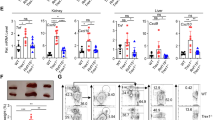
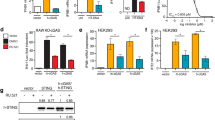
Abstract
Efforts to advance RNA aptamers as a new therapeutic modality have been limited by their susceptibility to degradation and immunogenicity. In a previous study, we demonstrated synthesized short double-stranded region-containing circular RNAs (ds-cRNAs) with minimal immunogenicity targeted to dsRNA-activated protein kinase R (PKR). Here we test the therapeutic potential of ds-cRNAs in a mouse model of imiquimod-induced psoriasis. We find that genetic supplementation of ds-cRNAs leads to inhibition of PKR, resulting in alleviation of downstream interferon-α and dsRNA signals and attenuation of psoriasis phenotypes. Delivery of ds-cRNAs by lipid nanoparticles to the spleen attenuates PKR activity in examined splenocytes, resulting in reduced epidermal thickness. These findings suggest that ds-cRNAs represent a promising approach to mitigate excessive PKR activation for therapeutic purposes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this study are available in the paper, Source Data and at https://doi.org/10.17632/zfm9kmfghs.2 (ref. 77). All sequencing data reported in this paper have been deposited in the Gene Expression Omnibus (GEO). High-throughput datasets generated in this study are available at GSE248680 (ref. 78), including circSHAPE-MaP data (GSE248679) and mouse RNA-seq data (IMQ-treated Pkr−/− mouse data, GSE248678; mouse spleens delivered EPIC-LNPs data, GSE253346); published RNA-seq data of patients with psoriasis can be downloaded from GEO under accession number GSE121212 (ref. 79). Source data are provided with this paper.
Code availability
This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact on request.
References
Hause, A. M. et al. Safety monitoring of bivalent COVID-19 mRNA vaccine booster doses among persons aged >/=12 years—United States, August 31-October 23, 2022. MMWR Morb. Mortal Wkly Rep. 71, 1401–1406 (2022).
Winkle, M., El-Daly, S. M., Fabbri, M. & Calin, G. A. Noncoding RNA therapeutics—challenges and potential solutions. Nat Rev. Drug Discov. 20, 629–651 (2021).
Enuka, Y. et al. Circular RNAs are long-lived and display only minimal early alterations in response to a growth factor. Nucleic Acids Res. 44, 1370–1383 (2016).
Zhang, Y. et al. The biogenesis of nascent circular RNAs. Cell Rep. 15, 611–624 (2016).
Fischer, J. W., Busa, V. F., Shao, Y. & Leung, A. K. L. Structure-mediated RNA decay by UPF1 and G3BP1. Mol. Cell. 78, 70–84.e76 (2020).
Liu, C. X. et al. Structure and degradation of circular RNAs regulate PKR activation in innate immunity. Cell 177, 865–880.e821 (2019).
Wesselhoeft, R. A. et al. RNA circularization diminishes immunogenicity and can extend translation duration in vivo. Mol. Cell. 74, 508–520.e504 (2019).
Liu, C. X. et al. RNA circles with minimized immunogenicity as potent PKR inhibitors. Mol. Cell. 82, 420–434.e426 (2022).
Liu, C. X. & Chen, L. L. Circular RNAs: characterization, cellular roles, and applications. Cell 185, 2390 (2022).
Bou-Nader, C., Gordon, J. M., Henderson, F. E. & Zhang, J. The search for a PKR code-differential regulation of protein kinase R activity by diverse RNA and protein regulators. RNA 25, 539–556 (2019).
Cao, S. S., Song, B. & Kaufman, R. J. PKR protects colonic epithelium against colitis through the unfolded protein response and prosurvival signaling. Inflamm. Bowel Dis. 18, 1735–1742 (2012).
Grolleau, A., Kaplan, M. J., Hanash, S. M., Beretta, L. & Richardson, B. Impaired translational response and increased protein kinase PKR expression in T cells from lupus patients. J. Clin. Invest. 106, 1561–1568 (2000).
Rath, E. et al. Induction of dsRNA-activated protein kinase links mitochondrial unfolded protein response to the pathogenesis of intestinal inflammation. Gut 61, 1269–1278 (2012).
