Abstract
A central goal in ecology is to understand what maintains species diversity in local communities. Classic ecological theory1,2 posits that niches dictate the maximum number of species that can coexist in a community and that the richness of observed species will be below this maximum only where immigration is very low. A new alternative theory3,4 is that niches, instead, dictate the minimum number of coexisting species and that the richness of observed species will usually be well above this because of ongoing immigration. We conducted an experimental test to discriminate between these two unified theories using a manipulative field experiment with tropical intertidal communities. We found, consistent with the new theory, that the relationship of species richness to immigration rate stabilized at a low value at low immigration rates and did not saturate at high immigration rates. Our results suggest that tropical intertidal communities have low niche diversity and are typically in a dispersal-assembled regime where immigration is high enough to overfill the niches. Observational data from other studies3,5 suggest that these conclusions may generalize to other ecological systems. Our new experimental approach can be adapted for other systems and be used as a ‘niche detector’ and a tool for assessing when communities are niche versus dispersal assembled.
Similar content being viewed by others
Main
A central unresolved question in ecology concerns the factors that drive and maintain species diversity in local communities. Under niche-assembly theory, species richness is explained in terms of stable coexistence attributable to properties of the local environment: species differ in their niches and these differences collectively stabilize community dynamics. Under dispersal-assembly theory, including MacArthur and Wilson’s theory of island biogeography1,2, the neutral theory of biodiversity6 and mass effects7, local diversity arises from an immigration–extinction balance and is ultimately dependent on regional processes.
Which of these two distinct perspectives is more correct is likely to depend on the context8,9,10,11,12,13. One critical mediating factor is the immigration rate to a community. Under pure niche assembly, there should be no relationship between species richness and the immigration rate (Fig. 1a). Under pure dispersal assembly, the relationship should be monotonically increasing (Fig. 1b). A classic unification of the two theories postulates that dispersal assembly applies only in cases where immigration is too low to fill all the niches (Fig. 1c). Indeed, MacArthur14 and Wilson15 speculated that the immigration–extinction balance and dispersal assembly central to their theory of island biogeography1,2 applied only below some threshold immigration rate, above which species richness would be determined mainly by habitat diversity, that is, niche assembly14 or by the diversity of the source species pool15. Under this hypothesis, the relationship between species richness and the immigration rate is predicted to increase for low immigration rates and then saturate at high immigration rates15,16,17 (Fig. 1c). This saturation point reflects the maximum possible number of species or the niche diversity of the island or patch—a ceiling that cannot be exceeded even with greater levels of immigration14.
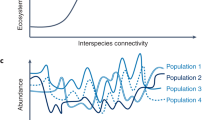
a, The niche-assembly hypothesis predicts constant species richness (\(S\)) with immigration rate (\(h\)). b, The dispersal-assembly hypothesis predicts a monotonically increasing relationship. c, A classic unifying hypothesis proposes that dispersal assembly applies under low immigration and niche assembly at high immigration. d, A new unifying hypothesis predicts the opposite. The dashed lines in panels c and d represent the transition points between the two assembly regimes.
A recently formulated new unified hypothesis3,4, however, predicts just the opposite: that niche assembly applies where immigration is low and dispersal assembly applies where immigration is high (Fig. 1d). Related ideas go back to Diamond’s community assembly rules18, the core–satellite hypothesis19 and source–sink dynamics20. Under this hypothesis, niches stabilize diversity at some fixed level and provide a floor on species richness where immigration is low, but, as immigration increases, communities undergo a transition from a niche-assembled regime to a dispersal-assembled regime (Fig. 1d). The relationship of species richness to immigration rate is predicted to be nearly flat for low immigration rates and increasing for high immigration rates (Fig. 1d): that is, species richness asymptotes at low immigration rates rather than high immigration rates as in the classic hypothesis (Fig. 1c). The transition point occurs where immigration becomes sufficient to overwhelm the niche constraints, which leads to a dynamic immigration–extinction equilibrium with more species than niches.
Which of these two contrasting unified hypotheses is correct has fundamental consequences for our understanding of the forces that structure ecological communities. The two hypotheses make very different predictions about the shape of the immigration–species richness curve, but there has not yet, to the best of our knowledge, been any experimental test to discriminate between them. A key challenge in experimentally testing the transition between dispersal- and niche-assembled regimes is the lack of foreknowledge as to the range over which the immigration rate should be varied to reveal both regimes (Fig. 1). In deterministic theoretical models, the niche-assembled regime can be uncovered by simply setting immigration to zero, but, in stochastic models or real experimental settings, this strategy fails because even in the niche-assembled regime, occasional immigrants are needed to offset stochastic extinctions. One feasible experimental approach is to vary the immigration rate across treatments with the rate approaching but not equal to zero in the low-immigration treatments. This is analogous to the mathematical approach of taking the limit as immigration tends to zero to avoid a singularity at zero. Ecologists acknowledge that immigration can in general influence community species richness2,7,11, but the key to our experimental approach is that we systematically vary immigration over a wide range in this way to reveal the functional form of the species richness versus immigration relationship and thus explain underlying mechanisms. Here, we use this approach to conduct a crucial experimental test of the predictions of the classic and new hypotheses to assess the conditions under which ecological communities are primarily niche versus dispersal assembled (Fig. 1).
