Abstract
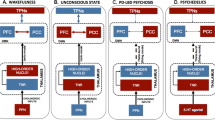
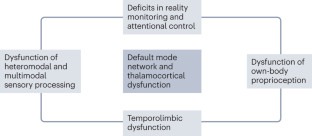
Parkinson disease (PD) psychosis (PDP) is a spectrum of illusions, hallucinations and delusions that are associated with PD throughout its disease course. Psychotic phenomena can manifest from the earliest stages of PD and might follow a continuum from minor hallucinations to structured hallucinations and delusions. Initially, PDP was considered to be a complication associated with dopaminergic drug use. However, subsequent research has provided evidence that PDP arises from the progression of brain alterations caused by PD itself, coupled with the use of dopaminergic drugs. The combined dysfunction of attentional control systems, sensory processing, limbic structures, the default mode network and thalamocortical connections provides a conceptual framework to explain how new incoming stimuli are incorrectly categorized, and how aberrant hierarchical predictive processing can produce false percepts that intrude into the stream of consciousness. The past decade has seen the publication of new data on the phenomenology and neurobiological basis of PDP from the initial stages of the disease, as well as the neurotransmitter systems involved in PDP initiation and progression. In this Review, we discuss the latest clinical, neuroimaging and neurochemical evidence that could aid early identification of psychotic phenomena in PD and inform the discovery of new therapeutic targets and strategies.
Key points
-
Parkinson disease (PD) psychosis (PDP) comprises a spectrum of illusions, hallucinations and delusions that are associated with PD throughout its course.
-
PDP is attributable not only to the use of dopaminergic drugs but also to inherent disruptions linked to the disease, which lead to dysfunction of neural systems governing visual perception, multimodal sensory integration, reality monitoring and attention.
-
Both minor and structured hallucinations in PD are associated with a pattern of cortical atrophy that includes the cuneus, precuneus, middle occipital gyrus, lingual and fusiform gyri, supramarginal gyrus, angular gyrus, anterior cingulate cortex, hippocampal regions and thalamus.
-
Functional neuroimaging studies indicate that PDP is associated with failure of top-down processing of attentional networks, aberrant coupling of the default mode network with visual networks and disconnection between the thalamus and posterior brain areas, leading to aberrant disinhibition of the default mode network.
-
Cortical cholinergic denervation and elevated levels of 5-HT2A serotonergic receptor binding in the ventral visual pathway, medial orbitofrontal cortex and insula have prominent roles in the development of visual hallucinations.
-
An important advance in the treatment of PDP has been the development of drugs that reduce the activity of cortical postsynaptic 5-HT2A receptors, of which pimavanserin is the most notable.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
ffytche, D. H. et al. The psychosis spectrum in Parkinson disease. Nat. Rev. Neurol. 13, 81–95 (2017).
Diederich, N. J., Fénelon, G., Stebbins, G. & Goetz, C. G. Hallucinations in Parkinson disease. Nat. Rev. Neurol. 5, 331–342 (2009).
Damasio, A. R., Lobo-Antunes, J. & Macedo, C. Psychiatric aspects in Parkinsonism treated with L-dopa. J. Neurol. Neurosurg. Psychiatry 34, 502–507 (1971).
Moskovitz, C., Moses, H. 3rd & Klawans, H. L. Levodopa-induced psychosis: a kindling phenomenon. Am. J. Psychiatry 135, 669–675 (1978).
Rinne, U. K., Sonninen, V. & Marttila, R. Dopaminergic agonist effects on Parkinsonian clinical features and brain monamine metabolism. Adv. Neurol. 9, 383–392 (1975).
Parkes, J. D. et al. Bromocriptine treatment in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 39, 184–193 (1976).
Holroyd, S. Prospective study of hallucinations and delusions in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 70, 734–738 (2001).
Goetz, C. G., Leurgans, S., Pappert, E. J., Raman, R. & Stemer, A. B. Prospective longitudinal assessment of hallucinations in Parkinson’s disease. Neurology 57, 2078–2082 (2001).
Williams, D. R. & Lees, A. J. Visual hallucinations in the diagnosis of idiopathic Parkinson’s disease: a retrospective autopsy study. Lancet Neurol. 4, 605–610 (2005).
Fénelon, G. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain 123, 733–745 (2000).
Fénelon, G., Goetz, C. G. & Karenberg, A. Hallucinations in Parkinson disease in the prelevodopa era. Neurology 66, 93–98 (2006).
Biousse, V. et al. Ophthalmologic features of Parkinson’s disease. Neurology 62, 177–180 (2004).
