Abstract
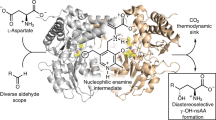
Aromatic amino acids and their derivatives are diverse primary and secondary metabolites with critical roles in protein synthesis, cell structure and integrity, defense and signaling. All de novo aromatic amino acid production relies on a set of ancient and highly conserved chemistries. Here we introduce a new enzymatic transformation for l-tyrosine synthesis by demonstrating that the β-subunit of tryptophan synthase—which natively couples indole and l-serine to form l-tryptophan—can act as a latent ‘tyrosine synthase’. A single substitution of a near-universally conserved catalytic residue unlocks activity toward simple phenol analogs and yields exclusive para carbon–carbon bond formation to furnish l-tyrosines. Structural and mechanistic studies show how a new active-site water molecule orients phenols for a nonnative mechanism of alkylation, with additional directed evolution resulting in a net >30,000-fold rate enhancement. This new biocatalyst can be used to efficiently prepare valuable l-tyrosine analogs at gram scales and provides the missing chemistry for a conceptually different pathway to l-tyrosine.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data, including the 18,719 annotated TrpB-like sequences, are available in the main text or supplementary materials. Full protein crystallographic data have been deposited with the PDB (https://www.rcsb.org) under accession codes 8EGY, 8EGZ, 8EH0 and 8EH1. Source data are provided with this paper.
References
Lynch, J. H. & Dudareva, N. Aromatic amino acids: a complex network ripe for future exploration. Trends Plant Sci. 25, 670–681 (2020).
Rodriguez, A. et al. Engineering Escherichia coli to overproduce aromatic amino acids and derived compounds. Microb. Cell Fact. 13, 126 (2014).
Lütke-Eversloh, T., Santos, C. N. S. & Stephanopoulos, G. Perspectives of biotechnological production of l-tyrosine and its applications. Appl. Microbiol. Biotechnol. 77, 751–762 (2007).
Dodd, D. et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 551, 648–652 (2017).
Yoo, H. et al. An alternative pathway contributes to phenylalanine biosynthesis in plants via a cytosolic tyrosine:phenylpyruvate aminotransferase. Nat. Commun. 4, 2833 (2013).
Merino, E., Jensen, R. A. & Yanofsky, C. Evolution of bacterial trp operons and their regulation. Curr. Opin. Microbiol. 11, 78–86 (2008).
Richards, T. A. et al. Evolutionary origins of the eukaryotic shikimate pathway: gene fusions, horizontal gene transfer, and endosymbiotic replacements. Eukaryot. Cell 5, 1517–1531 (2006).
Porat, I., Waters, B. W., Teng, Q. & Whitman, W. B. Two biosynthetic pathways for aromatic amino acids in the archaeon Methanococcus maripaludis. J. Bacteriol. 186, 4940–4950 (2004).
Parmeggiani, F., Weise, N. J., Ahmed, S. T. & Turner, N. J. Synthetic and therapeutic applications of ammonia-lyases and aminomutases. Chem. Rev. 118, 73–118 (2018).
Almhjell, P. J., Boville, C. E. & Arnold, F. H. Engineering enzymes for noncanonical amino acid synthesis. Chem. Soc. Rev. 47, 8980–8997 (2018).
Nagasawa, T. et al. Syntheses of l-tyrosine-related amino acids by tyrosine phenol-lyase of Citrobacter intermedius. Eur. J. Biochem. 117, 33–40 (1981).
Phillips, R. S., Sundararaju, B. & Faleev, N. G. Proton transfer and carbon–carbon bond cleavage in the elimination of indole catalyzed by Escherichia coli tryptophan indole-lyase. J. Am. Chem. Soc. 122, 1008–1014 (2000).
Seyedsayamdost, M. R., Reece, S. Y., Nocera, D. G. & Stubbe, J. Mono-, di-, tri-, and tetra-substituted fluorotyrosines: new probes for enzymes that use tyrosyl radicals in catalysis. J. Am. Chem. Soc. 128, 1569–1579 (2006).