Experimental design
We performed a manipulative field experiment on intertidal communities21,22,23. We focused on intertidal seawalls—a well-studied tractable model system24,25,26 (Methods). We experimentally manipulated the level of immigration across experimental habitat patches using custom-built set-ups (Fig. 2). Each set-up was a treatment replicate with two components: (1) a topographically complex concrete habitat tile that provided a standardized level of niche diversity across all replicates (Fig. 2a and Methods); and (2) a stainless-steel cage, with clear square polycarbonate sheet panels (slotted into the cage), that fitted over each tile to create a seal to ensure that the immigration of organisms to and from each unit was only via fixed-size holes (40 mm diameter) cut into the clear panels (Fig. 2b and Methods). Each set-up was submerged at high tide (95.2% of the time) and exposed at low tide (4.8% of the time; see Methods). We created a total of 12 treatments by varying the number of holes (\(h\)) in the clear polycarbonate panels (Fig. 2c).
a,b, Each set-up comprised two components: a habitat tile, used to standardize habitat surface area and structural complexity across replicates (a); and a custom-built cage that fitted securely over the habitat tile to create a seal, with slots that allowed five clear polycarbonate sheet panels to be inserted and removed for regular maintenance (b). c, The level of immigration was controlled by varying the total number of holes h on the clear polycarbonate panels. We had 12 \(h\)-treatments (grey boxes; \(h\,\)= 1, 2, 3, 4, 5, 8, 10, 12, 15, 20, 30, 45) and five replicates (\(n\,\) = 5) of each treatment set-up. The five white squares within each grey box show the number of holes and their arrangements for each of the five panels used to create the corresponding treatment. Panels in the first row were the top panels.
We installed a total of 60 experimental units (12 \(h\)-treatments × 5 replicates) in randomized order along the base of intertidal seawalls (approximately 0.5 m above chart datum) across a 400 m stretch at our study site in Singapore (Methods). To maintain the set-ups over the duration of the experiment, polycarbonate panels were removed and replaced with clean new ones every two to four weeks during low tide. We censused the number of species, species identities and abundances of the macrofaunal intertidal communities within each set-up every month for a year (Methods). Although we focused on macrofaunal intertidal species for practical reasons, we expect that qualitatively similar results would be obtained if we included algal and microbial communities. In total, we recorded 10,156 individuals of 64 different species: major taxa included gastropods, bivalves, polychaetes, tunicates and crustaceans (see full list in Supplementary Table 1). Species richness stabilized in all but the highest immigration treatments after six months (Extended Data Fig. 1), consistent with findings from previous studies carried out at the same study site and at nearby locations25,26,27. We thus used data from months 7 to 12 in our analyses (Extended Data Fig. 1). We fitted mechanistic mathematical models corresponding to each of the two hypotheses to the data by generalized nonlinear least squares (Methods). The classic model has two fitted parameters (the ratio of the immigration and extinction rates and metacommunity richness) and the new model has three (immigration rate parameter, niche diversity and the fundamental biodiversity number; see Methods). We assumed that immigration was linearly related to the number of holes (\(h\)), a parsimonious assumption that, if relaxed, only strengthens our results (Methods). We performed 1,000 bootstraps stratified by hole treatments to obtain 95% confidence intervals on the parameter estimates and fitted values (Methods).
Evidence of the transition
The relationship of species richness to immigration rate across experimental treatments was consistent with the new hypothesis, with a nearly flat phase at low immigration rates and an increasing phase at high immigration rates (Fig. 3; Akaike information criterion, AIC = 974.0). The fit of the classic model to the data was substantially poorer (Fig. 3, AIC = 1066.3; see also Extended Data Fig. 2, for a variant of the classic model). The new model explained 83% of the variation in the data. This provides experimental evidence of the transition from niche to dispersal assembly in ecology. The estimated niche diversity was \({n}^{* }=4.4\) with a 95% confidence interval of [4.2, 5.2], indicating that approximately four or five species can stably coexist in the seawall tile communities in the absence of substantial immigration, with species richness close to this minimum value being realized in the lowest immigration treatments (\(h=1,\,2,\,3,\,4\) and \(5\)). The transition to the dispersal-assembly regime occurred at intermediate immigration rates (\(h\approx 10\)) and roughly three times the minimum number of species were present in the highest immigration treatment (\(h=45\)). The effect of increasing species richness was partly mediated by increasing total abundance with immigration among the high-immigration treatments and partly by increasing species richness given total abundance (Fig. 4).