Ibarretxe-Bilbao, N. et al. Differential progression of brain atrophy in Parkinson’s disease with and without visual hallucinations. J. Neurol. Neurosurg. Psychiatry 81, 650–657 (2010).
Goldman, J. G. et al. Visuoperceptive region atrophy independent of cognitive status in patients with Parkinson’s disease with hallucinations. Brain 137, 849–859 (2014).
Meppelink, A. M. et al. Impaired visual processing preceding image recognition in Parkinson’s disease patients with visual hallucinations. Brain 132, 2980–2993 (2009).
Pagonabarraga, J. et al. Neural correlates of minor hallucinations in non-demented patients with Parkinson’s disease. Parkinsonism Relat. Disord. 20, 290–296 (2014).
Lenka, A., Pagonabarraga, J., Pal, P. K., Bejr-Kasem, H. & Kulisvesky, J. Minor hallucinations in Parkinson disease. Neurology 93, 259–266 (2019).
Fénelon, G., Soulas, T., de Langavant, L. C., Trinkler, I. & Bachoud-Levi, A.-C. Feeling of presence in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 82, 1219–1224 (2011).
Pagonabarraga, J. et al. Minor hallucinations occur in drug-naive Parkinson’s disease patients, even from the premotor phase. Mov. Disord. 31, 45–52 (2016).
Ravina, B. et al. Diagnostic criteria for psychosis in Parkinson’s disease: report of an NINDS, NIMH work group. Mov. Disord. 22, 1061–1068 (2007).
Fénelon, G., Soulas, T., Zenasni, F. & de Langavant, L. C. The changing face of Parkinson’s disease-associated psychosis: a cross-sectional study based on the new NINDS–NIMH criteria. Mov. Disord. 25, 763–766 (2010).
Zhong, M. et al. Prevalence and risk factors for minor hallucinations in patients with Parkinson’s disease. Behav. Neurol. 2021, 3469706 (2021).
Wood, R. A., Hopkins, S. A., Moodley, K. K. & Chan, D. Fifty percent prevalence of extracampine hallucinations in Parkinson’s disease patients. Front. Neurol. 6, 263 (2015).
Bejr‐Kasem, H. et al. Minor hallucinations reflect early gray matter loss and predict subjective cognitive decline in Parkinson’s disease. Eur. J. Neurol. 28, 438–447 (2021).
Doé de Maindreville, A., Fénelon, G. & Mahieux, F. Hallucinations in Parkinson’s disease: a follow‐up study. Mov. Disord. 20, 212–217 (2005).
Sasaki, C. et al. Visual illusions in Parkinson’s disease: an interview survey of symptomatology. Psychogeriatrics 22, 38–48 (2022).
Nishio, Y. et al. Defining visual illusions in Parkinson’s disease: kinetopsia and object misidentification illusions. Parkinsonism Relat. Disord. 55, 111–116 (2018).
Kulisevsky, J., Pagonabarraga, J., Pascual-Sedano, B., García-Sánchez, C. & Gironell, A. Prevalence and correlates of neuropsychiatric symptoms in Parkinson’s disease without dementia. Mov. Disord. 23, 1889–1896 (2008).
Gibson, G. et al. Frequency, prevalence, incidence and risk factors associated with visual hallucinations in a sample of patients with Parkinson’s disease: a longitudinal 4-year study. Int. J. Geriatr. Psychiatry 28, 626–631 (2013).
Stang, C. D. et al. Incidence, prevalence, and mortality of psychosis associated with Parkinson’s disease (1991–2010). J. Parkinsons Dis. 12, 1319–1327 (2022).
Aarsland, D., Ballard, C., Larsen, J. P. & McKeith, I. A comparative study of psychiatric symptoms in dementia with Lewy bodies and Parkinson’s disease with and without dementia. Int. J. Geriatr. Psychiatry 16, 528–536 (2001).
Chendo, I. et al. High frequency of psychosis in late-stage Parkinson’s disease. Clin. Park. Relat. Disord. 5, 100119 (2021).
Forsaa, E. B. et al. A 12-year population-based study of psychosis in Parkinson disease. Arch. Neurol. 67, 996–1001 (2010).
O’Brien, J. et al. Visual hallucinations in neurological and ophthalmological disease: pathophysiology and management. J. Neurol. Neurosurg. Psychiatry 91, 512–519 (2020).
Goetz, C. G. & Stebbins, G. T. Mortality and hallucinations in nursing home patients with advanced Parkinson’s disease. Neurology 45, 669–671 (1995).
Montagnese, M. et al. Cognition, hallucination severity and hallucination-specific insight in neurodegenerative disorders and eye disease. Cogn. Neuropsychiatry 27, 105–121 (2022).