Zheng, X. & Bevilacqua, P. C. Activation of the protein kinase PKR by short double-stranded RNAs with single-stranded tails. RNA 10, 1934–1945 (2004).
Ingrand, S. et al. The oxindole/imidazole derivative C16 reduces in vivo brain PKR activation. FEBS Lett. 581, 4473–4478 (2007).
Mouton-Liger, F. et al. PKR downregulation prevents neurodegeneration and β-amyloid production in a thiamine-deficient model. Cell Death Dis. 6, e1594 (2015).
Watanabe, T. et al. Therapeutic effects of the PKR inhibitor C16 suppressing tumor proliferation and angiogenesis in hepatocellular carcinoma in vitro and in vivo. Sci. Rep. 10, 5133 (2020).
Griffiths, C. E. M., Armstrong, A. W., Gudjonsson, J. E., & Barker, J. Psoriasis. Lancet 397, 1301–1315 (2021).
Moldovan, L. I. et al. High-throughput RNA sequencing from paired lesional- and non-lesional skin reveals major alterations in the psoriasis circRNAome. BMC Med. Genomics 12, 174 (2019).
Moldovan, L. I. et al. Characterization of circular RNA transcriptomes in psoriasis and atopic dermatitis reveals disease-specific expression profiles. Exp. Dermatol. 30, 1187–1196 (2021).
Seeler, S. et al. Global circRNA expression changes predate clinical and histological improvements of psoriasis patients upon secukinumab treatment. PLoS ONE 17, e0275219 (2022).
Swindell, W. R. et al. Imiquimod has strain-dependent effects in mice and does not uniquely model human psoriasis. Genome Med. 9, 24 (2017).
Obi, P. & Chen, Y. G. The design and synthesis of circular RNAs. Methods 196, 85–103 (2021).
Puttaraju, M. & Been, M. D. Group I permuted intron-exon (PIE) sequences self-splice to produce circular exons. Nucleic Acids Res. 20, 5357–5364 (1992).
Wesselhoeft, R. A., Kowalski, P. S. & Anderson, D. G. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat. Commun. 9, 2629 (2018).
Guo, S. K., Nan, F., Liu, C. X., Yang, L. & Chen, L. L. Mapping circular RNA structures in living cells by SHAPE-MaP. Methods 196, 47–55 (2021).
Kuhen, K. L. et al. Structural organization of the human gene (PKR) encoding an interferon-inducible RNA-dependent protein kinase (PKR) and differences from its mouse homolog. Genomics 36, 197–201 (1996).
Samuel, C. E. The eIF-2 alpha protein kinases, regulators of translation in eukaryotes from yeasts to humans. J. Biol. Chem. 268, 7603–7606 (1993).
Wu, M. et al. lncRNA SLERT controls phase separation of FC/DFCs to facilitate Pol I transcription. Science 373, 547–555 (2021).
Yang, X. W. et al. MutS functions as a clamp loader by positioning MutL on the DNA during mismatch repair. Nat. Commun. 13, 5808 (2022).
Hummert, J. et al. Photobleaching step analysis for robust determination of protein complex stoichiometries. Mol. Biol. Cell. 32, ar35 (2021).
Yuan, J., He, K., Cheng, M., Yu, J. & Fang, X. Analysis of the steps in single-molecule photobleaching traces by using the hidden Markov model and maximum-likelihood clustering. Chem. Asian J. 9, 2303–2308 (2014).
Nallagatla, S. R., Toroney, R. & Bevilacqua, P. C. Regulation of innate immunity through RNA structure and the protein kinase PKR. Curr. Opin. Struct. Biol. 21, 119–127 (2011).
Heinicke, L. A., Nallagatla, S. R., Hull, C. M. & Bevilacqua, P. C. RNA helical imperfections regulate activation of the protein kinase PKR: effects of bulge position, size, and geometry. RNA 17, 957–966 (2011).
Yan, Y., Tao, H., He, J. & Huang, S. Y. The HDOCK server for integrated protein-protein docking. Nat. Protoc. 15, 1829–1852 (2020).
