Abstract
PIONEER is a European network of excellence for big data in prostate cancer consisting of 37 private and public stakeholders from 9 countries across Europe. Many progresses have been done in prostate cancer management, but unanswered questions in the field still exist, and big data could help to answer these questions. The PIONEER consortium conducted a two-round modified Delphi survey aiming at building consensus between two stakeholder groups — health-care professionals and patients with prostate cancer — about the most important questions in the field of prostate cancer to be answered using big data. Respondents were asked to consider what would be the effect of answering the proposed questions on improving diagnosis and treatment outcomes for patients with prostate cancer and to score these questions on a scale of 1 (not important) to 9 (critically important). The mean percentage of participants who scored each of the proposed questions as critically important was calculated across the two stakeholder groups and used to rank the questions and identify the highest scoring questions in the critically important category. The identification of questions in prostate cancer that are important to various stakeholders will help the PIONEER consortium to provide answers to these questions to improve the clinical care of patients with prostate cancer.
Similar content being viewed by others
Introduction
Prostate cancer is the most common cancer diagnosed in men in Europe, with >1,400,000 estimated diagnoses in 2020, and the fifth cause of mortality for cancer worldwide, with >375,000 new deaths per year1. Prostate cancer is characterized by a relatively prolonged natural history, but patients’ outcomes are heterogeneous and vary profoundly according to disease features as well as individual characteristics2. Over the past few years, the introduction of novel imaging modalities, biomarkers, genomics and personalized medicine revolutionized the management of patients with prostate cancer3,4,5. However, several questions on the most suitable management option for patients with different stages of prostate cancer remain unanswered, and further research is needed to develop approaches that will improve oncological control and survival for patients at all stages of the disease and minimize the detrimental effects of therapy on health-related quality of life.
Prostate cancer management is typically based on patient stratification into risk categories, which estimate patient probability of experiencing recurrence after primary treatment, or the likelihood of disease progression — in case a management strategy with non-curative intent such as active surveillance (AS) was adopted. However, this classification mainly relies on clinical factors such as PSA values, clinical stage and biopsy grade group6. With this stratification, the identification of men who would die from prostate cancer or who would experience cancer adverse effects versus patients who have an increased probability of dying from other causes and do not experience any burden form prostate cancer is suboptimal. Thus, the effects of novel available tools (such as the five-tier prostate cancer risk stratification tools) on patient risk stratification during diagnosis still need to be clarified.
For patients with clinically localized disease, valid management options include deferred treatment, which mainly consists of AS and watchful waiting (WW), as well as curative intent treatments such as surgery to remove the prostate (radical prostatectomy) and radiation treatment2. AS and WW are both aimed at avoiding unnecessary therapies and treatment-related adverse effects, but differ substantially. AS is an option for selected patients with low-risk or intermediate-risk localized disease to avoid treatment-related adverse effects without missing the correct timing for the delivery of curative-intent therapies7. Several selection criteria have been proposed for patient inclusion in AS protocols. However, patient-specific and tumour-specific factors that could accurately predict the prognosis in this setting and help to identify the optimal candidates for AS are still unknown8. For example, multiparametric MRI and genetic testing have been proposed to identify men suitable for inclusion in AS protocols9,10. However, the role of these factors in prostate cancer management and the effect on patient survival still need to be elucidated. Similarly, to date, the optimal timing for follow-up monitoring and triggers for intervention in patients enrolled in AS protocols have been poorly addressed.
Patients considered for WW are deemed unsuitable for curative treatments owing to estimated life expectancy or the presence of substantial comorbidities, and are, therefore, typically monitored until the development of local or systemic symptoms2. The natural history of contemporary patients managed with WW and the rates of disease progression and survival still need to be investigated, as the available studies only rely on historical cohorts11. Moreover, the improved life expectancy and the different effect of prostate cancer comorbidities on survival would preclude the generalizability of the results from these studies to contemporary cohorts.
With regard to men with advanced disease (such as locally advanced or metastatic prostate cancer), several questions remain unanswered. Results from some studies suggested that the treatment of the primary tumour in patients with oligometastatic disease at diagnosis, as well as the delivery of metastases-directed therapies in the oligo-recurrence setting, might improve patient outcomes such as overall survival12,13. However, the effect of these local therapies on long-term outcomes in the metastatic setting remains unknown.