Romei, M. G., Lin, C.-Y., Mathews, I. I. & Boxer, S. G. Electrostatic control of photoisomerization pathways in proteins. Science 367, 76–79 (2020).
Beber, M. E. et al. eQuilibrator 3.0: a database solution for thermodynamic constant estimation. Nucleic Acids Res. 50, D603–D609 (2022).
Watkins-Dulaney, E., Straathof, S. & Arnold, F. Tryptophan synthase: biocatalyst extraordinaire. ChemBioChem 22, 5–16 (2021).
Rix, G. et al. Scalable continuous evolution for the generation of diverse enzyme variants encompassing promiscuous activities. Nat. Commun. 11, 5644 (2020).
Smith, D. R. M. et al. The first one-pot synthesis of l-7-iodotryptophan from 7-iodoindole and serine, and an improved synthesis of other l-7-halotryptophans. Org. Lett. 16, 2622–2625 (2014).
Buller, A. R. et al. Directed evolution of the tryptophan synthase β-subunit for stand-alone function recapitulates allosteric activation. Proc. Natl Acad. Sci. USA 112, 14599–14604 (2015).
Herger, M. et al. Synthesis of β-branched tryptophan analogues using an engineered subunit of tryptophan synthase. J. Am. Chem. Soc. 138, 8388–8391 (2016).
Romney, D. K., Murciano-Calles, J., Wehrmüller, J. E. & Arnold, F. H. Unlocking reactivity of TrpB: a general biocatalytic platform for synthesis of tryptophan analogues. J. Am. Chem. Soc. 139, 10769–10776 (2017).
Romney, D. K., Sarai, N. S. & Arnold, F. H. Nitroalkanes as versatile nucleophiles for enzymatic synthesis of noncanonical amino acids. ACS Catal. 9, 8726–8730 (2019).
Dick, M., Sarai, N. S., Martynowycz, M. W., Gonen, T. & Arnold, F. H. Tailoring tryptophan synthase TrpB for selective quaternary carbon bond formation. J. Am. Chem. Soc. 141, 19817–19822 (2019).
Watkins, E. J., Almhjell, P. J. & Arnold, F. H. Direct enzymatic synthesis of a deep-blue fluorescent noncanonical amino acid from azulene and serine. ChemBioChem 21, 80–83 (2020).
Watkins-Dulaney, E. J. et al. Asymmetric alkylation of ketones catalyzed by engineered TrpB. Angew. Chem. Int. Ed. 60, 21412–21417 (2021).
Saito, Y., Sato, T., Nomoto, K. & Tsuji, H. Identification of phenol- and p-cresol-producing intestinal bacteria by using media supplemented with tyrosine and its metabolites. FEMS Microbiol. Ecol. 94, fiy125 (2018).
Álvarez-Rodríguez, M. L. et al. Degradation of vanillic acid and production of guaiacol by microorganisms isolated from cork samples. FEMS Microbiol. Lett. 220, 49–55 (2003).
Iwasaki, Y. et al. Novel metabolic pathway for salicylate biodegradation via phenol in yeast Trichosporon moniliiforme. Biodegradation 21, 557–564 (2010).
Kuzuyama, T., Noel, J. P. & Richard, S. B. Structural basis for the promiscuous biosynthetic prenylation of aromatic natural products. Nature 435, 983–987 (2005).
Krastanov, A., Alexieva, Z. & Yemendzhiev, H. Microbial degradation of phenol and phenolic derivatives. Eng. Life Sci. 13, 76–87 (2013).
Milić, D. et al. Crystallographic snapshots of tyrosine phenol-lyase show that substrate strain plays a role in C–C bond cleavage. J. Am. Chem. Soc. 133, 16468–16476 (2011).
Chen, Z. & Zhao, H. Rapid creation of a novel protein function by in vitro coevolution. J. Mol. Biol. 348, 1273–1282 (2005).