Husain, B., Hesler, S. & Cole, J. L. Regulation of PKR by RNA: formation of active and inactive dimers. Biochemistry 54, 6663–6672 (2015).
Mayo, C. B. et al. Structural basis of protein kinase R autophosphorylation. Biochemistry 58, 2967–2977 (2019).
Dörner, T. & Furie, R. Novel paradigms in systemic lupus erythematosus. Lancet 393, 2344–2358 (2019).
Tsokos, G. C., Lo, M. S., Costa Reis, P. & Sullivan, K. E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 12, 716–730 (2016).
van der Fits, L. et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 182, 5836–5845 (2009).
Taylor, S. S., Haste, N. M. & Ghosh, G. PKR and eIF2alpha: integration of kinase dimerization, activation, and substrate docking. Cell 122, 823–825 (2005).
Ma, X. K. et al. CIRCexplorer3: a CLEAR pipeline for direct comparison of circular and linear RNA expression. Genom. Proteom. Bioinform. 17, 511–521 (2019).
Futschik, M. E. & Carlisle, B. Noise-robust soft clustering of gene expression time-course data. J. Bioinform. Comput. Biol. 3, 965–988 (2005).
Kumar, L. & M, E. F. Mfuzz: a software package for soft clustering of microarray data. Bioinformation 2, 5–7 (2007).
Lande, R. & Gilliet, M. Plasmacytoid dendritic cells: key players in the initiation and regulation of immune responses. Ann. N. Y. Acad. Sci. 1183, 89–103 (2010).
Chiricozzi, A., Romanelli, P., Volpe, E., Borsellino, G. & Romanelli, M. Scanning the immunopathogenesis of psoriasis. Int. J. Mol. Sci. 19, 179 (2018).
Yang, Y. L. et al. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 14, 6095–6106 (1995).
Nestle, F. O. et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J. Exp. Med. 202, 135–143 (2005).
Rácz, E. et al. Narrowband ultraviolet B inhibits innate cytosolic double-stranded RNA receptors in psoriatic skin and keratinocytes. Br. J. Dermatol. 164, 838–847 (2011).
Zhang, L. J. et al. Antimicrobial peptide LL37 and MAVS signaling drive interferon-β production by epidermal keratinocytes during skin injury. Immunity 45, 119–130 (2016).
Chen, L. L. et al. A guide to naming eukaryotic circular RNAs. Nat. Cell Biol. 25, 1–5 (2023).
Zhang, X. O. et al. Complementary sequence-mediated exon circularization. Cell 159, 134–147 (2014).
Naidu, G. S. et al. A combinatorial library of lipid nanoparticles for cell type-specific mRNA delivery. Adv. Sci. 10, e2301929 (2023).
Jones, S. A. et al. GILZ regulates Th17 responses and restrains IL-17-mediated skin inflammation. J. Autoimmun. 61, 73–80 (2015).
Fredriksson, T. & Pettersson, U. Severe psoriasis-oral therapy with a new retinoid. Dermatologica 157, 238–244 (1978).
Langley, R. G. & Ellis, C. N. Evaluating psoriasis with psoriasis area and severity index, psoriasis global assessment, and lattice system physician’s global assessment. J. Am. Acad. Dermatol. 51, 563–569 (2004).
Chen, Y. G. et al. N6-methyladenosine modification controls circular RNA immunity. Mol. Cell. 76, 96–109.e109 (2019).
Qu, L. et al. Circular RNA vaccines against SARS-CoV-2 and emerging variants. Cell 185, 1728–1744.e1716 (2022).
Gal-Ben-Ari, S., Barrera, I., Ehrlich, M. & Rosenblum, K. PKR: a kinase to remember. Front. Mol. Neurosci. 11, 480 (2018).
Tronel, C., Page, G., Bodard, S., Chalon, S. & Antier, D. The specific PKR inhibitor C16 prevents apoptosis and IL-1beta production in an acute excitotoxic rat model with a neuroinflammatory component. Neurochem. Int. 64, 73–83 (2014).
Chang, R. C. et al. Involvement of double-stranded RNA-dependent protein kinase and phosphorylation of eukaryotic initiation factor-2alpha in neuronal degeneration. J. Neurochem. 83, 1215–1225 (2002).