Over the past few years, several novel systemic therapies have been introduced for the treatment of metastatic hormone-sensitive and castration-resistant prostate cancer (CRPC), such as novel androgen-receptor targeted therapies, novel chemotherapeutics, PARP inhibitors or immunotherapy14. However, the best sequence of treatments with these molecules is still largely unknown. Similarly, little is known regarding the use of biomarkers in an individualized therapeutic approach.
Lastly, each local or systemic therapy for prostate cancer is associated with specific treatment-related adverse effects, which profoundly affect patient health-related quality of life. One of the main challenges in the management of patients with prostate cancer in the next decade will be to identify therapeutic approaches with the best trade-off between toxicity and efficacy for each patient to improve oncological control without affecting quality of life.
The PIONEER project
Prostate Cancer Diagnosis and Treatment Enhancement through the power of big data in Europe (PIONEER) is a European network of excellence for big data in prostate cancer, consisting of 37 private and public stakeholders from nine countries across Europe14. PIONEER was launched by the Innovative Medicines Initiative 2 and is part of the Big Data for Better Outcomes (BD4BO) programme. The overarching goal of PIONEER is to provide high-quality evidence on prostate cancer management to improve health outcomes and health-care systems in Europe by unlocking the potential of big data14.
Prostate cancer is the most common cancer diagnosed in men in Europe and is the cause of death in one tenth of all men with cancer15. Prostate cancer health-care costs were estimated at 8.43 billion euros per year in the European Union in 2009 and accounted for 7% of all cancer costs in Europe16. To date, many knowledge gaps exist about the screening, diagnosis and treatment of patients with prostate cancer, including: lack of standardization of prostate cancer outcomes’ definitions across all stages of the disease; insufficient knowledge about the risk factors for developing prostate cancer; insufficient knowledge about appropriate patient stratification and prognostic factors, including genetic profiles, for an optimal stratification of patients at the time of diagnosis; lack of meaningful engagement of all crucial stakeholders, including patients, when disease-specific core outcome sets are defined; ineffective implementation of knowledge and real-world clinical data into clinical practice.
The vision of PIONEER is to transform the management and clinical practice of prostate cancer across all disease stages (stage I—IV) towards a data-driven and outcome-driven, value-based and patient-centric health-care system. PIONEER will use advanced big data analytics and will develop a data platform of unparalleled scale, quality and diversity to drive meaningful improvement in clinical practice, prostate cancer disease-related outcomes and health economic outcomes across the European health-care landscape14. Specific objectives of the PIONEER project include: improving disease understanding and delivering a core set of clinically relevant standardized prostate cancer-related outcomes; optimizing diagnosis and therapeutic management of patients with prostate cancer across different stages of the disease and across multiple geographic locations by delivering valuable insights from real-world data and sharing best practices; providing unique tools for the standardization and analysis of complex prostate cancer datasets from a variety of sources, using different data models and terminologies, and including various layers of information (such as genetic, omics, imaging, biomarkers); developing a large and harmonized repository of prostate cancer data that can be used to improve evidence-based decision making for all patients with prostate cancer, and to enable data re-use in a wide variety of scenarios.
Knowledge gaps in prostate cancer management and the PIONEER approach
PIONEER’s ultimate vision is to re-orient the management and clinical practice of prostate cancer across all stages of the disease towards an outcome-driven, value-based and patient-centric health-care system. Clinical research is traditionally led by scientists and clinical professionals, or by commercial interest. In 2009, Chalmers and Glasziou, among others, strongly argued for an efficient research culture in which scientists should study health conditions that have the greatest burden on the population but also address questions about interventions and outcomes that are considered the most important by patients and clinicians17. The distinction between a scientific problem and a research question is perhaps not always clear. Scientific problem refers to a gap in knowledge, whereas a research question can be considered as the identification of a particular piece of knowledge that researchers seek to generate through a project to (partially) solve a problem. Generating relevant research questions in terms of novelty, scientific and practical effect, feasibility and clarity requires different types of pre-existing knowledge, for example the function of the prostate gland. PIONEER will rely on the availability of ample datasets, but researchers should consider what will be actually feasible to address. In general, available patient-centred prostate cancer datasets can be divided into three categories — clinical, genomics and imaging — and the availability of data in each category will influence the feasibility of solving a specific research question. However, the success of big data analysis does not solely depend on access to data. The interaction between researchers, clinicians, patients, people from information technology and data experts is crucial and requires a multidisciplinary approach18,19.