Each small grey point indicates a single experimental unit (seawall tile; 12 \(h\)-treatments, \(n\,\) = 5) in a given month (x coordinates are jittered to improve visibility); each large black point shows a treatment mean. Fitted models are shown by the solid curves (mean estimates) with shaded 95% confidence intervals from 1,000 bootstraps; the new model (equation (6)) is shown in red whereas the classic model is in blue (equation (3); see Methods). Consistent with the new model, the relationship of species richness to immigration in experimental intertidal seawall communities is biphasic and asymptotes to a low value at low immigration rates, indicating that, for low immigration rates, communities are primarily niche assembled and comprise approximately four or five species (dashed red horizontal line) and that, for high immigration rates, communities are primarily dispersal assembled. Under the new model, hypothetical species richness due to niche assembly alone is constant and close to richness in the low-immigration treatments (dashed red horizontal line). The classic model was substantially poorer than the new model (judged by Akaike information criterion, AIC). The dashed blue horizontal line indicates the expected species richness at high levels of immigration under the classic model, as estimated by the fitted model.
a, Average number of individuals (\(N\)) for each value of immigration (\(h\)). b, Average rarefied species richness for each value of immigration (\(h\)), using various rarefaction sample sizes (\(n\)).
Discussion
Our experimental results are consistent with the new hypothesis, that is, that communities are niche assembled under low immigration and are otherwise primarily dispersal assembled3,4. This is consistent with previous observational evidence of a biphasic species–area relationship in island archipelagos, where area is interpreted as a proxy for immigration3. However, the island data, being observational, are subject to alternative interpretations5 because confounding variables, such as niche diversity, can covary with area7. Our experimental approach avoids this by standardizing area across all treatments and thus provides a more direct test of theoretical principles about the effects of immigration on community assembly. We note that species richness was still increasing slowly and did not fully equilibrate in our highest immigration treatments (\(h=30,\,45\)) after six months (Extended Data Fig. 1): allowing it to equilibrate would only strengthen our conclusions by accentuating the biphasic relationship of species richness to immigration rate (species richness would be higher towards the right of Fig. 3).
Our new experimental approach can be adapted to other systems such as annual plant communities28 and microbial communities29 in laboratory conditions. In addition to providing additional tests of our two central hypotheses (Fig. 1c,d), the experimental approach can be used to assess when communities are niche versus dispersal assembled—thereby shedding light on a question that has perplexed ecologists for decades. The experiments also act as a ‘niche detector’, that is, a tool for assessing how many species can coexist without substantial immigration—another long-standing ecological conundrum.
Our experimental data, taken together with past observational and theoretical studies, suggest that niche diversity in ecological systems is typically low. The estimated niche diversity of our intertidal seawall model system was low (\({n}^{* }=4.4\)) and we predict that similar values would be obtained in experiments on the natural analogues of our system, tropical rocky shores. Low niche diversity for a variety of ecological communities is also implied by island species–area relationships, which typically exhibit a similar shape to our species–immigration relationship (Fig. 3), with a flat low-richness phase at low island areas attributable to niche assembly under low immigration3. This flat phase has long been known as the ‘small-island effect’30,31, but lacked a satisfactory dynamical explanation until recently3. Theoretical models also suggest that the number of species that can coexist via local niche mechanisms is generally low. Although, in some theoretical models, a large number of species can stably coexist via temporal niches arising from relative nonlinearity32,33 and storage effects34, such coexistence is fragile and not robust to stochasticity and perturbations, which raises doubt about its relevance in nature35.
We predict that applications of our experimental approach to other systems will lend further support to the hypothesis that niche diversity is generally much lower than the typical total species richness of natural habitats, which implies that most species are not stably coexisting but, instead, are transiently co-occurring and are reliant on continued immigration8 (that is, mass effects7). A corollary is that most natural communities are in the dispersal-assembly regime. In rocky intertidal communities characteristic of our study site, species richness is invariably much higher than observed in our niche-assembly regime36,37. We conjecture that these conclusions also apply to highly diverse systems such as tropical rainforest tree communities, where hundreds of species may be present in a single hectare38. A prominent niche-assembly explanation for high tree species richness is that predators and pathogens are largely host-specific, which leads to a high diversity of enemy-escape niches39. By contrast, the dispersal-assembly explanation attributes high local tree species richness to immigration, as well as to potentially larger-scale niche processes (for example, source–sink dynamics20, with species being adapted to distinct habitats across the landscape) and, ultimately, to diversification processes that play out over geological timescales and continental spatial scales. Although our experimental approach would be impractical in tropical forests because of long tree generation times, the question of what determines high tree diversity could be indirectly informed by evidence from other, more experimentally tractable, high-diversity communities, such as plankton communities33,40. If plankton communities collapse to a small number of species under low immigration, similar to our seawall communities (Fig. 3), this would cast doubt on the general notion that an observation of high species diversity implies high niche diversity.
