up HIV testing service for gay men in Sydney, Australia, attracts high-risk men. Sex. Health 11, 345–350 (2014).
Mutch, A. J. et al. Increasing HIV testing among hard-to-reach groups: examination of RAPID, a community-based testing service in Queensland, Australia. BMC Health Serv. Res. 17, 310 (2017).
Cooke, G. S. et al. Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol. Hepatol. 4, 135–184 (2019).
Hajarizadeh, B. et al. Hepatitis C treatment as prevention: evidence, feasibility, and challenges. Lancet Gastroenterol. Hepatol. 1, 317–327 (2016).
Wu, D. et al. Effectiveness of a pay-it-forward intervention compared with user-paid vaccination to improve influenza vaccine uptake and community engagement among children and older adults in China: a quasi-experimental pragmatic trial. Lancet Infect. Dis. 22, 1484–1492 (2022).
Bao, Y. et al. Prevalence of HIV, HCV and HBV infection and sociodemographic characteristics of people who inject drugs in China: a systematic review and meta-analysis. Int J. Drug Policy 70, 87–93 (2019).
Ge, L. et al. Preplanned studies: HIV and HCV infection status among drug users—China, 2010–2018. China CDC Wkly 2, 109–112 (2020).
Barocas, J. A. et al. Barriers and facilitators of hepatitis C screening among people who inject drugs: a multi-city, mixed-methods study. Harm Reduct. J. 11, 1 (2014).
Li, K. T. et al. A secondary mixed methods analysis of a pay-it-forward gonorrhea/chlamydia testing program among men who have sex with men in China. Sex. Transm. Dis. 47, 395–401 (2020).
Young, S. D. et al. Effect of a community popular opinion leader HIV/STI intervention on stigma in urban, coastal Peru. AIDS Behav. 15, 930–937 (2011).
Iryawan, A. R., Stoicescu, C., Sjahrial, F., Nio, K. & Dominich, A. The impact of peer support on testing, linkage to and engagement in HIV care for people who inject drugs in Indonesia: qualitative perspectives from a community-led study. Harm Reduct. J. 19, 16 (2022).
Robotin, M. C. & George, J. Community-based hepatitis Bscreening: what works? Hepatol. Int. 8, 478–492 (2014).
Cheng, K. K., Lam, T. H. & Leung, C. C. Wearing face masks in the community during the COVID-19 pandemic: altruism and solidarity. Lancet 399, e39–e40 (2022).
Genschel, P. & Jachtenfuchs, M. Postfunctionalism reversed: solidarity and rebordering during the COVID-19 pandemic. J. Eur. Public Policy 28, 350–369 (2021).
Rothman, K. J. No adjustments are needed for multiple comparisons. Epidemiology 1, 43–46 (1990).
Wang, R., Lagakos, S. W., Ware, J. H., Hunter, D. J. & Drazen, J. M. Statistics in medicine—reporting of subgroup analyses in clinical trials. N. Engl. J. Med. 357, 2189–2194 (2007).
Lu, H., Cole, S. R., Howe, C. J. & Westreich, D. Toward a clearer definition of selection bias when estimating causal effects. Epidemiology 33, 699–706 (2022).
Zhao, J. K. et al. Jiangsu Four Cancers Study: a large case–control study of lung, liver, stomach, and esophageal cancers in Jiangsu Province, China. Eur. J. Cancer Prev. 26, 357–364 (2017).
Lee, C. Y., Wu, P. H., Lu, M. W., Chen, T. C. & Lu, P. L. High prevalence of unawareness of HCV infection status among both HCV-seronegative and seropositive people living with human immunodeficiency virus in Taiwan. PLoS ONE 16, e0251158 (2021).
Gao, Y. et al. Prevalence of anti-HCV antibody among the general population in mainland China between 1991 and 2015: a systematic review and meta-analysis. Open Forum Infect. Dis. 6, ofz040 (2019).
Shirin, T., Ahmed, T., Iqbal, A., Islam, M. & Islam, M. N. Prevalence and risk factors of hepatitis B virus, hepatitis C virus, and human immunodeficiency virus infections among drug addicts in Bangladesh. J. Health Popul. Nutr. 18, 145–150 (2000).
Neaigus, A. et al. Sexual and other noninjection risks for HBV and HCV seroconversions among noninjecting heroin users. J. Infect. Dis. 195, 1052–1061 (2007).
Zu, J. et al. Estimating the impact of test-and-treat strategies on hepatitis B virus infection in China by using an age-structured mathematical model. Medicine 97, e0484 (2018).
Kauermann, G. & Carroll, R. J. A note on the efficiency of sandwich covariance matrix estimation. J. Am. Stat. Assoc. 96, 1387–1396 (2001).