Mosimann, U. P. et al. Characteristics of visual hallucinations in Parkinson disease dementia and dementia with Lewy bodies. Am. J. Geriatr. Psychiatry 14, 153–160 (2006).
Onofrj, M., Thomas, A. & Bonanni, L. New approaches to understanding hallucinations in Parkinson’s disease: phenomenology and possible origins. Expert Rev. Neurother. 7, 1731–1750 (2007).
Eversfield, C. L. & Orton, L. D. Auditory and visual hallucination prevalence in Parkinson’s disease and dementia with Lewy bodies: a systematic review and meta-analysis. Psychol. Med. 49, 2342–2353 (2019).
Fénelon, G., Thobois, S., Bonnet, A.-M., Broussolle, E. & Tison, F. Tactile hallucinations in Parkinson’s disease. J. Neurol. 249, 1699–1703 (2002).
Goetz, C. G., Vogel, C., Tanner, C. M. & Stebbins, G. T. Early dopaminergic drug-induced hallucinations in parkinsonian patients. Neurology 51, 811–814 (1998).
Goetz, C. G., Stebbins, G. T. & Ouyang, B. Visual plus nonvisual hallucinations in Parkinson’s disease: development and evolution over 10 years. Mov. Disord. 26, 2196–2200 (2011).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association Publishing, 2022).
Factor, S. A. et al. Cognitive correlates of hallucinations and delusions in Parkinson’s disease. J. Neurol. Sci. 347, 316–321 (2014).
Warren, N., O’Gorman, C., Hume, Z., Kisely, S. & Siskind, D. Delusions in Parkinson’s disease: a systematic review of published cases. Neuropsychol. Rev. 28, 310–316 (2018).
Stefanis, N. et al. Isolated delusional syndrome in Parkinson’s disease. Parkinsonism Relat. Disord. 16, 550–552 (2010).
Kiziltan, G., Özekmekçi, S., Ertan, S., Ertan, T. & Erginöz, E. Relationship between age and subtypes of psychotic symptoms in Parkinson’s disease. J. Neurol. 254, 448–452 (2007).
Poletti, M. et al. Dopamine agonists and delusional jealousy in Parkinson’s disease: a cross-sectional prevalence study. Mov. Disord. 27, 1679–1682 (2012).
De Michele, G. et al. Othello syndrome in Parkinson’s disease: a systematic review and report of a case series. Neurol. Sci. 42, 2721–2729 (2021).
Hashimoto, M., Sakamoto, S. & Ikeda, M. Clinical features of delusional jealousy in elderly patients with dementia. J. Clin. Psychiatry 76, 691–695 (2015).
Ballard, C. et al. Psychiatric morbidity in dementia with Lewy bodies: a prospective clinical and neuropathological comparative study with Alzheimer’s disease. Am. J. Psychiatry 156, 1039–1045 (1999).
Perini, G. et al. Misidentification delusions. Alzheimer Dis. Assoc. Disord. 30, 331–337 (2016).
Christodoulou, G. N., Margariti, M., Kontaxakis, V. P. & Christodoulou, N. G. The delusional misidentification syndromes: strange, fascinating, and instructive. Curr. Psychiatry Rep. 11, 185–189 (2009).
Pagonabarraga, J. et al. A prospective study of delusional misidentification syndromes in Parkinson’s disease with dementia. Mov. Disord. 23, 443–448 (2008).
Roane, D. M., Rogers, J. D., Robinson, J. H. & Feinberg, T. E. Delusional misidentification in association with parkinsonism. J. Neuropsychiatry Clin. Neurosci. 10, 194–198 (1998).
Hermanowicz, N. Delusional misidentification in Parkinson’s disease: report of two cases and a review. Postgrad. Med. 130, 280–283 (2018).
Moro, A., Munhoz, R. P., Moscovich, M., Arruda, W. O. & Teive, H. A. G. Delusional misidentification syndrome and other unusual delusions in advanced Parkinson’s disease. Parkinsonism Relat. Disord. 19, 751–754 (2013).
Nagahama, Y., Okina, T., Suzuki, N. & Matsuda, M. Neural correlates of psychotic symptoms in dementia with Lewy bodies. Brain 133, 557–567 (2010).
Mentis, M. J. et al. Abnormal brain glucose metabolism in the delusional misidentification syndromes: a positron emission tomography study in Alzheimer disease. Biol. Psychiatry 38, 438–449 (1995).