Given the evidence for two distinct assembly regimes, we encourage future experimental work—whether on intertidal systems or other communities—to carefully distinguish which regime prevails in any given context and to study each regime separately. In the niche-assembly regime, although we observed some consistency in the set of coexisting species (for example, the gastropod Drupella margariticola and the tunicate Didemnum psammatodes), our experimental set-up was not designed to unravel the precise niche-assembly rules. Figuring out the exact niche mechanisms operating in this regime is a priority for future research. Experiments that directly manipulate the species identities of immigrants can potentially address this (as done in microbial communities29). An alternative would be to spread the experimental units over a much larger geographical area to capture a larger species pool. For our study system, our estimate of the transition point between the two regimes (\(h\approx 10\)) can inform the design of future studies focused on one regime or the other. Our results can also inform sample size determination for future studies focusing on more-detailed biodiversity patterns including co-occurrence matrices and species abundance distributions. One assumption of our method is consistency of abiotic conditions across experimental immigration treatments. We confirmed this for light and temperature (Methods), but it remains conceivable that other variables (for example, pH) are important and we encourage future similar experiments to measure as wide a range of abiotic variables as possible. More broadly, to understand the dispersal-assembly regime, ecologists must design more cross-scale studies that link local and regional dynamics: it is increasingly clear that the factors driving the diversity of a small local community cannot be unravelled by studying the system in isolation, that is, without accounting for immigration9,11. A holistic assessment of the dispersal-assembly regime should also account for niche processes that may not on their own permit stable coexistence (as in the niche-assembly regime) but nevertheless slow competitive exclusion and thus act synergistically with immigration to increase species richness41,42. Such synergies between immigration and niche processes may turn out to be more important for diversity than traditional stable coexistence.
Our results have practical implications for the use of concrete tiles to augment diversity on man-made coastal defences in many parts of the world—a strategy known as eco-engineering or nature-based solutions43,44. The predominant role of dispersal assembly in our system indicates that such efforts may be ineffective unless attention is also paid to habitat preservation and connectivity in the broader landscape to ensure a large, diverse, continued supply of immigrants. Regardless of their topographic complexity, concrete tiles placed in a depauperate intertidal landscape will harbour few species and this needs to be considered more explicitly, especially in real-world applications. More broadly, the importance of dispersal assembly and the apparent low number of stabilized niches cautions against conservation approaches that rely too strongly on niche mechanisms for preserving species diversity45. Efforts to protect biodiversity must account for large-scale landscape connectivity to be successful—although we encourage further experimental tests of the new hypothesis in other systems and locations to establish the generality of this conclusion.
The emerging picture, not only from our experimental study but also from observations on island archipelagos and from theoretical work, is that dispersal assembly is the prevailing regime for most ecological communities and that the number of stabilized niches is low. This may explain the relative success of niche theory in low-diversity experimental systems28,46,47 but its failure to provide a predictive framework in most natural systems, especially in high-diversity systems such as tropical rainforests and coral reefs48,49,50. The quest for a general understanding of biological diversity would benefit from more experimental tests of which assembly regime—niche or dispersal—prevails in different contexts48.
Methods
Study system
We conducted the study on granite rip-rap seawalls in Singapore. Singapore is a small (728.6 km²) tropical island city-state in Southeast Asia: it comprises a mainland (approximately 710 km²), which is approximately 50 km from east to west and 26 km from north to south, and 64 smaller offshore islands, including Sentosa island where the present study was conducted (specifically, at 1° 15′ 00.4′′ N, 103° 49′ 04.0′′ E). More than 80% of Singapore’s natural coastline has been modified and replaced by granite rip-rap seawalls51. Natural rocky shores are now limited to a short 300-m stretch along the southern shore and a few islands south of Singapore’s mainland (including parts of Sentosa island). Located just one degree above the equator, our study system experiences a typical tropical climate: high and uniform temperatures, abundant rainfall and high humidity all year round. For the duration of the study, from March 2021 to March 2022, the average reported temperature was 27.9 °C and the average monthly precipitation was 19.6 cm at our study site52. Facing the Singapore Strait, our study system is an open hard-bottomed system in a highly urbanized marine environment that experiences a mixed semi-diurnal tidal regime51,53.
Monthly biodiversity surveys over a year conducted in ref. 36, which included our study location, found that, whereas composition of the communities colonizing seawalls and rocky shores differed, the two habitats harboured similar suites of species. This pattern is consistent with hard-bottom benthic systems around the world, including those in temperate and sub-tropical regions24,54,55. The study36 also found no significant temporal patterns in species assemblages (that is, our study system can be considered aseasonal), with molluscs, crustaceans and algae dominating all year round (see also ref. 56). Although rocky shores often host greater species richness compared with seawalls, many of the species unique to either habitat are rare species: for instance, in their monthly surveys over a year, ref. 36 found less than ten individuals for all unique species in either habitat. What is notable is that, although seawalls are artificial, in the tropics they can still harbour relatively high species numbers: in Singapore, the authors of ref. 36 found a total of 138 species on natural rocky shores versus 105 species on seawalls through their year-long survey. Communities on aseasonal tropical hard shores also undergo relatively rapid rates of succession27,57,58 compared with temperate and seasonal tropical hard shores59,60.
The seawalls at our study location were constructed from boulders approximately 50 years ago and are not grouted with concrete or any other fill material61. These boulders are not regularly replaced or maintained, due to the ideal strength and material properties afforded by granite and the relatively low wave energy in the meso-tidal coastal environment of Singapore51,62,63. The biota on these hard structures, which act as the source pool for immigrants to our experimental units, can thus be considered climax communities24,57.