Boville, C. E., Romney, D. K., Almhjell, P. J., Sieben, M. & Arnold, F. H. Improved synthesis of 4-cyanotryptophan and other tryptophan analogues in aqueous solvent using variants of TrpB from Thermotoga maritima. J. Org. Chem. 83, 7447–7452 (2018).
Ruvinov, S. B., Ahmed, S. A., McPhie, P. & Miles, E. W. Monovalent cations partially repair a conformational defect in a mutant tryptophan synthase α2β2 complex (β-E109A). J. Biol. Chem. 270, 17333–17338 (1995).
Wittmann, B. J., Johnston, K. E., Almhjell, P. J. & Arnold, F. H. evSeq: cost-effective amplicon sequencing of every variant in a protein library. ACS Synth. Biol. 11, 1313–1324 (2022).
Busch, F. et al. TrpB2 enzymes are O-phospho-l-serine dependent tryptophan synthases. Biochemistry 53, 6078–6083 (2014).
Tzin, V. & Galili, G. The biosynthetic pathways for shikimate and aromatic amino acids in Arabidopsis thaliana. Arabidopsis Book 8, e0132 (2010).
Nonhebel, H. M. Tryptophan-independent indole-3-acetic acid synthesis: critical evaluation of the evidence. Plant Physiol. 169, 1001–1005 (2015).
Yin, R., Frey, M., Gierl, A. & Glawischnig, E. Plants contain two distinct classes of functional tryptophan synthase β proteins. Phytochemistry 71, 1667–1672 (2010).
Buller, A. R. et al. Directed evolution mimics allosteric activation by stepwise tuning of the conformational ensemble. J. Am. Chem. Soc. 140, 7256–7266 (2018).
Kraut, D. A., Sigala, P. A., Fenn, T. D. & Herschlag, D. Dissecting the paradoxical effects of hydrogen bond mutations in the ketosteroid isomerase oxyanion hole. Proc. Natl Acad. Sci. USA 107, 1960–1965 (2010).
Pinney, M. M. et al. Parallel molecular mechanisms for enzyme temperature adaptation. Science 371, eaay2784 (2021).
Smith, A. J. T. et al. Structural reorganization and preorganization in enzyme active sites: comparisons of experimental and theoretically ideal active site geometries in the multistep serine esterase reaction cycle. J. Am. Chem. Soc. 130, 15361–15373 (2008).
Knör, S., Laufer, B. & Kessler, H. Efficient enantioselective synthesis of condensed and aromatic-ring-substituted tyrosine derivatives. J. Org. Chem. 71, 5625–5630 (2006).
Tang, M.-C., Fu, C.-Y. & Tang, G.-L. Characterization of SfmD as a heme peroxidase that catalyzes the regioselective hydroxylation of 3-methyltyrosine to 3-hydroxy-5-methyltyrosine in saframycin A biosynthesis. J. Biol. Chem. 287, 5112–5121 (2012).
Schmidt, E. W., Nelson, J. T. & Fillmore, J. P. Synthesis of tyrosine derivatives for saframycin MX1 biosynthetic studies. Tetrahedron Lett. 45, 3921–3924 (2004).
Thompson, B., Machas, M. & Nielsen, D. R. Engineering and comparison of non-natural pathways for microbial phenol production. Biotechnol. Bioeng. 113, 1745–1754 (2016).
Contente, M. L., Roura Padrosa, D., Molinari, F. & Paradisi, F. A strategic Ser/Cys exchange in the catalytic triad unlocks an acyltransferase-mediated synthesis of thioesters and tertiary amides. Nat. Catal. 3, 1020–1026 (2020).
Coelho, P. S. et al. A serine-substituted P450 catalyzes highly efficient carbene transfer to olefins in vivo. Nat. Chem. Biol. 9, 485–487 (2013).
Xia, Y., Chu, W., Qi, Q. & Xun, L. New insights into the QuikChangeTM process guide the use of Phusion DNA polymerase for site-directed mutagenesis. Nucleic Acids Res. 43, e12 (2015).