The PIONEER consortium initiated a research prioritization exercise aimed at identifying the major unmet questions in the field. First, the PIONEER consortium identified important prostate cancer evidence gaps from the perspectives of academic and industry professionals as well as patients, and then used modified Delphi methods to come to a consensus on a prioritized list of research questions.
Methods
The most important stakeholder groups for identifying the top unanswered questions in prostate cancer are health-care professionals (HCPs), who design and administer therapies and drive the research agenda, and patients, who are the recipients of the benefits and harms of care and research. The modified Delphi method was identified as appropriate to assess agreement within and between these stakeholder groups and to facilitate consensus20. The modified Delphi method is characterized by anonymous controlled feedback, as participants are first asked to score a series of items and, in subsequent rounds, are shown a summary of the scores that other participants attributed to each item in the previous round. Participants are then asked to re-score the items21.
Key opinion leaders, including members of the prostate cancer guideline panel of the European Association of Urology (EAU) and other urologists, oncologists, radiologists, nurses, health economists and researchers were consulted to propose the most important questions in the field of prostate cancer to be answered using big data. These key opinion leaders work in a variety of different settings including academic and/or university environments, hospitals and primary care. These experts were asked to provide crucial unanswered research questions for prostate cancer, considering what is not known about prostate cancer but would be important to know, and how answering these questions could transform practice and patient outcomes. Through this process, 44 key questions were identified. Afterwards, the PIONEER consortium conducted a two-round modified Delphi survey to build consensus between the two stakeholder groups: HCPs (including representatives from pharmaceutical companies who are medically qualified and work in either research and development or medical affairs branches of industry, excluding people from marketing departments) and patients with prostate cancer. Several organizations helped to disseminate the surveys including the EAU, EAUN, ecancer, ECPC, EUROPA UOMO and Prostate Cancer UK. Respondents were asked to score the proposed questions on a scale of 1 (not important) to 9 (critically important) in both the Delphi rounds, considering what would be the effect of answering these questions on improving diagnosis and treatment outcomes for patients with prostate cancer. The results were analysed by calculating the percentage of respondents scoring each question as not important (score 1–3), important (score 4–6) or critically important (score 7–9). In the second round, participants were shown a summary of the percentage of other participants (patients and HCPs) who had considered the question critically important in round one and were asked to score the questions on a scale of 1 to 9.
Results
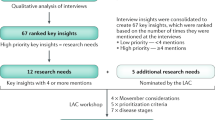
In total, 73 HCPs and 57 patients participated in round 1 of the modified Delphi survey. During this first round, 12 additional questions were proposed. For the second round, patients’ surveys were translated into French, German, Italian and Spanish. Overall, 49 HCPs and 169 patients (including 53 English, 19 French, 31 German, 53 Italian and 13 Spanish patients) participated in round 2 of the survey (Fig. 1).
The mean of the percentages of participants scoring each of the 56 questions as critically important was calculated across the two stake-holder groups (prioritization score) and used to rank the questions to identify the highest scoring questions in the critically important category (Box 1).
The five questions with the highest prioritization were overall deemed critically important by >85% of all respondents (Table 1 and Supplementary Information). None of the questions that were added after the first modified Delphi round were retained within the final top ten prioritized questions. All top five questions were also among the top ten questions that emerged from the first modified Delphi voting round. Three questions (Q1, Q2 and Q4) focused on prognostic factors, and two questions (Q4 and Q5) on the role of medical interventions on patient outcomes. The disease stages that were considered also varied and included localized (Q1, Q2, Q3), recurrent (Q4) and metastatic (Q5) disease. Thus, prioritization of questions does not seem to be biased towards the opinion of a subgroup of HCPs (for example, urologists versus medical oncologists). The prioritization of the first five questions was overall similar between HCPs and patients, but for two questions (Q3 and Q4) a ~10% difference was observed in the percentage of respondents who categorized the question as critically important (91.8% of HCPs versus 82.3% of patients for Q3, and 79.6% of HCPs versus 92.5% of patients for Q4). The remaining five questions (Q6–Q10) had an overall prioritization score of ~85%, with the exception of Q10, which had a score of 80.5%. Overall, three of these questions (Q6, Q7 and Q9) were among the top ten questions identified by HCPs and patients after the first modified Delphi voting round, whereas two questions (Q8 and Q10) were among the ten questions prioritized only by the HCP group during round 1. Among these five questions, Q6, Q9 and Q10 were focused on treatment-related benefits and harms, and on the most appropriate sequence of treatment with the available therapeutic options, whereas Q7 and Q8 covered the optimization of patient selection for treatment at various clinical stages, and the use of genetic profiles to predict patient response to treatment and, in turn, maximize treatment effect. Prioritization scores were similar between the groups of patients and HCPs for Q7 and Q8 (~85%), whereas patient prioritization scores for Q6, Q9 and Q10 were ~10–15% higher than the scores provided by HCPs (91.4%, 90.8% and 88.1% versus 79.6%, 77.6% and 72.9%, respectively). These results indicate that the views of the two stakeholder groups were similar.