Weilnhammer, V. A., Stuke, H., Sterzer, P. & Schmack, K. The neural correlates of hierarchical predictions for perceptual decisions. J. Neurosci. 38, 5008–5021 (2018).
Pezzoli, S. et al. Neuroanatomical and cognitive correlates of visual hallucinations in Parkinson’s disease and dementia with Lewy bodies: voxel-based morphometry and neuropsychological meta-analysis. Neurosci. Biobehav. Rev. 128, 367–382 (2021).
Aarsland, D. et al. Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Primers 7, 47 (2021).
Martinez-Horta, S. & Kulisevsky, J. Mild cognitive impairment in Parkinson’s disease. J. Neural Transm. 126, 897–904 (2019).
Anang, J. B. et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology 83, 1253–1260 (2014).
Goetz, C. G., Fan, W., Leurgans, S., Bernard, B. & Stebbins, G. T. The malignant course of “benign hallucinations” in Parkinson disease. Arch. Neurol. 63, 713–716 (2006).
Llebaria, G. et al. Neuropsychological correlates of mild to severe hallucinations in Parkinson’s disease. Mov. Disord. 25, 2785–2791 (2010).
Bronnick, K., Emre, M., Tekin, S., Haugen, S. B. & Aarsland, D. Cognitive correlates of visual hallucinations in dementia associated with Parkinson’s disease. Mov. Disord. 26, 824–829 (2011).
Thomas, G. E. C. et al. Changes in both top-down and bottom-up effective connectivity drive visual hallucinations in Parkinson’s disease. Brain Commun. 5, fcac329 (2023).
Serre, T., Oliva, A. & Poggio, T. A feedforward architecture accounts for rapid categorization. Proc. Natl Acad. Sci. USA 104, 6424–6429 (2007).
Blanke, O. Multisensory brain mechanisms of bodily self-consciousness. Nat. Rev. Neurosci. 13, 556–571 (2012).
Bernasconi, F. et al. Robot-induced hallucinations in Parkinson’s disease depend on altered sensorimotor processing in fronto-temporal network. Sci. Transl. Med. 13, eabc8362 (2021).
Bar, M. A cortical mechanism for triggering top-down facilitation in visual object recognition. J. Cogn. Neurosci. 15, 600–609 (2003).
Collerton, D., Perry, E. & McKeith, I. Why people see things that are not there: a novel perception and attention deficit model for recurrent complex visual hallucinations. Behav. Brain Sci. 28, 737–757 (2005).
Shine, J. M. et al. The role of dysfunctional attentional control networks in visual misperceptions in Parkinson’s disease. Hum. Brain Mapp. 35, 2206–2219 (2014).
Schendan, H. E. & Ganis, G. Top-down modulation of visual processing and knowledge after 250 ms supports object constancy of category decisions. Front. Psychol. 6, 1289 (2015).
Schendan, H. E. & Maher, S. M. Object knowledge during entry-level categorization is activated and modified by implicit memory after 200 ms. Neuroimage 44, 1423–1438 (2009).
Bejr-Kasem, H. et al. The role of attentional control over interference in minor hallucinations in Parkinson’s disease. Parkinsonism Relat. Disord. 102, 101–107 (2022).
Barnes, J., Boubert, L., Harris, J., Lee, A. & David, A. S. Reality monitoring and visual hallucinations in Parkinson’s disease. Neuropsychologia 41, 565–574 (2003).
Johnson, M. K., Hashtroudi, S. & Lindsay, D. S. Source monitoring. Psychol. Bull. 114, 3–28 (1993).
Muller, A. J., Shine, J. M., Halliday, G. M. & Lewis, S. J. G. Visual hallucinations in Parkinson’s disease: theoretical models. Mov. Disord. 29, 1591–1598 (2014).
Collerton, D. et al. Understanding visual hallucinations: a new synthesis. Neurosci. Biobehav. Rev. 150, 105208 (2023).
Geddes, M. R. et al. Altered functional connectivity in lesional peduncular hallucinosis with REM sleep behavior disorder. Cortex 74, 96–106 (2016).
Nishio, Y. et al. Deconstructing psychosis and misperception symptoms in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 88, 722–729 (2017).
Bejr-Kasem, H. et al. Disruption of the default mode network and its intrinsic functional connectivity underlies minor hallucinations in Parkinson’s disease. Mov. Disord. 34, 78–86 (2019).
Zhong, M. et al. Aberrant gray matter volume and functional connectivity in Parkinson’s disease with minor hallucination. Front. Aging Neurosci. 14, 923560 (2022).
Nagahama, Y. et al. Classification of psychotic symptoms in dementia with Lewy bodies. Am. J. Geriatr. Psychiatry 15, 961–967 (2007).
