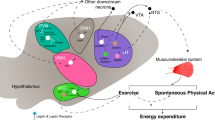
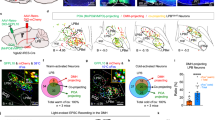
Abstract
The mammalian brain controls heat generation and heat loss mechanisms that regulate body temperature and energy metabolism. Thermoeffectors include brown adipose tissue, cutaneous blood flow and skeletal muscle, and metabolic energy sources include white adipose tissue. Neural and metabolic pathways modulating the activity and functional plasticity of these mechanisms contribute not only to the optimization of function during acute challenges, such as ambient temperature changes, infection and stress, but also to longitudinal adaptations to environmental and internal changes. Exposure of humans to repeated and seasonal cold ambient conditions leads to adaptations in thermoeffectors such as habituation of cutaneous vasoconstriction and shivering. In animals that undergo hibernation and torpor, neurally regulated metabolic and thermoregulatory adaptations enable survival during periods of significant reduction in metabolic rate. In addition, changes in diet can activate accessory neural pathways that alter thermoeffector activity. This knowledge may be harnessed for therapeutic purposes, including treatments for obesity and improved means of therapeutic hypothermia.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cramer, M. N., Gagnon, D., Laitano, O. & Crandall, C. G. Human temperature regulation under heat stress in health, disease, and injury. Physiol. Rev. 102, 1907–1989 (2022).
Song, N. J., Chang, S. H., Li, D. Y., Villanueva, C. J. & Park, K. W. Induction of thermogenic adipocytes: molecular targets and thermogenic small molecules. Exp. Mol. Med. 49, e353 (2017).
Fedorenko, A., Lishko, P. V. & Kirichok, Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 151, 400–413 (2012).
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 (2004). This review summarizes important aspects of molecular mechanisms that regulate brown adipose tissue function.
Matthias, A. et al. Thermogenic responses in brown fat cells are fully UCP1-dependent. UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty acid-induced thermogenesis. J. Biol. Chem. 275, 25073–25081 (2000).
Golozoubova, V. et al. Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J. 15, 2048–2050 (2001).
Nicholls, D. G. & Locke, R. M. Thermogenic mechanisms in brown fat. Physiol. Rev. 64, 1–64 (1984).
Cao, W. et al. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol. Cell. Biol. 24, 3057–3067 (2004).
Mukaida, S., Evans, B. A., Bengtsson, T., Hutchinson, D. S. & Sato, M. Adrenoceptors promote glucose uptake into adipocytes and muscle by an insulin-independent signaling pathway involving mechanistic target of rapamycin complex 2. Pharmacol. Res. 116, 87–92 (2017).
Olsen, J. M. et al. Glucose uptake in brown fat cells is dependent on mTOR complex 2-promoted GLUT1 translocation. J. Cell Biol. 207, 365–374 (2014).
Sell, H. et al. Peroxisome proliferator-activated receptor gamma agonism increases the capacity for sympathetically mediated thermogenesis in lean and ob/ob mice. Endocrinology 145, 3925–3934 (2004).
Sepa-Kishi, D. M., Jani, S., Da Eira, D. & Ceddia, R. B. Cold acclimation enhances UCP1 content, lipolysis, and triacylglycerol resynthesis, but not mitochondrial uncoupling and fat oxidation, in rat white adipocytes. Am. J. Physiol. Cell Physiol. 316, C365–C376 (2019).
Yao, T. et al. Ire1alpha in Pomc neurons is required for thermogenesis and glycemia. Diabetes 66, 663–673 (2017).
Madden, C. J. & Morrison, S. F. A high-fat diet impairs cooling-evoked brown adipose tissue activation via a vagal afferent mechanism. Am. J. Physiol. Endocrinol. Metab. 311, E287–292, (2016). This study shows that rats maintained on a high-fat diet showed a supression of brown adipose tissue thermogenesis that was mediated by vagal afferents during cooling.
Conceicao, E. P. S., Reynolds, C., Morrison, S. F. & Madden, C. J. Activation of transient receptor potential vanilloid 1 channels in the nucleus of the solitary tract and activation of dynorphin input to the median preoptic nucleus contribute to impaired BAT thermogenesis in diet induced obesity. eNeuro https://doi.org/10.1523/ENEURO.0048-21.2021 (2021). This study shows that rats maintained on a high-fat diet showed increased lipid levels in the nucleus tractus solitarius and that transient receptor potential vanilloid 1 and κ-opioid receptor activation mediated the suppression of brown adipose tissue activity during cooling.
Mota, C. M. D. & Madden, C. J. High fat diet suppresses energy expenditure via neurons in the brainstem. Neuroscience 520, 84–94 (2023).
Olsen, J. M. et al. beta(3)-Adrenergically induced glucose uptake in brown adipose tissue is independent of UCP1 presence or activity: mediation through the mTOR pathway. Mol. Metab. 6, 611–619 (2017).
Hankir, M. K. et al. Dissociation between brown adipose tissue [18F-FDG] uptake and thermogenesis in uncoupling protein 1-deficient mice. J. Nucl. Med. 58, 1100–1103 (2017).