Habitat tile design and fabrication
Concrete tiles (Fig. 2a) measuring 0.2 m × 0.2 m × 0.06 m (width × length × depth) were used as habitat units from which we sampled our ecological communities. This approach is advantageous as it allows us to standardize habitat area and niche diversity across all replicates. Only one tile design was used and it comprised two microhabitat component types relevant to intertidal organisms: square pits and grooves25,26,64. These structural components varied in their sizes and depths from 0.01 m to 0.04 m; specifically, pits were first positioned randomly on the tile, after which we overlaid the remaining unoccupied space with grooves using a Truchet tiling algorithm. This was done using the three-dimensional modelling software Rhino 6. Once the tile design was finalized, the model was three-dimensional printed and from this rubber moulds were made. The actual experimental tiles were then cast from the moulds using concrete (1:3 cement to sand mix)25. During casting, four stainless-steel M8 hex nuts were embedded into the tile to act as insets for attaching the stainless-steel cage set-up to each tile unit using stainless-steel bolts (Fig. 2b). Temperature and light conditions in the experimental units were comparable with the surrounding seawall (Supplementary Fig. 1). Previous studies conducted worldwide65 and in Singapore (including our study location; for example, refs. 25,26,27,57,58) have shown tiles to be an effective experimental device for sampling the benthic macrofaunal diversity of these tropical intertidal communities.
Determining diameter of hole on panels
To determine the diameter of holes on panels used in the experimental set-up (Fig. 2), we conducted a field survey of the study site (before the start of the experiment, in February 2021), as well as an adjacent natural rocky shore site located further along the same shoreline approximately 1 km away. Five 1 m2 quadrats were placed randomly along a 100 m transect at the mid-tidal height (approximately 0.5 m above chart datum; that is, along the same tidal height as in our experiment) at each site. From each quadrat, we counted the number of individuals of all macrofauna (greater than 1 mm) found within the 1 m2 quadrat and measured the maximum body length of each individual using a digital Vernier caliper (with a resolution of 0.01 mm). A total of 245 individuals were recorded. The mean maximum body length of individuals was 16.8 mm (the mean was 15.0 mm for seawalls and 18.4 mm for rocky shores; Supplementary Fig. 2). Only two of the 245 individuals (less than 1%) had maximum body lengths greater than 40 mm (these two were gastropod species: Chicoreus capucinus (a whelk), measuring 42.8 mm, and Peronia verruculata (an onch slug), measuring 40.4 mm). Note, however, that their widths were less than 40 mm and that both of these species were present in the actual experimental communities.
Data analyses
We fitted formulas arising from mechanistic mathematical models corresponding to each of the two hypotheses to the data on species richness S versus number of holes h, using generalized nonlinear least squares (function gnls in the R programming language66). The classic model formula arises from a dynamic model describing how the rate of change of species richness on an island depends on the immigration and extinction rates, yielding the equilibrium solution15,16,67.
where \(P\) is the number of species in the metacommunity, \(\lambda \) is the per-species immigration rate and \(\mu \) is the per-species extinction rate. In the first version of this model15, the extinction rate is assumed to be inversely proportional to the area of the island (\(\mu ={\mu }^{{\prime} }/A\)), giving
In our study system, area \(A\) is constant and we assume that the immigration rate is proportional to the number of holes \(h\), so that \(\lambda =\alpha h\) for some parameter \(\alpha \). Writing \({\alpha }^{{\prime} }=\alpha A/{\mu }^{{\prime} }\) then gives a two-parameter relationship between \(S\) and \(h\):
In the second version of this model16,67, the extinction rate is, instead, assumed to be proportional to the number of species currently on the island (\(\mu ={\mu }^{{\prime\prime} }S\)), giving
Again setting \(\lambda =\alpha h\) and writing \({\alpha }^{{\prime\prime} }=\alpha /{\mu }^{{\prime\prime} }\), gives another two-parameter relationship between \(S\) and \(h\):
Under the alternative new hypothesis, a mathematical formula for the relationship of species richness to number of holes arises from a master equation describing neutral drift within each of a fixed number of equal-sized non-overlapping niches in a local community68,69. Occasional stochastic extinction is balanced by immigration of new individuals from a larger metacommunity. The model has four parameters: the number of niches \({n}^{* }\), the local community size \(J\), the probability that a new individual is an immigrant \(m\) and the fundamental biodiversity number \(\theta \) of the metacommunity from which immigrants are drawn. Species richness at the dynamic equilibrium is3
where \({\psi }_{0}\) is the digamma function70, \({\gamma }^{* }=(\,{J}^{* }-1)m/(1-m)\) is a composite immigration parameter and \({J}^{* }=J/{n}^{* }\) is the number of individuals in each niche. The original derivation of equation (6) assumed death–birth dynamics, where a death event leads to a vacated space that can be filled by the birth of a new individual, but the same equilibrium solution arises from birth–death dynamics where a newly born individual displaces and kills an established individual. Other variants of the standard model, for example, with abundance measured as biomass instead of number of individuals, lead to similar equilibrium solutions. To relate the composite immigration parameter \({\gamma }^{* }\) to the number of holes in our seawall experiment, we assume that the number of immigrants per unit time is proportional to the number of holes and that the number of local births in a given niche is proportional to the number of individuals residing there (minus one for the individual that just died to vacate a space), so that the probability of immigration is \(m=bh/(bh+c(\,{J}^{* }-1))\) for some constants \(b\) and \(c\), which leads to \({\gamma }^{* }=bh/(c{n}^{* })\). We then define the composite parameter \(\alpha =b/(c{n}^{* })\) to get \({\gamma }^{* }=\alpha h\). Given data on \(J\), the model then has three free parameters, \(\alpha ,\,{n}^{* }\) and \(\theta \), which can be estimated from data on \(S\) and \(h\).