Vignando, M. et al. Mapping brain structural differences and neuroreceptor correlates in Parkinson’s disease visual hallucinations. Nat. Commun. 13, 519 (2022).
Barrett, M. J., Blair, J. C., Sperling, S. A., Smolkin, M. E. & Druzgal, T. J. Baseline symptoms and basal forebrain volume predict future psychosis in early Parkinson disease. Neurology 90, e1618–e1626 (2018).
Lenka, A. et al. Hippocampal subfield atrophy in patients with Parkinson’s disease and psychosis. J. Neural Transm. 125, 1361–1372 (2018).
Ramírez-Ruiz, B. et al. Cerebral atrophy in Parkinson’s disease patients with visual hallucinations. Eur. J. Neurol. 14, 750–756 (2007).
Shin, S. et al. Neuroanatomical substrates of visual hallucinations in patients with non-demented Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 83, 1155–1161 (2012).
Watanabe, H. et al. Cortical and subcortical brain atrophy in Parkinson’s disease with visual hallucination. Mov. Disord. 28, 1732–1736 (2013).
Okada, K., Suyama, N., Oguro, H., Yamaguchi, S. & Kobayashi, S. Medication-induced hallucination and cerebral blood flow in Parkinson’s disease. J. Neurol. 246, 365–368 (1999).
Stebbins, G. T. et al. Altered cortical visual processing in PD with hallucinations: an fMRI study. Neurology 63, 1409–1416 (2004).
Oishi, N. et al. Regional cerebral blood flow in Parkinson disease with nonpsychotic visual hallucinations. Neurology 65, 1708–1715 (2011).
Matsui, H. et al. Hypoperfusion of the visual pathway in parkinsonian patients with visual hallucinations. Mov. Disord. 21, 2140–2144 (2006).
Boecker, H., Ceballos-Baumann, A. O., Volk, D. & Conrad, B. Metabolic alterations in patients with Parkinson disease and visual hallucinations. Arch. Neurol. 64, 984–988 (2015).
Goetz, C. G., Vaughan, C. L., Goldman, J. G. & Stebbins, G. T. I finally see what you see: Parkinson’s disease visual hallucinations captured with functional neuroimaging. Mov. Disord. 29, 115–117 (2014).
Gasca-Salas, C., Clavero, P., García-García, D., Obeso, J. A. & Rodríguez-Oroz, M. C. Significance of visual hallucinations and cerebral hypometabolism in the risk of dementia in Parkinson’s disease patients with mild cognitive impairment. Hum. Brain Mapp. 37, 968–977 (2016).
Ramírez-Ruiz, B., Junqué, C., Martí, M.-J., Valldeoriola, F. & Tolosa, E. Neuropsychological deficits in Parkinson’s disease patients with visual hallucinations. Mov. Disord. 21, 1483–1487 (2006).
Nagano-Saito, A. et al. Visual hallucination in Parkinson’s disease with FDG PET. Mov. Disord. 19, 801–806 (2004).
Ramirez-Ruiz, B. et al. Brain response to complex visual stimuli in Parkinson’s patients with hallucinations: a functional magnetic resonance imaging study. Mov. Disord. 23, 2335–2343 (2008).
Sanchez-Castaneda, C. et al. Frontal and associative visual areas related to visual hallucinations in dementia with Lewy bodies and Parkinson’s disease with dementia. Mov. Disord. 25, 615–622 (2010).
Gama, R. L. et al. Structural brain abnormalities in patients with Parkinson’s disease with visual hallucinations: a comparative voxel-based analysis. Brain Cogn. 87, 97–103 (2014).
Ibarretxe-Bilbao, N. et al. Hippocampal head atrophy predominance in Parkinson’s disease with hallucinations and with dementia. J. Neurol. 255, 1324–1331 (2008).
Janzen, J. et al. The pedunculopontine nucleus is related to visual hallucinations in Parkinson’s disease: preliminary results of a voxel-based morphometry study. J. Neurol. 259, 147–154 (2012).
Shine, J. M., Halliday, G. M., Naismith, S. L. & Lewis, S. J. G. Visual misperceptions and hallucinations in Parkinson’s disease: dysfunction of attentional control networks? Mov. Disord. 26, 2154–2159 (2011).
Shine, J. M. et al. Imagine that: elevated sensory strength of mental imagery in individuals with Parkinson’s disease and visual hallucinations. Proc. Biol. Sci. 282, 20142047 (2015).
Shine, J. M. et al. Abnormal connectivity between the default mode and the visual system underlies the manifestation of visual hallucinations in Parkinson’s disease: a task-based fMRI study. Parkinsons Dis. 1, 15003 (2015).