Inokuma, K. et al. Uncoupling protein 1 is necessary for norepinephrine-induced glucose utilization in brown adipose tissue. Diabetes 54, 1385–1391 (2005).
Ma, S. W. & Foster, D. O. Uptake of glucose and release of fatty acids and glycerol by rat brown adipose tissue in vivo. Can. J. Physiol. Pharmacol. 64, 609–614 (1986).
Schweizer, S., Oeckl, J., Klingenspor, M. & Fromme, T. Substrate fluxes in brown adipocytes upon adrenergic stimulation and uncoupling protein 1 ablation. Life Sci. Alliance 1, e201800136 (2018).
Orava, J. et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 14, 272–279 (2011).
Wu, Q. et al. Fatty acid transport protein 1 is required for nonshivering thermogenesis in brown adipose tissue. Diabetes 55, 3229–3237 (2006).
Henkin, A. H. et al. Real-time noninvasive imaging of fatty acid uptake in vivo. ACS Chem. Biol. 7, 1884–1891 (2012).
Shabalina, I. G. et al. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep. 5, 1196–1203 (2013).
Jiang, W., Li, J., Gallowitsch-Puerta, M., Tracey, K. J. & Pisetsky, D. S. The effects of CpG DNA on HMGB1 release by murine macrophage cell lines. J. Leukoc. Biol. 78, 930–936 (2005).
Dieckmann, S. et al. Fatty acid metabolite profiling reveals oxylipins as markers of brown but not brite adipose tissue. Front. Endocrinol. 11, 73 (2020).
Jiang, H., Ding, X., Cao, Y., Wang, H. & Zeng, W. Dense intra-adipose sympathetic arborizations are essential for cold-induced beiging of mouse white adipose tissue. Cell Metab. 26, 686–692.e3 (2017).
Granneman, J. G., Burnazi, M., Zhu, Z. & Schwamb, L. A. White adipose tissue contributes to UCP1-independent thermogenesis. Am. J. Physiol. Endocrinol. Metab. 285, E1230–E1236 (2003).
Xu, X. et al. Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R1115–R1125 (2011).
Bertholet, A. M. et al. Mitochondrial patch clamp of beige adipocytes reveals UCP1-positive and UCP1-negative cells both exhibiting futile creatine cycling. Cell Metab. 25, 811–822 e814 (2017).
Kazak, L. et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163, 643–655 (2015).
Madden, C. J. & Morrison, S. F. Central nervous system circuits that control body temperature. Neurosci. Lett. 696, 225–232 (2019).
Morrison, S. F. & Nakamura, K. Central mechanisms for thermoregulation. Annu. Rev. Physiol. 81, 285–308 (2019).
Benarroch, E. E. Thermoregulation: recent concepts and remaining questions. Neurology 69, 1293–1297 (2007).
Janig, W. Peripheral thermoreceptors in innocuous temperature detection. Handb. Clin. Neurol. 156, 47–56 (2018).
McKemy, D. D., Neuhausser, W. M. & Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416, 52–58 (2002). This study shows that the TRPM8 receptor from trigeminal sensory neurons is activated by cool and cold thermal stimuli.
McKie, G. L., Medak, K. D., Shamshoum, H. & Wright, D. C. Topical application of the pharmacological cold mimetic menthol stimulates brown adipose tissue thermogenesis through a TRPM8, UCP1, and norepinephrine dependent mechanism in mice housed at thermoneutrality. FASEB J. 36, e22205 (2022).
Tajino, K. et al. Application of menthol to the skin of whole trunk in mice induces autonomic and behavioral heat-gain responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R2128–R2135 (2007).
Almeida, M. C. et al. Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J. Neurosci. 32, 2086–2099 (2012).
Bautista, D. M. et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448, 204–208 (2007).
Colburn, R. W. et al. Attenuated cold sensitivity in TRPM8 null mice. Neuron 54, 379–386 (2007).
Dhaka, A. et al. TRPM8 is required for cold sensation in mice. Neuron 54, 371–378 (2007).
Reimundez, A. et al. Deletion of the cold thermoreceptor TRPM8 increases heat loss and food intake leading to reduced body temperature and obesity in mice. J. Neurosci. 38, 3643–3656 (2018).
Ran, C., Hoon, M. A. & Chen, X. The coding of cutaneous temperature in the spinal cord. Nat. Neurosci. 19, 1201–1209 (2016).
Craig, A. D., Krout, K. & Andrew, D. Quantitative response characteristics of thermoreceptive and nociceptive lamina I spinothalamic neurons in the cat. J. Neurophysiol. 86, 1459–1480 (2001).
Li, J. et al. Medullary dorsal horn neurons providing axons to both the parabrachial nucleus and thalamus. J. Comp. Neurol. 498, 539–551 (2006).
Cechetto, D. F., Standaert, D. G. & Saper, C. B. Spinal and trigeminal dorsal horn projections to the parabrachial nucleus in the rat. J. Comp. Neurol. 240, 153–160 (1985).
Hylden, J. L., Anton, F. & Nahin, R. L. Spinal lamina I projection neurons in the rat: collateral innervation of parabrachial area and thalamus. Neuroscience 28, 27–37 (1989).
Craig, A. D. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 3, 655–666 (2002).