Stern, E., Chinnakkaruppan, A., David, O., Sonenberg, N. & Rosenblum, K. Blocking the eIF2alpha kinase (PKR) enhances positive and negative forms of cortex-dependent taste memory. J. Neurosci. 33, 2517–2525 (2013).
Zhu, P. J. et al. Suppression of PKR promotes network excitability and enhanced cognition by interferon-gamma-mediated disinhibition. Cell 147, 1384–1396 (2011).
Feng, X. et al. Circular RNA aptamers ameliorate AD-relevant phenotypes by targeting PKR. Preprint at bioRxiv https://doi.org/10.1101/2024.03.27.583257 (2024).
Kaushik, S. B. & Lebwohl, M. G. Psoriasis: which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J. Am. Acad. Dermatol. 80, 27–40 (2019).
Manfreda, V., Esposito, M., Campione, E., Bianchi, L. & Giunta, A. Apremilast efficacy and safety in a psoriatic arthritis patient affected by HIV and HBV virus infections. Postgrad. Med. 131, 239–240 (2019).
Guimaraes, C. P. et al. Site-specific C-terminal and internal loop labeling of proteins using sortase-mediated reactions. Nat. Protoc. 8, 1787–1799 (2013).
Donovan, J., Rath, S., Kolet-Mandrikov, D. & Korennykh, A. Rapid RNase L-driven arrest of protein synthesis in the dsRNA response without degradation of translation machinery. RNA 23, 1660–1671 (2017).
Xiao, M. S. & Wilusz, J. E. An improved method for circular RNA purification using RNase R that efficiently removes linear RNAs containing G-quadruplexes or structured 3′ ends. Nucleic Acids Res. 47, 8755–8769 (2019).
Matsui, T., Tanihara, K. & Date, T. Expression of unphosphorylated form of human double-stranded RNA-activated protein kinase in Escherichia coli. Biochem. Biophys. Res. Commun. 284, 798–807 (2001).
Smola, M. J., Rice, G. M., Busan, S., Siegfried, N. A. & Weeks, K. M. Selective 2′-hydroxyl acylation analyzed by primer extension and mutational profiling (SHAPE-MaP) for direct, versatile and accurate RNA structure analysis. Nat. Protoc. 10, 1643–1669 (2015).
Senavirathne, G. et al. Widespread nuclease contamination in commonly used oxygen-scavenging systems. Nat. Methods 12, 901–902 (2015).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Kim, D. & Salzberg, S. L. TopHat-Fusion: an algorithm for discovery of novel fusion transcripts. Genome Biol. 12, R72 (2011).
Zhang, Y., Wang, J. & Xiao, Y. 3dRNA: 3D structure prediction from linear to circular RNAs. J. Mol. Biol. 434, 167452 (2022).
Othniel, J. “doi: 10.17632/j6fmfjrc5y.1”, Mendeley Data, V1. Mendely Data https://doi.org/10.17632/94jg7jkt6n.1 (2020).
Guo, S. K. et al. Therapeutic Application of Circular RNA Aptamers in a Mouse Model of Psoriasis (Gene Expression Omnibus, 2024); http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE248680
Weidinger S., Rodriguez E., Tsoi L. C. & Gudjonsson J. Atopic Dermatitis, Psoriasis and Healthy Control RNA-seq Cohort (Gene Expression Omnibus, 2019); https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE121212
Acknowledgements
We thank F. Nan at Shanghai Institute of Materia Medica for support in LNPs, and the Chen laboratory members for critical discussion. This work was supported by National Key R&D Program of China (grant no. 2021YFA1300501), Strategic Priority Research Program of the Chinese Academy of Science (grant no. XDB0570000) and Science and Technology Commission of Shanghai Municipality (STCSM) (grant nos. 23DX1900100 and 23DX1900101) to L.-L.C.; National Natural Science Foundation of China (NSFC) (grant no. 31925011), National Key R&D Program of China (grant nos. 2021YFA1300503 and 2019YFA0802804) and STCSM (grant nos. 23DX1900102 and 23JS1400300) to L.Y.; NSFC (grant no. 32371349) and Shanghai Rising-Star Program (grant no. 23QA1410200) to C.-X.L.; China National Postdoctoral Program for Innovative Talents (grant nos. BX20220298 and BX20220077) and Shanghai Postdoctoral Excellence Program (grant nos. 2022757 and 2022728) to X.W. and F.N. D.P. acknowledges the support from the European Research Council (advance grant no. 101055029). This work has been supported by the New Cornerstone Science Foundation through the New Cornerstone Investigator Program and the XPLORER PRIZE.