Corbetta, M. & Shulman, G. L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3, 201–215 (2002).
Fox, M. D. et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl Acad. Sci. USA 102, 9673–9678 (2005).
Buckner, R. L. & DiNicola, L. M. The brain’s default network: updated anatomy, physiology and evolving insights. Nat. Rev. Neurosci. 20, 593–608 (2019).
Lewis, G. J. & Bates, T. C. The long reach of the gene. Psychologist 26, 194–198 (2013).
Fox, M. D., Corbetta, M., Snyder, A. Z., Vincent, J. L. & Raichle, M. E. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl Acad. Sci. USA 103, 10046–10051 (2006).
Eckert, M. A. et al. At the heart of the ventral attention system: the right anterior insula. Hum. Brain Mapp. 30, 2530–2541 (2009).
Shine, J. M., Halliday, G. H., Carlos, M., Naismith, S. L. & Lewis, S. J. G. Investigating visual misperceptions in Parkinson’s disease: a novel behavioral paradigm. Mov. Disord. 27, 500–505 (2012).
Hepp, D. H. et al. Loss of functional connectivity in patients with Parkinson disease and visual hallucinations. Radiology 285, 896–903 (2017).
Miloserdov, K. et al. Aberrant functional connectivity of resting state networks related to misperceptions and intra-individual variability in Parkinson’s disease. Neuroimage Clin. 25, 102076 (2020).
Dujardin, K. et al. What can we learn from fMRI capture of visual hallucinations in Parkinson’s disease? Brain Imaging Behav. 14, 329–335 (2020).
Walpola, I. C. et al. Mind-wandering in Parkinson’s disease hallucinations reflects primary visual and default network coupling. Cortex 125, 233–245 (2020).
Knolle, F. et al. Altered subcortical emotional salience processing differentiates Parkinson’s patients with and without psychotic symptoms. Neuroimage Clin. 27, 10227 (2020).
Zarkali, A. et al. Changes in dynamic transitions between integrated and segregated states underlie visual hallucinations in Parkinson’s disease. Commun. Biol. 5, 928 (2022).
Onofrj, M., Espay, A. J., Bonanni, L., Delli Pizzi, S. & Sensi, S. L. Hallucinations, somatic‐functional disorders of PD‐DLB as expressions of thalamic dysfunction. Mov. Disord. 34, 1100–1111 (2019).
Zarkali, A. et al. Fiber-specific white matter reductions in Parkinson hallucinations and visual dysfunction. Neurology 94, E1525–E1538 (2020).
Zarkali, A., McColgan, P., Leyland, L. A., Lees, A. J. & Weil, R. S. Longitudinal thalamic white and grey matter changes associated with visual hallucinations in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 93, 169–179 (2022).
Zarkali, A. et al. Differences in network controllability and regional gene expression underlie hallucinations in Parkinson’s disease. Brain 143, 3435–3448 (2021).
Thomas, G. E. C. et al. Changes in both top-down and bottom-up effective connectivity drive visual hallucinations in Parkinson’s disease. Brain Commun. 5, fcac329 (2022).
Chaumon, M., Kveraga, K., Barrett, L. F. & Bar, M. Visual predictions in the orbitofrontal cortex rely on associative content. Cereb. Cortex 24, 2899–2907 (2014).
Zarkali, A. et al. Increased weighting on prior knowledge in Lewy body-associated visual hallucinations. Brain Commun. 1, fcz007 (2019).
Lefebvre, S. et al. Hallucinations and conscious access to visual inputs in Parkinson’s disease. Sci. Rep. 6, 36284 (2016).
Perinelli, A., Tabarelli, D., Miniussi, C. & Ricci, L. Dependence of connectivity on geometric distance in brain networks. Sci. Rep. 9, 13412 (2019).
Yao, N. et al. The default mode network is disrupted in parkinson’s disease with visual hallucinations. Hum. Brain Mapp. 35, 5658–5666 (2014).
Franciotti, R. et al. Default mode network links to visual hallucinations: a comparison between Parkinson’s disease and multiple system atrophy. Mov. Disord. 30, 1237–1247 (2015).
Yao, N. et al. Multimodal MRI of the hippocampus in Parkinson’s disease with visual hallucinations. Brain Struct. Funct. 221, 287–300 (2016).
Yao, N. et al. Resting activity in visual and corticostriatal pathways in Parkinson’s disease with hallucinations. Parkinsonism Relat. Disord. 21, 131–137 (2015).