Norrsell, U. & Craig, A. D. Behavioral thermosensitivity after lesions of thalamic target areas of a thermosensory spinothalamic pathway in the cat. J. Neurophysiol. 82, 611–625 (1999).
Yahiro, T., Kataoka, N., Nakamura, Y. & Nakamura, K. The lateral parabrachial nucleus, but not the thalamus, mediates thermosensory pathways for behavioural thermoregulation. Sci. Rep. 7, 5031 (2017).
Nakamura, K. & Morrison, S. F. A thermosensory pathway that controls body temperature. Nat. Neurosci. 11, 62–71 (2008). This study showed that cutaneous thermosensory signals activate brown adipose tissue thermogenesis via parabrachial neurons that send glutamatergic inputs to the preoptic area.
Craig, A. D. Central neural substrates involved in temperature discrimination, thermal pain, thermal comfort, and thermoregulatory behavior. Handb. Clin. Neurol. 156, 317–338 (2018).
Kobayashi, A. & Osaka, T. Involvement of the parabrachial nucleus in thermogenesis induced by environmental cooling in the rat. Pflug. Arch. Eur. J. Physiol. 446, 760–765 (2003).
Nakamura, K. & Morrison, S. F. Preoptic mechanism for cold-defensive responses to skin cooling. J. Physiol. 586, 2611–2620 (2008).
Nakamura, K. & Morrison, S. F. Central efferent pathways for cold-defensive and febrile shivering. J. Physiol. 589, 3641–3658 (2011).
da Conceicao, E. P. S., Morrison, S. F., Cano, G., Chiavetta, P. & Tupone, D. Median preoptic area neurons are required for the cooling and febrile activations of brown adipose tissue thermogenesis in rat. Sci. Rep. 10, 18072 (2020).
Blessing, W. W. & Nalivaiko, E. Raphe magnus/pallidus neurons regulate tail but not mesenteric arterial blood flow in rats. Neuroscience 105, 923–929 (2001).
Ootsuka, Y., Blessing, W. W. & McAllen, R. M. Inhibition of rostral medullary raphe neurons prevents cold-induced activity in sympathetic nerves to rat tail and rabbit ear arteries. Neurosci. Lett. 357, 58–62 (2004).
Tanaka, M., McKinley, M. J. & McAllen, R. M. Preoptic-raphe connections for thermoregulatory vasomotor control. J. Neurosci. 31, 5078–5088 (2011).
Ootsuka, Y. & Tanaka, M. Control of cutaneous blood flow by central nervous system. Temperature 2, 392–405 (2015).
Rathner, J. A., Madden, C. J. & Morrison, S. F. Central pathway for spontaneous and prostaglandin E2-evoked cutaneous vasoconstriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R343–R354 (2008).
Egan, G. F. et al. Cortical, thalamic, and hypothalamic responses to cooling and warming the skin in awake humans: a positron-emission tomography study. Proc. Natl Acad. Sci. USA 102, 5262–5267 (2005).
Fechir, M. et al. Cortical control of thermoregulatory sympathetic activation. Eur. J. Neurosci. 31, 2101–2111 (2010).
McAllen, R. M. et al. Human medullary responses to cooling and rewarming the skin: a functional MRI study. Proc. Natl Acad. Sci. USA 103, 809–813 (2006).
Daniels, R. L., Takashima, Y. & McKemy, D. D. Activity of the neuronal cold sensor TRPM8 is regulated by phospholipase C via the phospholipid phosphoinositol 4,5-bisphosphate. J. Biol. Chem. 284, 1570–1582 (2009).
Diver, M. M., Cheng, Y. & Julius, D. Structural insights into TRPM8 inhibition and desensitization. Science 365, 1434–1440 (2019).
Fujita, F., Uchida, K., Takaishi, M., Sokabe, T. & Tominaga, M. Ambient temperature affects the temperature threshold for TRPM8 activation through interaction of phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 33, 6154–6159 (2013).
Teliban, A., Bartsch, F., Struck, M., Baron, R. & Janig, W. Responses of intact and injured sural nerve fibers to cooling and menthol. J. Neurophysiol. 111, 2071–2083 (2014).
Hensel, H. & Banet, M. Adaptive changes in cats after long-term exposure to various temperatures. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 52, 1008–1012 (1982).
Blondin, D. P. et al. Four-week cold acclimation in adult humans shifts uncoupling thermogenesis from skeletal muscles to brown adipose tissue. J. Physiol. 595, 2099–2113 (2017).
van der Lans, A. A. et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J. Clin. Invest. 123, 3395–3403 (2013).
Blondin, D. P. et al. Increased brown adipose tissue oxidative capacity in cold-acclimated humans. J. Clin. Endocrinol. Metab. 99, E438–E446 (2014).
Morrison, S. F., Ramamurthy, S. & Young, J. B. Reduced rearing temperature augments responses in sympathetic outflow to brown adipose tissue. J. Neurosci. 20, 9264–9271 (2000).
Robertson, C. E. & McClelland, G. B. Ancestral and developmental cold alter brown adipose tissue function and adult thermal acclimation in Peromyscus. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 191, 589–601 (2021).