Overall, both stakeholder groups prioritized questions related to four specific topics among the top ten questions: comparison of treatments or diagnostic tools among patients with different disease stages such as CRPC; timing of treatment and care pathways; comparison of adverse effects among different treatments or genetic profile of patients; and understanding patient types and risk profiles. The main difference between the two groups was that in the top ten list of questions, patients also prioritized questions related to coordination of care and the skill of care providers. Examples of questions prioritized by patients include questions related to the comparison of adverse effect rates among different treatments, questions related to tumour-specific and patient-specific variables, questions about prognosis and AS and questions related to the ideal sequencing of therapeutic options to provide the best patient outcomes. The most rated question by both groups was around treatment options and timing of treatment following recurrence of prostate cancer (Table 1 and Supplementary Information). These questions are all important dimensions of evidence-based decision making, which would help to increase patient understanding of diagnosis and potential treatment options, and inform patient outcome expectations. Answering these questions would support appropriate decision making and could minimize patient decisional regret. The coordination of care and the skill sets of care providers are important dimensions of confidence and trust in the process of care.
Examples of questions prioritized by HCPs include questions related to best models for risk stratification, questions related to understanding which specific groups of patients would benefit from specific treatments such as upfront chemotherapy and questions related to diagnosis and use of pre-biopsy multiparametric MRI (Table 1 and Supplementary Information). Interestingly, a clear emphasis was placed by HCPs on gaining an improved understanding of treatment options and how to tailor these therapies to specific groups of patients; however, reduced emphasis was put on the delivery and coordination of care, or on the expertise and skill set of the HCPs involved in care. This evidence indicates that HCPs had different views from patients about some aspects of prostate cancer management.
In order to improve the clinical care of patients with prostate cancer and fill the gaps in the field, we recommend that expert clinicians, patients and researchers work together to answer the top ten unanswered questions in prostate cancer:
-
Q1) What are the relevant tumour-specific and patient-specific variables that affect the prognosis of patients with prostate cancer suitable for active surveillance?
-
Q2) What is the natural history of patients with prostate cancer undergoing conservative management (that is, watchful waiting) and what is the impact of comorbidities and life expectancy on long-term outcomes?
-
Q3) What differentiates patients with lethal versus non-lethal disease, irrespective of risk stratification?
-
Q4) When should we treat patients who experience prostate cancer recurrence after primary treatment, and which are the most effective therapeutic approaches?
-
Q5) Which specific patient groups benefit the most from upfront chemotherapy? What are the side effects and what is the impact of chemotherapy on quality of life in real-life practice? What is the benefit of chemotherapy in the subgroup of patients who experience recurrence after primary treatment?
-
Q6) How does the rate of side effects or local problems (including secondary or palliative treatments needed) compare among treatments (open, laparoscopic, robotic surgery with or without lymph node dissection, brachytherapy, different forms of external beam radiation therapy), and which patient-specific factors are associated with these adverse secondary end points?
-
Q7) What is the clinical benefit of determining a genetic risk profile of patients in prostate cancer management, especially in the screening setting?
-
Q8) Which patients benefit from different available treatment options for CRPC?
-
Q9) What is the therapeutic benefit of treating the local tumour in patients diagnosed with (oligo)metastatic prostate cancer?
-
Q10) How should the available therapeutic options be sequenced in order to achieve response and best outcomes in individual patients and in specific settings?