Author information
Authors and Affiliations
Contributions
L.-L.C. supervised and conceived the project. S.-K.G., C.-X.L., Y.-F.X. and X.W. designed and performed experiments. F.N. preformed computational analyses, supervised by L.Y. Y.H., S.L., L.L., E.K. and N.A. formulated LNPs, supervised by D.P. and L.-L.C. R.S. and S.P. provided samples from patients with psoriasis, supervised by T.C. C.L. helped with smTIRF experiments, supervised by J.L. J.W. generated the next-generation sequencing library. S.N. and M.-Y.W. helped with biochemical and mice experiments. L.-L.C., S.-K.G., C.-X.L., Y.-F.X., X.W. and F.N. wrote the paper with input from all authors. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
L.-L.C., S.-K.G., C.-X.L., S.L. and Y.-F.X. are named as inventors on patents related to circRNA held by CAS CEMCS. L.-L.C. is a scientific co-founder of RiboX Therapeutics. D.P. receives licensing fees (to patents on which he was an inventor) from, invested in, consults (or on scientific advisory boards or boards of directors) for, lectured (and received a fee) or conducts sponsored research at TAU for the following entities: ART Biosciences, BioNtech SE, Earli Inc., Kernal Biologics, Geneditor Biologics, Newphase Ltd, NeoVac Ltd, RiboX Therapeutics, Roche, SirTLabs Corporation and Teva Pharmaceuticals Inc.
Peer review
Peer review information
Nature Biotechnology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 EPIC synthesized by optimized strategy preserves its characteristics in minimized immunogenicity and folding status.
(a) Examination of circularization efficiency of two circular RNAs (circPOLR2A, 336 nt; circmCherry, 1452 nt) using Anabaena tRNALeu-derived PIE with varied lengths of extraneous nucleotides. Circularized RNA products are analyzed with denaturing PAGE, bands of RNA circles are verified with RNase R and marked with blue arrows. Representative results are shown from three replicates. Gels of each replicate are processed in parallel. (b) The 27 nt extraneous sequence tend to form stem-loop alone at the JS, independent of cargo sequences by predictions of in silico and SHAPE-MaP. (c) Ana_PIE_27nt version results in minimal induction of inflammatory factors. The same amounts of circular POLR2A (200 ng for each sample) with varied lengths of extraneous nucleotides were transfected into A549 cells. Relative expression of inflammatory factors after 6 hours transfection were examined by RT-qPCR. n = 3 biological repeats. (d) Secondary structures of circPOLR2A_Lig and circPOLR2A_J (EPIC). Left, the original and new junction sites are indicated in the secondary structure of circPOLR2A_Lig8. Right, the secondary structure of circPOLR2A_J (EPIC), in which the 27 nt extraneous sequences forms a stable step-loop. The predicted imperfect duplex regions are marked with gray and yellow shadows. (e) Human PKR protein purified from E. coli is shown by SDS-PAGE and Coomassie Blue staining. (f) The 27 extra nucleotides RNA circle doesn’t suppress PKR phosphorylation. Purified PKR (0.6 μM) is activated by 79 bp dsRNA (0.01 μM) in vitro, which is shown by autoradiography using γ-32P-ATP. 0.01 μM of RNA circles are used in the assays. (g) EPIC suppresses mouse PKR phosphorylation efficiently. Left, purified mouse PKR protein is shown by SDS-PAGE and Coomassie Blue staining. Right, the activation of mPkr (0.6 μM) by 79 bp dsRNA (0.01 μM) in vitro, is inhibited by EPIC (0.01 μM). c, f and g: n.s., p > 0.05, *p < 0.05, **p < 0.005, ***p < 0.001, two-tailed student’s t test, data are shown as mean ± SD.