Sun, W. et al. Cold-induced epigenetic programming of the sperm enhances brown adipose tissue activity in the offspring. Nat. Med. 24, 1372–1383 (2018).
Saito, M. et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58, 1526–1531 (2009).
Davies, V. S., Lindsund, E., Petrovic, N., Cannon, B. & Nedergaard, J. Repeated short excursions from thermoneutrality suffice to restructure brown adipose tissue. Biochimie 210, 40–49 (2023).
Heldmaier, G., Steinlechner, S., Rafael, J. & Latteier, B. Photoperiod and ambient temperature as environmental cues for seasonal thermogenic adaptation in the Djungarian hamster, Phodopus sungorus. Int. J. Biometeorol. 26, 339–345 (1982).
Geiser, F., Hiebert, S. & Kenagy, G. J. Torpor bout duration during the hibernation season of two sciurid rodents: interrelations with temperature and metabolism. Physiol. Zool. 63, 489–503 (1990).
Janský, L., Haddad, G., Kahlerová, Z. & Nedoma, J. Effect of external factors on hibernation of golden hamsters. J. Comp. Physiol. B 154, 427–433 (1984).
Bartness, T. J., Demas, G. E. & Song, C. K. Seasonal changes in adiposity: the roles of the photoperiod, melatonin and other hormones, and sympathetic nervous system. Exp. Biol. Med. 227, 363–376 (2002).
Vuarin, P., Dammhahn, M. & Henry, P.-Y. Individual flexibility in energy saving: body size and condition constrain torpor use. Funct. Ecol. 27, 793–799 (2013).
Florant, G. L., Nuttle, L. C., Mullinex, D. E. & Rintoul, D. A. Plasma and white adipose tissue lipid composition in marmots. Am. J. Physiol. 258, R1123–R1131 (1990).
Geiser, F., McAllan, B. M. & Kenagy, G. J. The degree of dietary fatty acid unsaturation affects torpor patterns and lipid composition of a hibernator. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 164, 299–305 (1994).
Ruf, T. & Arnold, W. Effects of polyunsaturated fatty acids on hibernation and torpor: a review and hypothesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1044–R1052 (2008).
Giroud, S. et al. Membrane phospholipid fatty acid composition regulates cardiac SERCA activity in a hibernator, the Syrian hamster (Mesocricetus auratus). PLoS ONE 8, e63111 (2013).
Conceicao, E. P. S., Reynolds, C. A., Morrison, S. F. & Madden, C. J. Activation of transient receptor potential vanilloid 1 channels in the nucleus of the solitary tract and activation of dynorphin input to the median preoptic nucleus contribute to impaired BAT thermogenesis in diet-induced obesity. eNeuro https://doi.org/10.1523/ENEURO.0048-21.2021 (2021).
Considine, R. V. et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 334, 292–295 (1996).
Xing, X., Yang, M. & Wang, D. H. The expression of leptin, hypothalamic neuropeptides and UCP1 before, during and after fattening in the Daurian ground squirrel (Spermophilus dauricus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 184, 105–112 (2015).
Haynes, W. G., Morgan, D. A., Walsh, S. A., Mark, A. L. & Sivitz, W. I. Receptor-mediated regional sympathetic nerve activation by leptin. J. Clin. Invest. 100, 270–278 (1997).
Morrison, S. F. Activation of 5-HT1A receptors in raphe pallidus inhibits leptin-evoked increases in brown adipose tissue thermogenesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R832–R837 (2004).
Ballinger, M. A., Hess, C., Napolitano, M. W., Bjork, J. A. & Andrews, M. T. Seasonal changes in brown adipose tissue mitochondria in a mammalian hibernator: from gene expression to function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 311, R325–R336 (2016).
Satinoff, E. Aberrations of regulation in ground squirrels following hypothalamic lesions. Am. J. Physiol. 212, 1215–1220 (1967).
Satinoff, E. Disruption of hibernation caused by hypothalamic lesions. Science 155, 1031–1033 (1967).
Heller, H. C. & Colliver, G. W. CNS regulation of body temperature during hibernation. Am. J. Physiol. 227, 583–589 (1974).
Kilduff, T. S., Miller, J. D., Radeke, C. M., Sharp, F. R. & Heller, H. C. 14C-2-deoxyglucose uptake in the ground squirrel brain during entrance to and arousal from hibernation. J. Neurosci. 10, 2463–2475 (1990).
Bratincsak, A. et al. Spatial and temporal activation of brain regions in hibernation: c-Fos expression during the hibernation bout in thirteen-lined ground squirrel. J. Comp. Neurol. 505, 443–458 (2007).
Conceicao, E. P. S., Madden, C. J. & Morrison, S. F. Neurons in the rat ventral lateral preoptic area are essential for the warm-evoked inhibition of brown adipose tissue and shivering thermogenesis. Acta Physiol. 225, e13213 (2019).
Madden, C. J. & Morrison, S. F. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R831–R843 (2009).
Amir, S., Shizgal, P. & Rompre, P. P. Glutamate injection into the suprachiasmatic nucleus stimulates brown fat thermogenesis in the rat. Brain Res. 498, 140–144 (1989).