Kille, S. et al. Reducing codon redundancy and screening effort of combinatorial protein libraries created by saturation mutagenesis. ACS Synth. Biol. 2, 83–92 (2013).
Zhao, H., Giver, L., Shao, Z., Affholter, J. A. & Arnold, F. H. Molecular evolution by staggered extension process (StEP) in vitro recombination. Nat. Biotechnol. 16, 258–261 (1998).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Lane, A. N. & Kirschner, K. The catalytic mechanism of tryptophan synthase from Escherichia coli. Eur. J. Biochem. 129, 571–582 (1983).
Bhushan, R. & Bruckner, H. Use of Marfey’s reagent and analogs for chiral amino acid analysis: assessment and applications to natural products and biological systems. J. Chromatogr. B 879, 3148–3161 (2011).
Hopf, T. A. et al. The EVcouplings Python framework for coevolutionary sequence analysis. Bioinformatics 35, 1582–1584 (2019).
Nguyen, L.-T., Schmidt, H. A., von Haeseler, A. & Minh, B. Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274 (2015).
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K. F., von Haeseler, A. & Jermiin, L. S. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods 14, 587–589 (2017).
Letunic, I. & Bork, P. Interactive Tree of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 49, W293–W296 (2021).
Kabsch, W. XDS. Acta Crystallogr. D 66, 125–132 (2010).
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D 67, 235–242 (2011).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010).
Laskowski, R. A., MacArthur, M. W., Moss, D. S. & Thornton, J. M. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Cryst. 26, 283–291 (1993).
Neese, F., Wennmohs, F., Becker, U. & Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 152, 224108 (2020).
Becke, A. D. Density‐functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Zheng, J., Xu, X. & Truhlar, D. G. Minimally augmented Karlsruhe basis sets. Theor. Chem. Acc. 128, 295–305 (2011).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Chai, J.-D. & Head-Gordon, M. Systematic optimization of long-range corrected hybrid density functionals. J. Chem. Phys. 128, 084106 (2008).
Barone, V. & Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 102, 1995–2001 (1998).
Best, R. B. et al. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone ϕ, ψ and side-chain χ1 and χ2 dihedral angles. J. Chem. Theory Comput. 8, 3257–3273 (2012).
Vanommeslaeghe, K. et al. CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 31, 671–690 (2010).
Eastman, P. et al. OpenMM 7: rapid development of high performance algorithms for molecular dynamics. PLoS Comput. Biol. 13, e1005659 (2017).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Acknowledgements
We thank the following past and present members of the Arnold Laboratory for helpful discussions on the manuscript and experimental assistance: A. Buller, T. Boville, D. Romney, E. Watkins-Dulaney, S. Brinkmann-Chen and N. Goldberg, among many others. We thank S. Du for helpful discussions and J. Kaiser from the Caltech Molecular Observatory for crystallography support. This work was supported by the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) under award R01GM125887. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Individual support comes from Nagendranath Reddy Graduate Fellowship (to P.J.A.); Caltech Biotechnology Leadership Program (BLP; NIH training grant 5T32GM112592-5 to K.E.J.); Caltech AI4Science/Amazon AWS Fellowship (to K.E.J.); Merck–Helen Hay Whitney Postdoctoral Fellowship (to N.J.P.); Ruth L. Kirschstein NIH Postdoctoral Fellowship (1F32GM143797-01A1 to J.L.K.); National Science Foundation Graduate Research Fellowship Program (GRFP; grant DGE‐1745301 to V.C.B.); Fonds de recherche du Québec Nature et technologie (FRQNT; to J.D.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
P.J.A. and F.H.A. conceptualized the project. P.J.A., K.E.J., N.J.P., J.L.K. and V.C.B. conducted the investigation. P.J.A., K.E.J., N.J.P., V.C.B., J.L.K. and J.D. performed validation. P.J.A. wrote the original draft. P.J.A., K.E.J., N.J.P., J.L.K., V.C.B., J.D. and F.H.A. reviewed and edited the manuscript. F.H.A. secured funding and provided supervision.