Discussion
The abandonment of the paternalistic model of the doctor–patient relationship and the increasing knowledge of prostate cancer biology has led to a change in how patients with prostate cancer are treated. A shift from general cancer treatments to patient-tailored treatments occurred, taking in consideration not only tumour features, but also patients’ quality of life, personal expectations and desires. Clinical practice has already strongly changed, but the plethora of unanswered questions identified from the prioritization exercise presented in this Consensus Statement clearly reflects that this transition is not yet complete. The prioritized questions reflect the main concerns of both patients and HCPs on the natural history of prostate cancer, on the importance of improving disease stratification, as well as on the different treatment options and the effectiveness and adverse effects or complications associated with these treatments.
Notably, the two highest ranked questions are focused on conservative strategies and on identifying patients who can be managed conservatively and safely with AS and WW. This aspect is important to guarantee the optimal treatment to each patient. Both management options are being used in daily practice, but many uncertainties still exist about the best way to conduct these strategies. These uncertainty are reflected by the publication of the DETECTIVE study, which was designed to formulate consensus statements on various aspects of AS including patient eligible for AS, and optimal timing of investigations and assessment owing to the lack of high-level evidence about AS management8.
Q3–5 and Q8 are a reflection of the increasing appreciation of disease and patient heterogeneity22,23. Big data will improve risk stratification of patients and disease categorization based on meaningful real world clinical end points. Moreover, big data could lead to optimized risk stratification using both clinical and omics data (Q7), which could ultimately lead to the development of clinical prediction models, supporting the increase of patient-tailored treatment strategies with reduced toxicity and increased efficacy.
Big data would not only facilitate the development of prognostic models, but could also improve prediction of therapeutic response. Management of the various stages of prostate cancer is becoming increasingly challenging as the knowledge on disease biology increases and new technologies and treatments are introduced. In an ever-changing field, understanding the safety profile of the available treatments and determining the optimal sequencing of the various types of multimodal treatments that are now part of the treatment landscape (Q6 and Q10) are crucial. Lastly, the best management strategy for complex and relatively uncommon clinical scenarios (such as the management of oligometastatic disease) is still unclear (Q9), and could also be addressed using big data.
Future directions
PIONEER is a consortium dedicated to improving the diagnosis, treatment and care of patients with prostate cancer through the development and implementation of research studies to address clinical knowledge gaps. Members of the PIONEER consortium can form research question (RQ) teams to focus on the prioritized questions. These RQ teams are dedicated to addressing specific research questions, and each data contributor who has produced relevant prostate cancer data has the right to participate in the research teams developing the protocols. Any PIONEER member or data contributor (including industry participants) can propose the creation of a new RQ team to focus on specific research questions either identified from the list of the 56 prioritized questions from this Consensus Statement, or by proposing a new question (non-prioritized questions must be justified).
A PIONEER RQ oversight committee was formed to support and sanction the establishment of RQ teams, with membership designated by the PIONEER executive committee. The RQ oversight committee is composed of senior clinicians and researchers from both public and private partners with the aim of ensuring transparency and efficiency when using the PIONEER big data platform to answer the most relevant questions pertaining to patients with prostate cancer. In this process, high-quality publications will be generated to provide evidence-based data to support clinical practice guideline recommendations as well as inform the decision-making processes by HCPs and patients.
The committee process is covered in the Research Committee Charter, which is available to all PIONEER members. Briefly, to initiate the formation of a new RQ team, the beneficiary or associated partner will submit an application to the chair of the RQ oversight committee at least 7 days before the subsequent research committee meeting, which are held monthly. A thorough review of the merits of the proposed application is made on the basis of four elements: scientific or clinical relevance of the proposed question; potential overlap with other teams’ activities; existence of sufficient data to support the proposed investigation; and verification that the proposed team meets the basic qualifications as set out in the application.
In addition to these criteria, several other points must be addressed before approval is given. To warrant a truly collaborative team, the RQ team membership must include a minimum of two public and two European Federation of Pharmaceutical Industries and Associations (EFPIA) partners. After the application is approved, membership to the RQ team is opened to all PIONEER partners. Within the proposal, the applicant should also clearly explain which RQ is to be tackled, address the knowledge gaps that are associated with that question, present the study design and methods to be used, state the crucial variables of the proposed project (inclusion and exclusion criteria, end points, covariates or controls) and indicate what the expected findings are, including how these findings will be used to improve patient care, outcomes and lives. Last, the applicant must identify a list of at least three datasets that will be used to answer the question along with a timeline and publication and dissemination plan.