Ruby, N. F., Dark, J., Heller, H. C. & Zucker, I. Ablation of suprachiasmatic nucleus alters timing of hibernation in ground squirrels. Proc. Natl Acad. Sci. USA 93, 9864–9868 (1996).
Hrvatin, S. et al. Neurons that regulate mouse torpor. Nature 583, 115–121 (2020). Together with Takahashi et al. (2020), this study was one of the first to describe a specific population of neurons that can induce a torpor-like state.
Takahashi, T. M. et al. A discrete neuronal circuit induces a hibernation-like state in rodents. Nature 583, 109–114 (2020). Together with Hrvatin et al. (2020), this study was one of the first to describe a specific population of neurons that can induce a torpor-like state.
Zhang, Z. et al. Estrogen-sensitive medial preoptic area neurons coordinate torpor in mice. Nat. Commun. 11, 6378 (2020).
Upton, B. A., D’Souza, S. P. & Lang, R. A. QPLOT neurons-converging on a thermoregulatory preoptic neuronal population. Front. Neurosci. 15, 665762 (2021).
Tan, C. L. et al. Warm-sensitive neurons that control body temperature. Cell 167, 47–59 e15 (2016).
Yamaguchi, H. et al. Dorsomedial and preoptic hypothalamic circuits control torpor. Curr. Biol. 33, 5381–5389.e4 (2023).
Ambler, M., Hitrec, T., Wilson, A., Cerri, M. & Pickering, A. Neurons in the dorsomedial hypothalamus promote, prolong, and deepen torpor in the mouse. J. Neurosci. 42, 4267–4277 (2022).
Jinka, T. R., Toien, O. & Drew, K. L. Season primes the brain in an arctic hibernator to facilitate entrance into torpor mediated by adenosine A(1) receptors. J. Neurosci. 31, 10752–10758 (2011).
Tamura, Y., Shintani, M., Nakamura, A., Monden, M. & Shiomi, H. Phase-specific central regulatory systems of hibernation in Syrian hamsters. Brain Res. 1045, 88–96 (2005).
Tupone, D., Cano, G. & Morrison, S. F. Thermoregulatory inversion: a novel thermoregulatory paradigm. Am. J. Physiol. Regul. Integr. Comp. Physiol. 312, R779–R786 (2017).
Tupone, D., Madden, C. J. & Morrison, S. F. Central activation of the A1 adenosine receptor (A1AR) induces a hypothermic, torpor-like state in the rat. J. Neurosci. 33, 14512–14525 (2013).
Scammell, T. E. et al. An adenosine A2a agonist increases sleep and induces Fos in ventrolateral preoptic neurons. Neuroscience 107, 653–663 (2001).
Stierman, B. National Health and Nutrition Examination Survey 2017–March 2020 prepandemic data files — development of files and prevalence estimates for selected health outcomes. National Health Statistics Reports https://doi.org/10.15620/cdc:106273 (2021).
World Obesity Federation. World Obesity Atlas 2023 (2023).
Verga, S., Buscemi, S. & Caimi, G. Resting energy expenditure and body composition in morbidly obese, obese and control subjects. Acta Diabetol. 31, 47–51 (1994).
DeLany, J. P., Kelley, D. E., Hames, K. C., Jakicic, J. M. & Goodpaster, B. H. High energy expenditure masks low physical activity in obesity. Int. J. Obes. 37, 1006–1011 (2013).
Faria, S. L., Faria, O. P., Menezes, C. S., de Gouvea, H. R. & de Almeida Cardeal, M. Metabolic profile of clinically severe obese patients. Obes. Surg. 22, 1257–1262 (2012).
Maric, I. et al. Sex and species differences in the development of diet-induced obesity and metabolic disturbances in rodents. Front. Nutr. 9, 828522 (2022).
Assaad, H. et al. Analysis of energy expenditure in diet-induced obese rats. Front. Biosci. 19, 967–985 (2014).
Bastardot, F., Marques-Vidal, P. & Vollenweider, P. Association of body temperature with obesity. The CoLaus study. Int. J. Obes. 43, 1026–1033 (2019).
Waalen, J. & Buxbaum, J. N. Is older colder or colder older? The association of age with body temperature in 18,630 individuals. J. Gerontol. A Biol. Sci. Med. Sci. 66, 487–492 (2011).
Anderson, G. S. & Martin, A. D. Calculated thermal conductivities and heat flux in man. Undersea Hyperb. Med. 21, 431–441 (1994).
Fischer, A. W., Cannon, B. & Nedergaard, J. No insulating effect of obesity, neither in mice nor in humans. Am. J. Physiol. Endocrinol. Metab. 317, E952–E953 (2019).
Fischer, A. W., Csikasz, R. I., von Essen, G., Cannon, B. & Nedergaard, J. No insulating effect of obesity. Am. J. Physiol. Endocrinol. Metab. 311, E202–E213 (2016).
Brychta, R. J. et al. Quantification of the capacity for cold-induced thermogenesis in young men with and without obesity. J. Clin. Endocrinol. Metab. 104, 4865–4878 (2019).
Brychta, R. J., Cypess, A. M., Reitman, M. L. & Chen, K. Y. Reply to letter to the editor: ‘No insulating effect of obesity, neither in mice nor in humans’. Am. J. Physiol. Endocrinol. Metab. 317, E954–E956 (2019).