Corresponding author
Ethics declarations
Competing interests
P.J.A. and F.H.A. are inventors on patent applications filed by the California Institute of Technology that cover enzymatic synthesis of tyrosine analogs from analogs of phenol and serine (US17/985,033 and PCT/US2022/049617, pending). The other authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Shina Kamerlin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Mechanistic details of TrpB and Trpase/TPL.
a. Catalytic cycle of Thermotoga maritima TrpB (TmTrpB) and Trpase. The general reaction for TrpB is shown in black, which is mostly paralleled by Trpase with two main deviations that are highlighted with yellow boxes and/or arrows. Reaction arrows are shown as unidirectional in the synthetic direction for conceptual simplicity. b. Hypothetical reaction coordinates for β-elimination reactions that access and degrade the enzyme-bound amino-acrylate intermediate (E(A-A), shown as the same energy on the enzymes) by TrpB (purple) and TPL/Trpase (yellow). Left: A comparable rate of l-tryptophan (Trp)/l-tyrosine (Tyr) β-elimination by an enzyme (E) will show accumulation of E(A-A) in TrpB and transience in TPL/Trpase if the barriers for conversion to pyruvate and ammonia are sufficiently different. Experimentally, a different intermediate accumulates in TrpB that occurs prior to E(A-A) formation, generally E(Q3) or E(Q2). Right: TrpB accesses E(A-A) from l-serine (Ser) and the E(A-A) species accumulates in the absence of a nucleophile. In the presence of indole, the left half of each reaction coordinate is connected, providing a direct pathway from Ser to Trp with minimal degradation of the amino-acrylate, despite the thermodynamic driving force of pyruvate and ammonia formation (see main text). c. Active-site coordination and activation of phenol by Citrobacter freundii tyrosine phenol lyase (CfTPL) for reversible alkylation (and Tyr degradation). d. Active-site coordination and activation of indole for irreversible alkylation by TmTrpB.
Extended Data Fig. 2 Identification and characterization of the ncAA condensation product of TrpB and 1-naphthol.
a. Stand-alone variants of TmTrpB (Tm9D8*, teal) and Pyrococcus furiosus TrpB (Pf2B9, blue) synthesize a condensation product when provided 1-naphthol and Ser. b. Alignment of a co-crystal structure of Salmonella typhimurium TrpS (StTrpS, PDB 4HPX) bound with the non-reactive inhibitor benzimidazole and a homology model of Tm9D8*. Pairwise alignment of 1-naphthol to benzimidazole aligned for para C–C bond formation demonstrates that the conserved catalytic residue E105 likely is not optimal for the extended phenolic group of 1-naphthol and other phenol analogs. c. Preparative-scale synthesis and 2D NMR characterization of the 1-naphthol product of Tm9D8* E105G reveals that the para alkylation product NaphAla is the only reaction product. HSQC = heteronuclear single-quantum correlation; HMBC = heteronuclear multiple bond correlation.
Extended Data Fig. 3 Characterization of tyrosine synthase activity by enzymes engineered from TrpB.
a. Determination of enantioselectivity by Marfey’s analysis demonstrates that TyrS variants only make l-tyrosine (Tyr), consistent with the standard mechanism of TrpB. Traces are offset equally in x and y. The first peak is l-Tyr according to the differences between the enantiopure (teal) and racemic (blue) Tyr standards. Enzymatic Tyr (purple) produces this first peak. b. Chromatographic separation of Tyr (purple) and ortho-Tyr (teal), separately and coinjected (blue), via the standard gradient used in this study. Upper: extracted ion counts for 182 m/z, the molecular ion. Lower: absorbance at 280 nm. Despite the small constitutional difference between these isomers, their retention times are separated by ~0.7 min (1.1 min vs. 1.8 min) on the standard LCMS method and column used in this study (see General experimental methods). c. No significant ortho-Tyr is detectable in the enzymatic reaction (teal) compared to authentic Tyr (orange). d. Mass spectrometry (MS)-based calibration curve for Tyr synthesis, with a best fit for the logarithm of each MS peak area to the concentration of Tyr in the reaction (in µM). e. Analytic detection limit for Tyr. The MS peak areas deviate from the logarithmic relationship below 1 µM Tyr, at which point instrument noise and residual Tyr from non-exhaustive column washing make quantification impossible and present a limit of quantification for enzyme-produced Tyr.