Note that in the new model the per-individual immigration rate scales linearly with number of holes, but in the classic model it is the per-species immigration rate that scales in this way. We argue that the novel model is more realistic because the per-species rate is likely to exhibit a convex relationship with number of holes (similar to a species accumulation curve). Modifying the classic model to allow a convex relationship would be likely to reduce the quality of its fit to the data (because it would be harder for it to capture the observed upswing in species richness at high numbers of holes), but we chose not to do this because the linear relationship is consistent with Wilson’s original conceptualization15 and because we wanted to conduct a conservative test of the new hypothesis.
We fitted each of the three models (two versions of the classic model, equations (3) and (5); and the new model, equation (6)) to the data by generalized least squares. After fitting each model, we performed 1,000 bootstraps stratified by hole treatments to obtain 95% confidence intervals on the parameter estimates and fitted values of species richness. The distribution of errors for each model was close to Gaussian in all cases (Supplementary Fig. 3).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data generated in this study are available in the Zenodo repository: https://doi.org/10.5281/zenodo.7819940. Source data are provided with this paper.
Code availability
The R code used for data analysis and for producing the figures are available in the Zenodo repository: https://doi.org/10.5281/zenodo.7819940.
References
MacArthur, R. H. & Wilson, E. O. An equilibrium theory of insular zoogeography. Evolution 17, 373–387 (1963).
Macarthur, R. H. & Wilson, E. O. The Theory of Island Biogeography (Princeton Univ. Press, 1967).
Chisholm, R. A., Fung, T., Chimalakonda, D. & O’Dwyer, J. P. Maintenance of biodiversity on islands. Proc. R. Soc. B: Biol. Sci. 283, 20160102 (2016).
Chisholm, R. A. & Fung, T. Examining the generality of the biphasic transition from niche-structured to immigration-structured communities. Theor. Ecol. 15, 1–16 (2022).
Schrader, J., Moeljono, S., Keppel, G. & Kreft, H. Plants on small islands revisited: the effects of spatial scale and habitat quality on the species–area relationship. Ecography 42, 1405–1414 (2019).
Hubbell, S. P. The Unified Neutral Theory of Biodiversity and Biogeography (Princeton Univ. Press, 2001).
Shmida, A. V. I. & Wilson, M. V. Biological determinants of species diversity. J. Biogeogr. 12, 1–20 (1985).
Leibold, M. A. & McPeek, M. A. Coexistence of the niche and neutral perspectives in community ecology. Ecology 87, 1399–1410 (2006).
Chase, J. M. & Myers, J. A. Disentangling the importance of ecological niches from stochastic processes across scales. Philos. Trans. R. Soc. B 366, 2351–2363 (2011).
Chase, J. M. et al. Embracing scale‐dependence to achieve a deeper understanding of biodiversity and its change across communities. Ecol. Lett. 21, 1737–1751 (2018).
Leibold, M. A. et al. The metacommunity concept: a framework for multi‐scale community ecology. Ecol. Lett. 7, 601–613 (2004).
Tilman, D. Niche tradeoffs, neutrality, and community structure: a stochastic theory of resource competition, invasion, and community assembly. Proc. Natl Acad. Sci. USA 101, 10854–10861 (2004).
Kadmon, R. & Allouche, O. Integrating the effects of area, isolation, and habitat heterogeneity on species diversity: a unification of island biogeography and niche theory. Am. Nat. 170, 443–454 (2007).
MacArthur, R. H. Patterns of species diversity. Biol. Rev. 40, 510–533 (1965).
Wilson, E. O. The species equilibrium. Brookhaven Sym. Biol. 22, 38–47 (1969).
Wright, S. J. Intra-archipelago vertebrate distributions: the slope of the species-area relation. Am. Nat. 118, 726–748 (1981).
Lomolino, M. V. & Weiser, M. D. Towards a more general species-area relationship: diversity on all islands, great and small. J. Biogeogr. 28, 431–445 (2001).
Diamond, J. M. in Ecology and Evolution of Communities (eds Cody, M. L. & Diamond, J. M.) 342–444 (Harvard Univ. Press, 1975).
Hanski, I. Dynamics of regional distribution: the core and satellite species hypothesis. Oikos 38, 210–221 (1982).
Pulliam, H. R. Sources, sinks, and population regulation. Am. Nat. 132, 652–661 (1988).
Paine, R. T. & Vadas, R. L. The effects of grazing by sea urchins, Strongylocentrotus spp., on benthic algal populations 1. Limnol. Oceanogr. 14, 710–719 (1969).
Lubchenco, J. & Menge, B. A. Community development and persistence in a low rocky intertidal zone. Ecol. Monogr. 48, 67–94 (1978).
Bertness, M. D., Leonard, G. H., Levine, J. M., Schmidt, P. R. & Ingraham, A. O. Testing the relative contribution of positive and negative interactions in rocky intertidal communities. Ecology 80, 2711–2726 (1999).