Verbraecken, J., Van de Heyning, P., De Backer, W. & Van Gaal, L. Body surface area in normal-weight, overweight, and obese adults. A comparison study. Metabolism 55, 515–524 (2006).
Dow, C. A., Stauffer, B. L., Brunjes, D. L., Greiner, J. J. & DeSouza, C. A. Regular aerobic exercise reduces endothelin-1-mediated vasoconstrictor tone in overweight and obese adults. Exp. Physiol. 102, 1133–1142 (2017).
Barton, M., Baretella, O. & Meyer, M. R. Obesity and risk of vascular disease: importance of endothelium-dependent vasoconstriction. Br. J. Pharmacol. 165, 591–602 (2012).
Sivitz, W. I., Wayson, S. M., Bayless, M. L., Sinkey, C. A. & Haynes, W. G. Obesity impairs vascular relaxation in human subjects: hyperglycemia exaggerates adrenergic vasoconstriction arterial dysfunction in obesity and diabetes. J. Diabetes Complications 21, 149–157 (2007).
Schinzari, F. et al. Vasodilator responses and endothelin-dependent vasoconstriction in metabolically healthy obesity and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 309, E787–E792 (2015).
Komegae, E. N., Fonseca, M. T. & Steiner, A. A. Diet-induced obesity attenuates the hypothermic response to lipopolysaccharide independently of TNF-alpha production. Temperature 7, 270–276 (2020).
Sardjoe Mishre, A. S. D. et al. Association of shivering threshold time with body composition and brown adipose tissue in young adults. J. Therm. Biol. 108, 103277 (2022).
Zhou, H., Xie, D. & Xiao, P. Research on thermal comfort of obese and overweight people during indoor running exercise. Build. Environ. 242, 110574 (2023).
Sehl, P. L., Leites, G. T., Martins, J. B. & Meyer, F. Responses of obese and non-obese boys cycling in the heat. Int. J. Sports Med. 33, 497–501 (2012).
Loffler, H., Aramaki, J. U. & Effendy, I. The influence of body mass index on skin susceptibility to sodium lauryl sulphate. Skin. Res. Technol. 8, 19–22 (2002).
Cypess, A. M. et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517 (2009).
van Marken Lichtenbelt, W. D. et al. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360, 1500–1508 (2009). This study showed that glucose uptake into brown adipose tissue is inversely correlated with obesity.
Hollstein, T. et al. Reduced brown adipose tissue activity during cold exposure is a metabolic feature of the human thrifty phenotype. Metabolism 117, 154709 (2021).
Leitner, B. P. et al. Mapping of human brown adipose tissue in lean and obese young men. Proc. Natl Acad. Sci. USA 114, 8649–8654 (2017).
Morrison, S. F. RVLM and raphe differentially regulate sympathetic outflows to splanchnic and brown adipose tissue. Am. J. Physiol. 276, R962–R973 (1999).
Choi, K. M. et al. Defective brown adipose tissue thermogenesis and impaired glucose metabolism in mice lacking Letmd1. Cell Rep. 37, 110104 (2021).
Da Eira, D., Jani, S. & Ceddia, R. B. An obesogenic diet impairs uncoupled substrate oxidation and promotes whitening of the brown adipose tissue in rats. J. Physiol. 601, 69–82 (2023).
Sanchez-Rangel, E. et al. Norepinephrine transporter availability in brown fat is reduced in obesity: a human PET study with [(11)C] MRB. Int. J. Obes. 44, 964–967 (2020).
Davis, T. R. & Mayer, J. Imperfect homeothermia in the hereditary obese-hyperglycemic syndrome of mice. Am. J. Physiol. 177, 222–226 (1954).
Trayhurn, P., Thurlby, P. L. & James, W. P. Thermogenic defect in pre-obese ob/ob mice. Nature 266, 60–62 (1977).
Levin, B. E., Comai, K. & Sullivan, A. C. Metabolic and sympatho-adrenal abnormalities in the obese Zucker rat: effect of chronic phenoxybenzamine treatment. Pharmacol. Biochem. Behav. 14, 517–525 (1981).
Jung, R. T., Shetty, P. S., James, W. P., Barrand, M. A. & Callingham, B. A. Reduced thermogenesis in obesity. Nature 279, 322–323 (1979).
Knehans, A. W. & Romsos, D. R. Reduced norepinephrine turnover in brown adipose tissue of ob/ob mice. Am. J. Physiol. 242, E253–E261 (1982).
Young, J. B., Saville, E., Rothwell, N. J., Stock, M. J. & Landsberg, L. Effect of diet and cold exposure on norepinephrine turnover in brown adipose tissue of the rat. J. Clin. Invest. 69, 1061–1071 (1982).
Madden, C. J., Santos da Conceicao, E. P. & Morrison, S. F. Vagal afferent activation decreases brown adipose tissue (BAT) sympathetic nerve activity and BAT thermogenesis. Temperature 4, 89–96 (2017).
Cao, W. H., Madden, C. J. & Morrison, S. F. Inhibition of brown adipose tissue thermogenesis by neurons in the ventrolateral medulla and in the nucleus tractus solitarius. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R277–R290 (2010).