Extended Data Fig. 4 TyrS variants remain kinetically controlled.
a. A 1 mM solution of authentic Tyr (approximately saturating) was incubated with and without 10 µM TmTyrS6 for 20 hours, showing no significant degradation. (1 mM phenol shown for reference.) b. Like in a, saturated solutions of authentic NaphAla, 3-iodo-Tyr and 3-chloro-Tyr were incubated with 10 µM of a TyrS variant that displayed high activity toward the respective phenol analog. Under thermodynamic control, as with Trpase and TPL, this enzyme would efficiently degrade the respective Tyr analog. Approximate areas in which the phenol analogs elute are designated in gray boxes. Only NaphAla shows very minor degradation at a rate far less than its rate of NaphAla synthesis, indicating that the reaction of TyrS remains under kinetic control for Tyr analogs as well.
Extended Data Fig. 5 Effect of E105G on Trp and Tyr synthesis.
a. In addition to reducing the rate of Trp synthesis, the E105G mutation also reduces regioselectivity of indole alkylation from >99.5%. Upper: Tm9D8* E105G with indole forms nearly 1:1 Trp to isoTrp, the N-alkylation product. Lower: Pf2B9 E104G retains some regioselectivity, but still markedly decreased from its previously unquantifiable levels. b. 100 µM Tm9D8* does not make Tyr above the limit of quantification (1 µM) in 24 hours, but the equivalent amount of Tm9D8* E105G makes >50 µM Tyr. c. The same analysis for Pf2B9 E104G, with the addition of a 5 µM standard of Tyr for reference, as Pf2B9 E104G at 75 °C is slower than Tm9D8* E105G at 37 °C.
Extended Data Fig. 6 Phylogenetic analysis of TrpB sequences.
a. Conservation of E105 in 18,051 aligned TrpB-like sequences. Inset: axis-adjusted view. b. Conservation of G229 in 18,051 aligned TrpB-like sequences. Inset: axis-adjusted view. c. Pairwise correlations between mutations at E105 and G229. Those with E105G and G229A are TrpB2-like enzymes found primarily in Actinobacteria. Those with E105A and G229S/T are TrpB-like enzymes found in plants, primarily in malvids. d. Unrooted phylogenetic tree from Fig. 3b, built from 1,158 TrpB- and TrpB2-like sequences (all those with E105G/A, selected paralogs, and 1,000 randomly sampled sequences). Nodes are colored by residue identity at position 105 (fill) and 229 (outline).
Extended Data Fig. 7 X-ray crystallographic analysis of TyrS variants.
a. TmTyrS1 crystals. The morphology was fairly amorphous but yielded excellent diffraction and was amenable to small-molecule soaking experiments. b. Comparison of the internal aldimine E(Ain) forms of TmTyrS1 and another stand-alone TrpB (Pf2B9). The TmTyrS1 structure adopts a more ‘closed’ conformation with a disrupted COMM domain helix, but it is unclear if these are significant for TyrS, TmTrpB/Tm9D8*, or at all. c. Polder omit map contoured at 5σ for the amino-acrylate, formed by soaking E(Ain) crystals with Ser. The COMM domain helix is re-formed. This reduces the conformational heterogeneity between the two TrpB subunits in the asymmetric unit between the E(Ain) state (d) and the Ser-soaked E(A-A) state (e). f. The removal of E105 allows for the binding of new active-site water molecules, coordinated by G105, V183 and the side chain of Y182. It additionally makes an interaction with a separate water molecule. Placement of 1-naphthol into this structure (as performed in Extended Data Fig. 2b) overlays the hydroxyl of 1-naphthol with the second water molecule, which would align 1-naphthol for C–C bond formation. g. Polder omit map contoured at 7σ for TmTyrS1 E(A-A) bound with 4-hydroxyquinoline (4-HQ). h. Polder omit map contoured at 7.6σ for TmTyrS1 E(A-A) bound with quinoline N-oxide. i. Alignment of each subunit in the E(A-A) state and E(A-A)•4-HQ state (4 separate structures), showing minimal re-organization when 4-HQ binds. See Supplementary Table 4 for pairwise RMSDs.