Acknowledgements
This work was supported by the Key Technologies Research and Development Program (2022YFC2304900-4 to W.T.), National Institute of Health (R34MH119963 to W.T., and R01AI158826), National Nature Science Foundation of China (81903371 to W.T.) and CRDF Global (G-202104-67775 to W.T.). We thank all study participants, staff members from Rainbow and Zhinanzhen groups in Jiangsu, the Social Entrepreneurship to Spur Health Global, and Jiangsu Center for Diseases Prevention and Control, who contributed to this study.
Author information
Authors and Affiliations
Contributions
This manuscript is an original research paper that has not been published previously, nor is it under review with any other journal. W.T. and G.F. conceived the initial idea and designed this clinical trial. W.T. oversaw the study design, implementation, data analysis, results generation and manuscript write-up. J.L., Y.X., G.M. and G.F. implemented the study and collected data. W.T., G.F. and J.L. had access to the study’s raw data. Y.Z., J.L., Y.X. and H.L. were responsible for data cleaning and data analysis and generated the final analysis outputs. J.O. and F.Z. provided advice for data analysis. Y.Z., J.L. and Y.X. wrote the first draft of the paper, and J.D.T., J.O., D.W., A.K. and J.S.S. contributed to the interpretation of the results and provided expert advice on the draft. All co-authors provided constructive comments and approved the final draft of the submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks William Liu, Zixin Wang, Christian Bottomley and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary handling editor: Ming Yang, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Decision tree model.
Start_up_C: The training cost to start the study; Capital_fixed_costs: The capital fixed cost of the study; Variable_cost_P/S: variable cost in the PIF/SOC group, variable cost means the fees of sample collection, transportation, and testing; Donation_P: The money donated to the PIF program by the participants; Payment_S: The fees paid for the testing service by participants in the SOC; PIF: pay-it-forward; SOC: standard-of-care.
Extended Data Fig. 2 Univariate sensitivity analysis compared to PIF vs. SOC’s cost-effectiveness (ICER of financial cost per additional person tested).
Start_up_C: The training cost to start the study; Capital_fc: The capital fixed cost of the study; Variable_cost_P/S: variable cost in the PIF/SOC group, variable cost means the fees of sample collection, transportation, and testing. Donation_P: The money donated to the PIF program by the participants; Payment_S: The fees paid for the testing service by participants in the SOC; Test_S: The probability of participants tested in the SOC; Test_P: The probability of participants tested in the PIF; Positive_P: The probability of participants tested positive in the PIF; Positive_S: The probability of participants tested positive in the SOC; PIF: pay-it-forward; SOC: standard-of-care; PIF: pay-it-forward; SOC: standard-of-care.
Extended Data Fig. 3 Univariate sensitivity analysis compared to the cost-effectiveness of PIF vs. SOC (ICER of financial cost per additional case identified).
Start_up_C: The training cost to start the study; Capital_fc: The capital fixed cost of the study; Variable_costs_P/S: variable cost in the PIF/SOC group, variable cost means the fees of sample collection, transportation, and testing. Donation_P: The money donated to the PIF program by the participants; Payment_S: The fees paid for the testing service by participants in the SOC; Test_S: The probability of participants tested in the SOC; Test_P: The probability of participants tested in the PIF; Positive_P: The probability of participants tested positive in the PIF; Positive_S: The probability of participants tested positive in the SOC; PIF: pay-it-forward; SOC: standard-of-care.
Extended Data Fig. 4 Cost-effectiveness acceptability curve of the financial cost per person tested.
the PIF has a greater probability of being more cost-effective than SOC if the willingness to pay is greater than $20 per person tested. PIF: pay-it-forward; SOC: standard-of-care.
Extended Data Fig. 5 Cost-effectiveness acceptability curve of the financial cost per case identified.
the probability of PIF being more cost-effective than SOC may decrease as the willingness to pay increases from $0 to $2000 per identified case. PIF: pay-it-forward; SOC: standard-of-care.
Extended Data Fig. 6
Community-led intervention implementation timeline.
Extended Data Fig. 7
Community-led intervention implementation procedures.
Supplementary information
Supplementary Information
Consort 2010 checklist extension to cluster randomized trials, study ethical approval statement, randomization STATA code, study protocol, statistical analysis plan and EASE-SAGER Checklist.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Li, J., Xie, Y. et al. Pay-it-forward incentives for hepatitis virus testing in men who have sex with men: a cluster randomized trial. Nat Med 29, 2241–2247 (2023). https://doi.org/10.1038/s41591-023-02519-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-023-02519-w
This article is cited by
-
Innovation is needed to increase viral hepatitis testing globally
Nature Reviews Gastroenterology & Hepatology (2024)