Doyle, M. W., Bailey, T. W., Jin, Y. H. & Andresen, M. C. Vanilloid receptors presynaptically modulate cranial visceral afferent synaptic transmission in nucleus tractus solitarius. J. Neurosci. 22, 8222–8229 (2002).
Hofmann, M. E. & Andresen, M. C. Vanilloids selectively sensitize thermal glutamate release from TRPV1 expressing solitary tract afferents. Neuropharmacology 101, 401–411 (2016).
Green, D. et al. Central activation of TRPV1 and TRPA1 by novel endogenous agonists contributes to mechanical allodynia and thermal hyperalgesia after burn injury. Mol. Pain. 12, 1744806916661725 (2016).
Patwardhan, A. M. et al. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. J. Clin. Invest. 120, 1617–1626 (2010).
Osthues, T. & Sisignano, M. Oxidized lipids in persistent pain states. Front. Pharmacol. 10, 1147 (2019).
Mohammed, M., Madden, C. J., Andresen, M. C. & Morrison, S. F. Activation of TRPV1 in nucleus tractus solitarius reduces brown adipose tissue thermogenesis, arterial pressure, and heart rate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 315, R134–R143 (2018).
Okada, S. et al. Functional involvement of nucleus tractus solitarii neurons projecting to the parabrachial nucleus in trigeminal neuropathic pain. J. Oral Sci. 61, 370–378 (2019).
Yang, W. Z. et al. Parabrachial neuron types categorically encode thermoregulation variables during heat defense. Sci. Adv. 6, eabb9414 (2020).
Hermanson, O., Telkov, M., Geijer, T., Hallbeck, M. & Blomqvist, A. Preprodynorphin mRNA-expressing neurones in the rat parabrachial nucleus: subnuclear localization, hypothalamic projections and colocalization with noxious-evoked fos-like immunoreactivity. Eur. J. Neurosci. 10, 358–367 (1998).
Huang, D., Grady, F. S., Peltekian, L. & Geerling, J. C. Efferent projections of Vglut2, Foxp2, and Pdyn parabrachial neurons in mice. J. Comp. Neurol. 529, 657–693 (2021).
Norris, A. J., Shaker, J. R., Cone, A. L., Ndiokho, I. B. & Bruchas, M. R. Parabrachial opioidergic projections to preoptic hypothalamus mediate behavioral and physiological thermal defenses. eLife 10, e60779 (2021).
Cintron-Colon, R. et al. Activation of kappa opioid receptor regulates the hypothermic response to calorie restriction and limits body weight loss. Curr. Biol. 29, 4291–4299.e4 (2019).
Xin, L., Geller, E. B. & Adler, M. W. Body temperature and analgesic effects of selective mu and kappa opioid receptor agonists microdialyzed into rat brain. J. Pharmacol. Exp. Ther. 281, 499–507 (1997).
Geerling, J. C. et al. Genetic identity of thermosensory relay neurons in the lateral parabrachial nucleus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R41–R54 (2016).
Yang, W. Z. et al. A parabrachial-hypothalamic parallel circuit governs cold defense in mice. Preprint at bioRxiv https://doi.org/10.1101/2023.04.19.537288 (2023).
Nakamura, K. & Morrison, S. F. A thermosensory pathway that controls body temperature. Nat. Neurosci. 11, 62–71 (2008).
Yahiro, T., Kataoka, N. & Nakamura, K. Two ascending thermosensory pathways from the lateral parabrachial nucleus that mediate behavioral and autonomous thermoregulation. Preprint at bioRxiv https://doi.org/10.1101/2023.04.10.536301 (2023).
Cano, G. et al. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J. Comp. Neurol. 460, 303–326 (2003).
Bratincsak, A. & Palkovits, M. Activation of brain areas in rat following warm and cold ambient exposure. Neuroscience 127, 385–397 (2004).
Kasuga, R. et al. Role of TRPM8 in cold avoidance behaviors and brain activation during innocuous and nocuous cold stimuli. Physiol. Behav. 248, 113729 (2022).
Feng, C. et al. Cold-sensitive ventromedial hypothalamic neurons control homeostatic thermogenesis and social interaction-associated hyperthermia. Cell Metab. 34, 888–901.e5 (2022).
Chitravanshi, V. C., Kawabe, K. & Sapru, H. N. Stimulation of the hypothalamic arcuate nucleus increases brown adipose tissue nerve activity via hypothalamic paraventricular and dorsomedial nuclei. Am. J. Physiol. Heart Circ. Physiol. 311, H433–H444 (2016).
Mota, C. M. D. & Madden, C. J. Mediobasal hypothalamic neurons contribute to the control of brown adipose tissue sympathetic nerve activity and cutaneous vasoconstriction. J. Therm. Biol. 114, 103551 (2023).
Cowley, M. A. et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37, 649–661 (2003).
Cowley, M. A. et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, 480–484 (2001).
Horvath, T. L., Bechmann, I., Naftolin, F., Kalra, S. P. & Leranth, C. Heterogeneity in the neuropeptide Y-containing neurons of the rat arcuate nucleus: GABAergic and non-GABAergic subpopulations. Brain Res. 756, 283–286 (1997).