Lee, J. Y. et al. Lateral geniculate atrophy in Parkinson’s with visual hallucination: a trans-synaptic degeneration? Mov. Disord. 31, 547–554 (2016).
Miyata, M. et al. Optic radiation atrophy in Lewy body disease with visual hallucination on phase difference enhanced magnetic resonance images. Sci. Rep. 12, 18556 (2022).
Hepp, D. H. et al. Damaged fiber tracts of the nucleus basalis of Meynert in Parkinson’s disease patients with visual hallucinations. Sci. Rep. 7, 10112 (2017).
Yuki, N., Yoshioka, A., Mizuhara, R. & Kimura, T. Visual hallucinations and inferior longitudinal fasciculus in Parkinson’s disease. Brain Behav. 10, e01883 (2020).
Zhong, J. M. et al. Why psychosis is frequently associated with Parkinson’s disease? Neural Regen. Res. 8, 2548–2556 (2013).
Hall, J. M. et al. Changes in structural network topology correlate with severity of hallucinatory behavior in Parkinson’s disease. Netw. Neurosci. 3, 521–538 (2019).
Rootes-Murdy, K., Goldsmith, D. R. & Turner, J. A. Clinical and structural differences in delusions across diagnoses: a systematic review. Front. Integr. Neurosci. 15, 726321 (2022).
Factor, S. A., Molho, E. S., Podskalny, G. D. & Brown, D. Parkinson’s disease: drug-induced psychiatric states. Adv. Neurol. 65, 115–138 (1995).
Goetz, C., Tanner, C. & Klawans, H. Pharmacology of hallucinations induced by long-term drug therapy. Am. J. Psychiatry 139, 494–497 (1982).
de la Riva, P., Smith, K., Xie, S. X. & Weintraub, D. Course of psychiatric symptoms and global cognition in early Parkinson disease. Neurology 83, 1096–1103 (2014).
Neumann, J. et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain 132, 1783–1794 (2009).
Dotchin, C. L., Jusabani, A. & Walker, R. W. Non-motor symptoms in a prevalent population with Parkinson’s disease in Tanzania. Parkinsonism Relat. Disord. 15, 457–460 (2009).
Wolters, E. C. Dopaminomimetic psychosis in Parkinson’s disease patients: diagnosis and treatment. Neurology 52, S10–S13 (1999).
McCutcheon, R. A. et al. Mesolimbic dopamine function is related to salience network connectivity: an integrative positron emission tomography and magnetic resonance study. Biol. Psychiatry 85, 368–378 (2019).
Russo, M. et al. The pharmacology of visual hallucinations in synucleinopathies. Front. Pharmacol. 10, 1379 (2019).
van der Zee, S. et al. Altered cholinergic innervation in de novo Parkinson’s disease with and without cognitive impairment. Mov. Disord. 37, 713–723 (2022).
Bohnen, N. I. et al. Cholinergic system changes in Parkinson’s disease: emerging therapeutic approaches. Lancet Neurol. 21, 381–392 (2022).
Whitehouse, P. J., Hedreen, J. C., White, C. L. & Price, D. L. Basal forebrain neurons in the dementia of Parkinson disease. Ann. Neurol. 13, 243–248 (1983).
Perry, E. K. et al. Cholinergic correlates of cognitive impairment in Parkinson’s disease: comparisons with Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 48, 413–421 (1985).
Shimada, H. et al. Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology 73, 273–278 (2009).
Bohnen, N. I. et al. Progression of regional cortical cholinergic denervation in Parkinson’s disease. Brain Commun. 4, fcac320 (2022).
Ray, N. J., Kanel, P. & Bohnen, N. I. Atrophy of the cholinergic basal forebrain can detect presynaptic cholinergic loss in Parkinson’s disease. Ann. Neurol. 93, 991–998 (2023).
Manganelli, F. et al. Functional involvement of central cholinergic circuits and visual hallucinations in Parkinson’s disease. Brain 132, 2350–2355 (2009).
Johnson, M. W., Hendricks, P. S., Barrett, F. S. & Griffiths, R. R. Classic psychedelics: an integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacol. Ther. 197, 83–102 (2019).
Hofmann, A. Psychotomimetic drugs; chemical and pharmacological aspects. Acta Physiol. Pharmacol. Neerl. 8, 240–258 (1959).
Cheng, A. V. T. et al. Cortical serotonin-S2 receptor binding in Lewy body dementia, Alzheimer’s and Parkinson’s diseases. J. Neurol. Sci. 106, 50–55 (1991).
Chen, C. et al. Post-synaptic 5-HT1A and 5-HT2A receptors are increased in Parkinson’s disease neocortex. Ann. N. Y. Acad. Sci. 861, 288–289 (1998).