Extended Data Fig. 2 Stable interactions of ds-cRNAs and PKR resolved by single molecule imaging.
(a) Cy5 and biotin labeling did not alter the EPIC conformation in general. SHAPE reactivity of each group was quantified at the single nucleotide resolution, and ΔSHAPE was calculated accordingly and plotted in absolute values. (b) The binding frequency between PKR and examined RNAs by smTIRF. The numbers of RNAs counted in each column with three independent replicates: 79 bp dsRNA, n = 265, 108, 157; 33 bp dsRNA, n = 204, 179, 135; 73 nt hairpin RNA, n = 207, 281, 394; 23 bp duplex RNA, n = 178, 277, 363; EPIC, n = 134, 150, 178; circSMARCA5, n = 67, 101, 78. The binding frequency of PKR on each RNA: 79 bp dsRNA, 62.9%; 33 bp dsRNA, 37.3%; 73 nt hairpin RNA, 39.4%; 23 bp duplex RNA, 11.6%; EPIC, 22.4%; circSMARCA5, 1.5%. Data are shown as mean ± SD. (c) A similar robust binding between PKR and circCAMSAP1. Left, representative kymograph of circCAMSAP1-Cy5 binding with PKR-Cy3 in separated and merged channels. Right: quantification of the PKR-Cy3 intensity on a single RNA molecule within 120 s. (d) HMM has been applied to analysis single-step photobleaching data. Trace line represents raw PKR-Cy3 trajectory of one exemplary molecule recorded by smTIRF. Means line represents the idealized trajectory determined by HMM. (e) The interaction between EPIC and PKR-ΔIDR is similar with WT PKR. Left, the construction of WT PKR and PKR-ΔIDR. Middle, the binding frequency between EPIC and PKR (or PKR-ΔIDR). The numbers of RNAs counted in each column with three independent replicates: PKR, n = 287, 201, 350; PKR-ΔIDR, n = 237, 371, 299. Data are shown as mean ± SD. Right, the number of PKR-ΔIDR binding on single EPIC is calculated based on the step analysis of PKR-ΔIDR-Cy3 photobleaching. n = number of events examined.
Extended Data Fig. 3 Chemical probing and docked structure reveal the potential binding mode between one EPIC with two PKR molecules.
(a) The SHAPE signal difference (ΔSHAPE value) of EPIC with or without PKR protein in vitro. Top, SHAPE reactivity of each group is quantified. Bottom, ΔSHAPE value of EPIC with or without PKR protein in vitro. Yellow shadow indicates the nucleotides of 23 bp imperfect dsRNA-region. Regions with significant SHAPE augment upon PKR binding are in purple, regions with reduced SHAPE signals upon PKR binding are in green and marked by pink shadow. (b) Predicted secondary structure of EPIC based on circSHAPE-MaP signals. Of note, EPIC contains one imperfect dsRNA region sufficient for PKR binding (yellow shadow, 23 bp). The heavy pink lines denote the altered NAI accessibility in EPIC upon PKR addition. (c) Predicted EPIC structure and the binding with PKR. Top left, domains of PKR and the schematic pipeline for docking EPIC on PKR. Bottom left, the most possible 3D structure of EPIC predicted by 2D structure with circSHAPE-MaP signal. Right, the binding modes of 79 bp dsRNA (top) and EPIC (bottom) docked with two PKR molecules predicted by AlphaFold2. The 23 bp imperfect dsRNA region is labeled in yellow, and the nucleotides with altered SHAPE reactivities upon PKR binding are labeled in red.
Extended Data Fig. 4 CircRNAs are globally degraded at the initial inflammation stage in IMQ-induced psoriasis mice model.