The research committee bylaws state that for a proposal to be considered, a minimum of 80% of the RQ oversight committee members must be present at the meeting, and a decision to sanction a new RQ team will require at least a 60% majority of the committee members present at the meeting. The decision will be announced to the applicant within 3 days of the committee meeting.
For example, RQ1 from this Consensus Statement, which focuses on patients with prostate cancer and the effect of life expectancy and comorbidities on the outcome of conservative management strategies (that is, AS) was approved by the PIONEER research committee. The research team organized the PIONEER Study-A-Thon held in March 2020 in collaboration with the European Health Data Evidence Network (EHDEN)) and the Observational Health Data Sciences and Informatics (OHDSI) aimed at characterizing the long-term outcomes (clinical characterization) of patients with prostate cancer managed with conservative treatments and to build a prediction model to generate risk scores that could inform patients about possible risks of disease progression. At the time of the Study-A-Thon, 1,557,114 patients with prostate cancer (diagnosed between 1989 and 2021) were identified among 12 databases analysed. Overall, 896,318 of these patients received immediate treatment, whereas 536,235 patients underwent conservative management. Patients actively participated in the Study-A-Thon from start to finish, shared the experience of living with prostate cancer, the effect of treatment and the experiences of survivorship, including existing gaps in care and discussing outcomes considered of most importance. Results will be presented in a separate publication. PIONEER has formed other RQ teams to answer some of the top questions in prostate cancer. Patients will again be central to the planning, protocol development and execution of the RQs.
The expectation is that the real-world evidence obtained by successfully answering the prioritized research questions would be relevant to fill gaps in clinical practice guidelines (supporting guideline recommendations) and improve clinician–patient shared decision making.
Strengths and weaknesses of the study
The main strengths of our modified Delphi approach are that the online format facilitated the inclusion of a large and diverse sample and the anonymous feedback enabled participants to know both stakeholder groups’ scores without being influenced by dominant voices or people with perceived authority. A limitation of this approach is that additional patient group participants were added in round 2, whereas methods guidance supports not adding participants21. We did accept this inclusion as a limitation, but the decision to invite further participants was made to boost sample size, to obtain maximum opinion diversity and to mitigate against the anticipated critique that the original English-speaking-only sample might not have adequately included opinions from other native European language speakers, which might have deviated from the opinions of the English-speaking sample. Another limitation was the inclusion of pharmaceutical industry representatives, who might be considered to have a competing interest in driving the prioritization of research questions. However, the anonymous scoring process and the definition of consensus as a percentage applied to the stakeholder groups separately mean that the industry voice has been considered, but had no more weight than any other stakeholder group in the results.
Conclusions
PIONEER has conducted an international multi-stakeholder consensus to identify and prioritize the most important questions in the field of prostate cancer. The top ten questions prioritized by two stakeholder groups (HCPs and patients) focused on four specific topics: comparisons of treatments or diagnostic tools among patients with different disease stages; timing of treatment and care pathways; comparison of adverse effects among different treatments or genetic profile of patients; and understanding of patient types and risk profiles. Answering these questions will improve the clinical care of patients with prostate cancer.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Mottet, N. et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur. Urol. 79, 243–262 (2021).
Loeb, S. & Giri, V. N. Clinical implications of germline testing in newly diagnosed prostate cancer. Eur. Urol. Oncol. 4, 1–9 (2021).
Hofman, M. S. et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet 395, 1208–1216 (2020).
Kasivisvanathan, V. et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N. Engl. J. Med. 378, 1767–1777 (2018).
Gandaglia, G. et al. Prognostic implications of multiparametric magnetic resonance imaging and concomitant systematic biopsy in predicting biochemical recurrence after radical prostatectomy in prostate cancer patients diagnosed with magnetic resonance imaging-targeted biopsy. Eur. Urol. Oncol. 3, 739–747 (2020).
Klotz, L. et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J. Clin. Oncol. 33, 272–277 (2015).
Lam, T. B. L. et al. EAU-EANM-ESTRO-ESUR-SIOG prostate cancer guideline panel consensus statements for deferred treatment with curative intent for localised prostate cancer from an international collaborative study (DETECTIVE study). Eur. Urol. 76, 790–813 (2019).