Hawkins, S. J., Pack, K. E., Hyder, K., Benedetti-Cecchi, L. & Jenkins, S. R. Rocky shores as tractable test systems for experimental ecology. J. Mar. Biol. Assoc. UK 100, 1017–1041 (2020).
Loke, L. H. L. & Todd, P. A. Structural complexity and component type increase intertidal biodiversity independently of area. Ecology 97, 383–393 (2016).
Loke, L. H. L., Chisholm, R. A. & Todd, P. A. Effects of habitat area and spatial configuration on biodiversity in an experimental intertidal community. Ecology 100, e02757 (2019).
Hartanto, R. S. et al. Material type weakly affects algal colonisation but not macrofaunal community in an artificial intertidal habitat. Ecol. Eng. 176, 106514 (2022).
Levine, J. M. & HilleRisLambers, J. The importance of niches for the maintenance of species diversity. Nature 461, 254–257 (2009).
Friedman, J., Higgins, L. M. & Gore, J. Community structure follows simple assembly rules in microbial microcosms. Nat. Ecol. Evol. 1, 1–7 (2017).
Triantis, K. A. & Sfenthourakis, S. Island biogeography is not a single‐variable discipline: the small island effect debate. Divers. Distrib. 18, 92–96 (2012).
Preston, F. W. The canonical distribution of commonness and rarity: part I. Ecology 43, 185–215 (1962).
Armstrong, R. A. & McGehee, R. Competitive exclusion. Am. Nat. 115, 151–170 (1980).
Huisman, J. & Weissing, F. J. Biodiversity of plankton by species oscillations and chaos. Nature 402, 407–410 (1999).
Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343–366 (2000).
Schippers, P., Verschoor, A. M., Vos, M. & Mooij, W. M. Does “supersaturated coexistence” resolve the “paradox of the plankton”? Ecol. Lett. 4, 404–407 (2001).
Lai, S., Loke, L. H. L., Bouma, T. J. & Todd, P. A. Biodiversity surveys and stable isotope analyses reveal key differences in intertidal assemblages between tropical seawalls and rocky shores. Mar. Ecol. Prog. Ser. 587, 41–53 (2018).
Lim, L. J. W. et al. Diversity and distribution of intertidal marine species in Singapore. Singapore. Raffles Bull. Zool. 68, 396–403 (2020).
Turner, I. M. The Ecology of Trees in the Tropical Rain Forest (Cambridge Univ. Press, 2001).
Terborgh, J. Using Janzen–Connell to predict the consequences of defaunation and other disturbances of tropical forests. Biol. Conserv. 163, 7–12 (2013).
Descamps-Julien, B. & Gonzalez, A. Stable coexistence in a fluctuating environment: an experimental demonstration. Ecology 86, 2815–2824 (2005).
Levi, M. R. & Bestelmeyer, B. T. Digital soil mapping for fire prediction and management in rangelands. Fire Ecol. 14, 1–12 (2018).
Chisholm, R. A. & Fung, T. Janzen-Connell effects are a weak impediment to competitive exclusion. Am. Nat. 196, 649–661 (2020).
Morris, R. L. et al. Design options, implementation issues and evaluating success of ecologically engineered shorelines. Oceanogr. Mar. Biol. 57, 169–228 (2019).
Cohen-Shacham, E., Walters, G., Janzen, C. & Maginnis, S. Nature-based Solutions to Address Global Societal Challenges (IUCN, Gland, Switzerland, 2016).
Cordonnier, T., Kunstler, G., Courbaud, B. & Morin, X. Managing tree species diversity and ecosystem functions through coexistence mechanisms. Ann. For. Sci. 75, 1–11 (2018).
Tilman, D. Tests of resource competition theory using four species of Lake Michigan algae. Ecology 62, 802–815 (1981).
Fargione, J., Brown, C. S. & Tilman, D. Community assembly and invasion: an experimental test of neutral versus niche processes. Proc. Natl Acad. Sci. USA 100, 8916–8920 (2003).
Hubbell, S. P. Neutral theory in community ecology and the hypothesis of functional equivalence. Funct. Ecol. 19, 166–172 (2005).
Volkov, I., Banavar, J. R., Hubbell, S. P. & Maritan, A. Patterns of relative species abundance in rainforests and coral reefs. Nature 450, 45–49 (2007).
Dornelas, M., Connolly, S. R. & Hughes, T. P. Coral reef diversity refutes the neutral theory of biodiversity. Nature 440, 80–82 (2006).
Lai, S., Loke, L. H. L., Hilton, M. J., Bouma, T. J. & Todd, P. A. The effects of urbanisation on coastal habitats and the potential for ecological engineering: a Singapore case study. Ocean Coast. Manage. 103, 78–85 (2015).
Climate of Singapore. Meteorological Service Singapore http://www.weather.gov.sg/climate-climate-of-singapore/ (2022).
Van Maren, D. S. & Gerritsen, H. Residual flow and tidal asymmetry in the Singapore Strait, with implications for resuspension and residual transport of sediment. J. Geophys. Res. 117, C04021 (2012).