(a) Phenotypes of IMQ-induced psoriasis mice model. Left, representative image of mice showing skin inflammation after IMQ treatment on dorsal skin, compared to Vaseline treatment or blank control. Right, quantification of the spleen size at indicated time points upon IMQ treatment. n = 4 or 5 animals in each group. (b) Increased expression of psoriasis-related genes with distinct patterns. Relative expression levels of the psoriasis-related genes (mIl17a and mIl23a), mPkr and mIl6 were examined by RT-qPCR. The expression is normalized to D0 expression. n = 4 or 5 animals in each group and mActin is used as an internal control for normalization. (c) Activation of RNase L was measured by RT-qPCR combined with RctB ligation. (d) Increased epidermis thickness of dorsal skins from IMQ-treated mice. n = 3 animals in each group. (e) Expression level of 721 HC circRNAs and their cognate linear RNAs in IMQ treated mice. The value of each time point is the mean from two repeats, and each repeat includes 2 biologically independent animals. Data are shown as median and IQR. Wilcoxon rank-sum test, ***p < 0.001. (f) Validation of randomly selected circRNAs, their cognate mRNAs and pre-mRNAs at indicated time points in IMQ treated mice. Expression of these RNAs were examined by RT-qPCR and normalized to mActin. Data are shown as mean ± SD.
Extended Data Fig. 5 Enrichment analyses reveal up-regulated pathways in the initial stage of psoriasis pathogenesis.
(a) Schematic of clustering the fast-responding inflammatory factors including PKR. 787 DEGs are defined as over threefold up-regulation at D1 compared to D0 in two biological repeats. All DEGs are performed the KEGG pathway enrichment analysis. (b) The KEGG analyses of 151 genes from Cluster 1. (c) A short list of the most enriched genes of Cluster 1 which belong to the IFNα signaling pathways and the dsRNA-sensors related genes. (d)-(h) The enriched KEGG pathways of genes from the other 5 clusters of distinct gene expression patterns in IMQ-induced psoriasis mice model. b, d, e, f, g, h: all pathways are selected according p.adjust < 0.05 and the pathways related to viral or bacterial infection are not shown in Cluster 1.
Extended Data Fig. 6 Generation of Pkr-/- mice.
(a) Generation of Pkr-/- mice by CRISPR/Cas9. Top, the schematic of generating Pkr-/- mice. Bottom left, the wiggle track of RNA-seq data of total RNAs collected from WT and Pkr-/- mice spleens. Bottom right, WB verification of mPkr loss. (b) The expression of circRNAs and their cognate linear RNAs in Pkr-/- mice with IMQ treatment. FPBcirc values (top), FPBlinear value (middle) and CIRCscore (bottom) of 721 HC circRNAs in each indicated time points, related to Fig. 3c and Extended Data Fig. 4e. Each corresponding group is calculated mean from two biological repeats, and each repeat with 2 biologically independent animals. Data are shown as median and IQR. Wilcoxon rank-sum test, ***p < 0.001. (c) Heatmap of selected categories of psoriasis-related gene expression from the IMQ-treated spleens of Pkr-/- and paired WT mice. Each repeat includes two biologically independent animals. The expression of each gene is scaled by z-score and each category is shown in the same scale bar, indicated by intervals.
Extended Data Fig. 7 Generation of the in vivo over-expressed human circPOLR2A (9,10) mice model (C_OE mice).
(a) Schematic of generating C_OE mice carrying the circPOLR2A (9,10) constitutive expression cassette. (b) NB analysis showed constitutive human circPOLR2A (9, 10) production in all examined tissues in the EF1α promoter and CAG promoter driven C_OE mice. (c) NB analysis confirmed the expression of human circPOLR2A (9, 10) in the liver of EF1α and CAG C_OE mice, respectively. (d) EF1α C_OE mice exhibited unobservable abnormalities compared to WT mice, here showing the weight analysis of WT and the littermate EF1α C_OE mice from 4 to 60 weeks. Each dot represents one individual animal (n ≥ 3) at each examined time point. n.s., p > 0.05, two-tailed student’s t test, data are shown as mean ± SD. (e) Generation of the in vivo over-expressed human circPOLR2A (9,10) R1 cell lines. (f) Schematic of calculating the copy number of human circPOLR2A (9, 10) in C_OE mice, relative to OE_C R1 cells in e.
Extended Data Fig. 8 In vivo over-expression of the human circPOLR2A (9, 10) mitigates the IMQ-induced psoriasis pathogenesis.