Lantz, A. et al. Expanding active surveillance inclusion criteria: a novel nomogram including preoperative clinical parameters and magnetic resonance imaging findings. Eur. Urol. Oncol. 5, 187–194 (2020).
Carter, H. B. et al. Germline mutations in ATM and BRCA1/2 are associated with grade reclassification in men on active surveillance for prostate cancer. Eur. Urol. 75, 743–749 (2019).
Lu-Yao, G. L. et al. Fifteen-year outcomes following conservative management among men aged 65 years or older with localized prostate cancer. Eur. Urol. 68, 805–811 (2015).
Ost, P. et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J. Clin. Oncol. 36, 446–453 (2018).
Parker, C. C. et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet 392, 2353–2366 (2018).
Omar, M. I. et al. Introducing PIONEER: a project to harness big data in prostate cancer research. Nat. Rev. Urol. 17, 351–362 (2020).
Ferlay, J. et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur. J. Cancer 49, 1374–1403 (2013).
Luengo-Fernandez, R., Leal, J., Gray, A. & Sullivan, R. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol. 14, 1165–1174 (2013).
Chalmers, I. & Glasziou, P. Avoidable waste in the production and reporting of research evidence. Lancet 374, 86–89 (2009).
Beck, S. B. T., Poets, M. & Sauermann, H. What’s the problem? How crowdsourcing contributes to identifying scientific research questions. Acad. Manag. Proc. 1, 15282 (2019).
Hulsen, T. An overview of publicly available patient-centered prostate cancer datasets. Transl. Androl. Urol. 8 (Suppl. 1), S64–S77 (2019).
Fish, R., MacLennan, S., Alkhaffaf, B. & Williamson, P. R. “Vicarious thinking” was a key driver of score change in Delphi surveys for COS development and is facilitated by feedback of results. J. Clin. Epidemiol. 128, 118–129 (2020).
Williamson, P. R. et al. The COMET Handbook: version 1.0. Trials 18 (Suppl. 3), 280 (2017).
Joniau, S. et al. Stratification of high-risk prostate cancer into prognostic categories: a European multi-institutional study. Eur. Urol. 67, 157–164 (2015).
Van den Broeck, T. et al. Prognostic value of biochemical recurrence following treatment with curative intent for prostate cancer: a systematic review. Eur. Urol. 75, 967–987 (2019).
Acknowledgements
PIONEER is funded through the IMI2 Joint Undertaking and is listed under grant agreement No. 777492. IMI2 receives support from the European Union’s Horizon 2020 research and innovation programme and the European Federation of Pharmaceutical Industries and Associations (EFPIA). The views communicated within are those of PIONEER. Neither the IMI nor the European Union, EFPIA or any associated partners are responsible for any use that may be made of the information contained herein.
Author information
Authors and Affiliations
Consortia
Contributions
M.I.O., S.M., K.D., J.N.D. researched data for the article. M.I.O., S.M., M.J.R., M.J.Ro., K.D., T.V.D.B., S.J.M., S.E.A. G.G., P.P.W. and J.N.D. contributed substantially to discussion of the content. M.I.O., S.M., M.J.R., M.J.Ro., K.D., T.V.D.B., S.J.M., S.E.A. and G.G., wrote the article. M.I.O., P.P.W., K.M., J.B.R., Z.D., T.A., B.D.M., A.B., A.A. and J.N.D. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
T.A. is an employee of Astellas Pharma; S.E.A. and J.B.R. are employees of Bayer. Both companies make products used to treat prostate cancer. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks C. Moore, F. Saad, H. Goldberg and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
BD4BO: https://bd4bo.eu/
EAU: https://uroweb.org/
EAUN: https://nurses.uroweb.org/
ecancer: https://ecancer.org/en/
ECPC: https://ecpc.org/
EHDEN: https://www.ehden.eu/
EUROPA UOMO: https://www.europa-uomo.org/
Innovative Medicines Initiative 2: https://www.imi.europa.eu/about-imi
OHDSI: https://ohdsi.org/
PIONEER: https://prostate-pioneer.eu/Innovative
Prostate Cancer UK: https://prostatecanceruk.org/
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Omar, M.I., MacLennan, S., Ribal, M.J. et al. Unanswered questions in prostate cancer — findings of an international multi-stakeholder consensus by the PIONEER consortium. Nat Rev Urol 20, 494–501 (2023). https://doi.org/10.1038/s41585-023-00748-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-023-00748-9