Chapman, M. G. & Bulleri, F. Intertidal seawalls—new features of landscape in intertidal environments. Landsc. Urban Plan. 62, 159–172 (2003).
Davis, J., Levin, L. & Walther, S. Artificial armored shorelines: sites for open-coast species in a southern California bay. Mar. Biol. 140, 1249–1262 (2002).
Lee, A. C. & Sin, T. M. Intertidal assemblages on coastal defence structures in Singapore II. Contrasts between islands and the mainland. Raffles Bull. Zool. 22, 255–268 (2009).
Loke, L. H. L., Liao, L. M., Bouma, T. J. & Todd, P. A. Succession of seawall algal communities on artificial substrates. Raffles Bull. Zool. 32, 1–10 (2016).
Hsiung, A. R. et al. Little evidence that lowering the pH of concrete supports greater biodiversity on tropical and temperate seawalls. Mar. Ecol. Prog. Ser. 656, 193–205 (2020).
Kaehler, S. & Williams, G. A. Early development of algal assemblages under different regimes of physical and biotic factors on a seasonal tropical rocky shore. Mar. Ecol. Prog. Ser. 172, 61–71 (1998).
Williams, G. A., Davies, M. S. & Nagarkar, S. Primary succession on a seasonal tropical rocky shore: the relative roles of spatial heterogeneity and herbivory. Mar. Ecol. Prog. Ser. 203, 81–94 (2000).
Tan, S. K. Land Reclamation in Singapore (National University of Singapore, Singapore, 1976).
Hilton, M. J. & Chou, L. M. Sediment facies of a low‐energy, meso‐tidal, fringing reef, Singapore. Singap. J. Trop. Geogr. 20, 111–130 (1999).
Zhao, K. et al. Modelling surface temperature of granite seawalls in Singapore. Case Stud. Therm. Eng. 13, 100395 (2019).
Loke, L. H. L., Bouma, T. J. & Todd, P. A. The effects of manipulating microhabitat size and variability on tropical seawall biodiversity: field and flume experiments. J. Exp. Mar. Biol. Ecol. 492, 113–120 (2017).
Strain, E. M. et al. A global analysis of complexity–biodiversity relationships on marine artificial structures. Glob. Ecol. Biogeogr. 30, 140–153 (2021).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2022); https://www.r-project.org/.
Schoener, T. W. Competition and the form of habitat shift. Theor. Popul. Biol. 6, 265–307 (1974).
Chisholm, R. A. & Pacala, S. W. Niche and neutral models predict asymptotically equivalent species abundance distributions in high-diversity ecological communities. Proc. Natl Acad. Sci. USA 107, 15821–15825 (2010).
Chisholm, R. A. & Pacala, S. W. Theory predicts a rapid transition from niche-structured to neutral biodiversity patterns across a speciation-rate gradient. Theor. Ecol. 4, 195–200 (2011).
Abramowitz, M. & Stegun, I. A. Handbook of Mathematical Functions with Formulas, Graphs, and Mathematical Tables (Dover, 1972).
Acknowledgements
We thank E. Heery and N. Kristensen for helpful comments on the manuscript and H. Muller-Landau for helpful discussion. L.H.L.L. was supported by a Macquarie University Research Fellowship (no. 110042722) and a grant from Wildlife Reserves Singapore Conservation Fund. R.A.C. was supported by a grant from the James S. McDonnell Foundation (no. 220020470).
Author information
Authors and Affiliations
Contributions
L.H.L.L. conceived and conducted the experiment, collected the data, performed analyses and wrote the first draft of the manuscript. R.A.C. contributed ideas, performed analyses and edited the manuscript. All authors contributed substantially to revisions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks David Alonso and the other, anonymous, reviewers(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Trends in species richness in the experimental communities over a year.
Each plot shows species richness (\(S\)) against time (months) for one experimental treatment (panels), where treatments vary in the level of immigration (governed by number of holes, \(h\)). Experimental communities stabilized after 6–7 months for all but the highest immigration treatments. The horizontal and vertical coordinates of points have been jittered to improve visibility. Solid curves are mean estimates with shaded 95% confidence intervals; vertical dashed lines indicate the seventh month from the start of the experiment.
Extended Data Fig. 2 Fitting a variant of the classic model.
Each small grey point indicates a single experimental unit (seawall tile; 12 \(h\)-treatments, \(n\) = 5) in a given month (x-coordinates are jittered to improve visibility); each large black point shows a treatment mean. Fitted models are shown by the solid curves (mean estimates) with shaded 95% confidence intervals from 1,000 bootstraps; the novel model (Eq. 6) is shown in red while the variant of the classic model is in green (Eq. 5; see Methods). This variant of the classic model was substantially poorer than the novel model although better than the original classic model (judged by AIC). The dashed green horizontal line indicates the expected species richness at high levels of immigration under the variant of the classic model, as estimated from the fitted parameters.
Supplementary information
Supplementary Information
Supplementary Text, Figs. 1–3 and Table 1.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Loke, L.H.L., Chisholm, R.A. Unveiling the transition from niche to dispersal assembly in ecology. Nature 618, 537–542 (2023). https://doi.org/10.1038/s41586-023-06161-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06161-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.