(a) Human circPOLR2A (9, 10) dampens the expression of inflammatory factors in IMQ mice. Relative expression of representative IFNα signaling pathways in C_OE mice (n = 6), compared to WT mice (n = 5) on D1, examined by RT-qPCR. n.s., p > 0.05, *p < 0.05, **p < 0.005, two-tailed student’s t test, data are shown as mean ± SD. (b) Human circPOLR2A (9, 10) dampens PKR activation in IMQ mice. PKR activation kinetics were analyzed in C_OE and WT mice on D2, ActB was used as the internal control. (c) RNase L activation on D1 led to reduced circPOLR2A (9,10) in C_OE mice. (d) Relative expression levels of the circPOLR2A (9,10) were examined by RT-qPCR in IMQ mice. The expression of circPOLR2A (9,10) is normalized to D0 and mActin is used as an internal control for normalization.
Extended Data Fig. 9 Ds-cRNAs are encapsulated by ionizable LNPs.
(a) Schematic of a microfluidic mixing preparation of circular RNA-encapsulated ionizable-based LNPs. (b) Physicochemical characterization of ds-cRNA-encapsulated ionizable-based LNPs. The particle size, PDI, Zeta potential and encapsulation efficiency of EPIC-LNPs were measured in three replicates. Data are shown as mean ± SD. (c) Transmission electron micrographs of EPIC-LNPs. (d) Examination of endogenous circRNA expression upon ds-cRNA treatment by RNA-seq. Blue dots, quantification of total RNAs from spleens of IMQ mice without EPIC-LNPs. Orange triangles, quantification of total RNAs from spleens at 24 hrs post i.v. of 150 pmol EPIC-LNPs at D1.5 IMQ mice; of note, EPICs were counted by FPBcirc in these samples. Blue triangles, quantification of total endogenous RNAs from same samples shown by Orange triangles, but leaving out EPICs during calculation by FPBcirc. EPIC-LNPs treatment restored the reduced endogenous circRNA almost to the D0 level.
Extended Data Fig. 10 Expression of fast-responding inflammatory factors is elevated in psoriasis patients and the ds-cRNA treatment is beneficial in patient-derived PBMCs.
(a) The expression of circRNAs is reduced in the lesional skin samples, compared to the non-lesional skin samples. Total expression level of circRNAs from lesional and non-lesional skin samples derived from 27 psoriasis patients19,20 are analyzed. Top, total FPBcirc values of all circRNAs; median, total FPBlinear values of all circRNAs cognate linear RNAs; bottom, total CIRCscore values of all circRNAs. Wilcoxon rank-sum test, n.s., p > 0.05, ***p < 0.001. (b) Heatmap of the psoriasis-related genes of lesional and non-lesional skin in psoriasis patients. The expression level of each gene is scaled by z-score and each category is shown in the same scale bar, indicated by intervals. (c) EPIC-LNPs suppresses the expression of psoriatic signature genes in PBMCs isolated from psoriasis patients. 1 pmol of EPIC was delivered into roughly 106 cells. Each dot represents one patient sample. n.s., p > 0.05, *p < 0.05, paired student’s t test.
Supplementary information
Supplementary Table 1
The circRNA expression in IMQ-induced psoriasis mice.
Supplementary Table 2
The linear RNA expression in IMQ-induced psoriasis mice.
Supplementary Table 3
Gene expression cluster and KEGG pathway in IMQ-induced psoriasis mice.
Supplementary Table 4
RNA-seq profile of patients with psoriasis (linear RNA).
Supplementary Table 5
RNA-seq profile of patients with psoriasis (circRNA).
Supplementary Table 6
General information of psoriasis donors.
Supplementary Table 7
List of construct, probe and primer sequences.
Supplementary Table 8
Endogenous circRNA expression of psoriasis mice delivered EPIC-LNPs.
Source data
Source Data Fig. 1
Unprocessed western blots and/or gels associated with the data presented in main and extended data figures.
Source Data Fig. 2
Statistical source data associated with the data presented in main and extended data figures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, SK., Liu, CX., Xu, YF. et al. Therapeutic application of circular RNA aptamers in a mouse model of psoriasis. Nat Biotechnol (2024). https://doi.org/10.1038/s41587-024-02204-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41587-024-02204-4