Tang, Q. et al. MANF in POMC neurons promotes brown adipose tissue thermogenesis and protects against diet-induced obesity. Diabetes 71, 2344–2359 (2022).
Kong, D. et al. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell 151, 645–657 (2012).
Mota, C. M. D., Siler, D. A., Burchiel, K. J. & Madden, C. J. Acute deep brain stimulation of the paraventricular nucleus of the hypothalamus increases brown adipose tissue thermogenesis in rats. Neurosci. Lett. 799, 137130 (2023).
Munzberg, H. & Myers, M. G. Jr. Molecular and anatomical determinants of central leptin resistance. Nat. Neurosci. 8, 566–570 (2005).
Enriori, P. J., Sinnayah, P., Simonds, S. E., Garcia Rudaz, C. & Cowley, M. A. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J. Neurosci. 31, 12189–12197 (2011).
Munzberg, H., Flier, J. S. & Bjorbaek, C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145, 4880–4889 (2004).
El-Haschimi, K., Pierroz, D. D., Hileman, S. M., Bjorbaek, C. & Flier, J. S. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J. Clin. Invest. 105, 1827–1832 (2000).
Kaiyala, K. J., Ogimoto, K., Nelson, J. T., Muta, K. & Morton, G. J. Physiological role for leptin in the control of thermal conductance. Mol. Metab. 5, 892–902 (2016).
Mark, A. L. Selective leptin resistance revisited. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305, R566–R581 (2013).
Lin, X. et al. Dysregulation of insulin receptor substrate 2 in beta cells and brain causes obesity and diabetes. J. Clin. Invest. 114, 908–916 (2004).
Benedict, C. et al. Intranasal insulin enhances postprandial thermogenesis and lowers postprandial serum insulin levels in healthy men. Diabetes 60, 114–118 (2011).
Bruning, J. C. et al. Role of brain insulin receptor in control of body weight and reproduction. Science 289, 2122–2125 (2000).
Fosch, A., Zagmutt, S., Casals, N. & Rodriguez-Rodriguez, R. New insights of SF1 neurons in hypothalamic regulation of obesity and diabetes. Int. J. Mol. Sci. 22, 6186 (2021).
Tong, Q. et al. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 5, 383–393 (2007).
Brown, G. M. & Page, J. The effect of chronic exposure to cold on temperature and blood flow of the hand. J. Appl. Physiol. 5, 221–227 (1952).
Brown, G. M., Hatcher, J. D. & Page, J. Temperature and blood flow in the forearm of the Eskimo. J. Appl. Physiol. 5, 410–420 (1953).
Little, M. A., Thomas, R. B., Mazess, R. B. & Baker, P. T. Population differences and developmental changes in extremity temperature responses to cold among Andean Indians. Hum. Biol. 43, 70–91 (1971).
Nelms, J. D. & Soper, D. J. Cold vasodilatation and cold acclimatization in the hands of British fish filleters. J. Appl. Physiol. 17, 444–448 (1962).
Enander, A., Skoldstrom, B. & Holmer, I. Reactions to hand cooling in workers occupationally exposed to cold. Scand. J. Work Env. Health 6, 58–65 (1980).
Carman, K. W. & Knight, K. L. Habituation to cold-pain during repeated cryokinetic sessions. J. Athl. Train. 27, 223–230 (1992).
Tipton, M. J. et al. Habituation of the metabolic and ventilatory responses to cold-water immersion in humans. J. Therm. Biol. 38, 24–31 (2013).
Virtanen, K. A. et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360, 1518–1525 (2009).
Gerngross, C., Schretter, J., Klingenspor, M., Schwaiger, M. & Fromme, T. Active brown fat during (18)F-FDG PET/CT imaging defines a patient group with characteristic traits and an increased probability of brown fat redetection. J. Nucl. Med. 58, 1104–1110 (2017).
Orava, J. et al. Blunted metabolic responses to cold and insulin stimulation in brown adipose tissue of obese humans. Obesity 21, 2279–2287 (2013).
Blondin, D. P. et al. Selective impairment of glucose but not fatty acid or oxidative metabolism in brown adipose tissue of subjects with type 2 diabetes. Diabetes 64, 2388–2397 (2015).
Balkan, B., Strubbe, J. H., Bruggink, J. E. & Steffens, A. B. Overfeeding-induced obesity in rats: insulin sensitivity and autonomic regulation of metabolism. Metabolism 42, 1509–1518 (1993).
Bartelt, A. et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 17, 200–205 (2011).
Acknowledgements
The authors thank all authors whose work in thermoregulation and related areas contributed to this Review. The authors also thank the editor and reviewers for their constructive suggestions, which helped us to improve our Review. This work was supported by the National Institutes of Health (DK112198).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to this paper.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neuroscience thanks Jon Resch, Shaun F. Morrison and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mota, C.M.D., Madden, C.J. Neural circuits of long-term thermoregulatory adaptations to cold temperatures and metabolic demands. Nat. Rev. Neurosci. 25, 143–158 (2024). https://doi.org/10.1038/s41583-023-00785-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-023-00785-8