Extended Data Fig. 8 TmTyrS1 kinetics and regioselectivity.
a. The 1-naphthol chromophore is redshifted as it is converted to NaphAla, with an isosbestic point near 284 nm, and a maximal difference near 330 nm. b. The absorbance change at 335 nm can be used to monitor reaction progress (upper) and to determine the change in molar absorptivity as 1-naphthol is converted to NaphAla at pH 8.0 (lower, 0.85 AU mM−1 cm−1). c. Michaelis-Menten analysis of TmTyrS1 and 1-naphthol. The kcat value agrees with those determined by LCMS at saturating conditions (TOFs in Fig. 3a). Line represents the best-fit parameters, while colored areas represent fits determined from the 75 and 95% credible regions of kcat and KM. d. Michaelis-Menten analysis of TmTyrS1 G105E. While the kcat is ~10–30-fold lower than other stand-alone TrpBs and 5-fold lower than TmTyrS1 with 1-naphthol, its KM is quite low (nearly 2,000-fold lower than TmTyrS1 with 1-naphthol). Line represents the best-fit parameters, while colored areas represent fits determined from the 75% and 95% credible regions of kcat and KM. e. While all TyrS variants (and Tm9D8* E105G/Pf2B9 E104G) lose regioselectivity for indole alkylation (see Extended Data Fig. 5a and Fig. 3a source data), replacing the catalytic glutamate in TmTyrS1 (TmTyrS1 G105E) restores regioselectivity for indole alkylation.
Extended Data Fig. 9 Computational modeling of intermediates and transition states.
a. Quantum mechanics (QM)-calculated structure of the step-wise intermediate for indole alkylation, after C3–Cβ bond formation but before deprotonation (C3–H bond breakage). This structure readily converges, even in the absence of enzyme-mediated interactions. b. Initial structure of the step-wise intermediate for phenol alkylation, after C4–Cβ bond formation but before deprotonation (C4–H bond breakage) for QM optimization. This did not converge to a stable structure, regardless of interactions modeled. c. QM-calculated transition-state structure for concerted C4–Cβ bond formation and C4–H bond breakage (solid orange bonds) of phenol. This is mediated by the catalytic lysine (K83) and the active-site water installed with the E105G mutation.
Supplementary information
Supplementary Information
Supplementary Tables 1–4, Supplementary Methods, Supplementary Notes 1–3, enzyme DNA sequences and NMR spectra.
Supplementary Data 1
Two-dimensional NMR of NaphAla product.
Supplementary Data 2
Data for Supplementary Information standard curves for halophenol to halotyrosine conversion by LC–MS.
Supplementary Data 3
The full MSA of TrpB-like sequences with columns for their IDs, sequences, aligned sequences, organism information, residue identity and other annotations.
Supplementary Data 4
Data (.treefile and annotation files) and instructions for reproducing Fig. 3b and Extended Data Fig. 6d.
Supplementary Data 5
Atom coordinates for the QM-calculated intermediate structures in Fig. 4b,c and Extended Data Fig. 9.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Almhjell, P.J., Johnston, K.E., Porter, N.J. et al. The β-subunit of tryptophan synthase is a latent tyrosine synthase. Nat Chem Biol (2024). https://doi.org/10.1038/s41589-024-01619-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41589-024-01619-z