Rasmussen, N. B. et al. 5-HT2A receptor binding in the frontal cortex of Parkinson’s disease patients and alpha-synuclein overexpressing mice: a postmortem study. Parkinsons Dis. 2016, 3682936 (2016).
Huot, P. et al. Increased 5-HT2A receptors in the temporal cortex of parkinsonian patients with visual hallucinations. Mov. Disord. 25, 1399–1408 (2010).
Ballanger, B. et al. Serotonin 2A receptors and visual hallucinations in Parkinson disease. Arch. Neurol. 67, 416–421 (2010).
Burstein, E. S. Relevance of 5-HT2A receptor modulation of pyramidal cell excitability for dementia-related psychosis: implications for pharmacotherapy. CNS Drugs 35, 727–741 (2021).
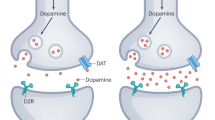
Papapetropoulos, S. Regional alpha-synuclein aggregation, dopaminergic dysregulation, and the development of drug-related visual hallucinations in Parkinson’s disease. J. Neuropsychiatry Clin. Neurosci. 18, 149–157 (2006).
Barnes, N. M., Hales, T. G., Lummis, S. C. R. & Peters, J. A. The 5-HT3 receptor — the relationship between structure and function. Neuropharmacology 56, 273–284 (2009).
Tsitsipa, E. et al. Selective 5HT3 antagonists and sensory processing: a systematic review. Neuropsychopharmacology 47, 880–890 (2022).
Zhang, Z.-J. et al. Beneficial effects of ondansetron as an adjunct to haloperidol for chronic, treatment-resistant schizophrenia: a double-blind, randomized, placebo-controlled study. Schizophr. Res. 88, 102–110 (2006).
Arnsten, A. F. T., Lin, C. H., Van Dyck, C. H. & Stanhope, K. J. The effects of 5-HT3 receptor antagonists on cognitive performance in aged monkeys. Neurobiol. Aging 18, 21–28 (1997).
Gil-Bea, F. J. et al. Facilitation of cholinergic transmission by combined treatment of ondansetron with flumazenil after cortical cholinergic deafferentation. Neuropharmacology 47, 225–232 (2004).
Garani, R., Watts, J. J. & Mizrahi, R. Endocannabinoid system in psychotic and mood disorders, a review of human studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 106, 110096 (2021).
Lu, H.-C. & Mackie, K. An introduction to the endogenous cannabinoid system. Biol. Psychiatry 79, 516–525 (2016).
Katona, I. & Freund, T. F. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat. Med. 14, 923–930 (2008).
Marco, E. Endocannabinoid system and psychiatry: in search of a neurobiological basis for detrimental and potential therapeutic effects. Front. Behav. Neurosci. 5, 63 (2011).
Van Laere, K. et al. Regional changes in type 1 cannabinoid receptor availability in Parkinson’s disease in vivo. Neurobiol. Aging 33, 620.e1–620.e8 (2012).
Halff, E. F., Rutigliano, G., Garcia-Hidalgo, A. & Howes, O. D. Trace amine-associated receptor 1 (TAAR1) agonism as a new treatment strategy for schizophrenia and related disorders. Trends Neurosci. 46, 60–74 (2023).
Dave, S., Weintraub, D., Aarsland, D. & ffytche, D. H. Drug and disease effects in Parkinson’s psychosis: revisiting the role of dopamine. Mov. Disord. Clin. Pract. 7, 32–36 (2020).
Friedman, J. H. & Factor, S. A. Atypical antipsychotics in the treatment of drug-induced psychosis in Parkinson’s disease. Mov. Disord. 15, 201–211 (2000).
Meco, G., Alessandria, A., Bonifati, V. & Giustini, P. Risperidone for hallucinations in levodopa-treated Parkinson’s disease patients. Lancet 343, 1370–1371 (1994).
Goetz, C. G., Blasucci, L. M., Leurgans, S. & Pappert, E. J. Olanzapine and clozapine. Neurology 55, 789–794 (2000).
Kashihara, K., Maeda, T. & Yoshida, K. Safety and tolerability of aripiprazole in patients with psychosis associated with Parkinson’s disease—results of a multicenter open trial. Neuropsychopharmacol. Rep. 42, 135–141 (2022).
Younce, J. R., Davis, A. A. & Black, K. J. A systematic review and case series of ziprasidone for psychosis in Parkinson’s disease. J. Parkinsons Dis. 9, 63–71 (2019).
Seppi, K. et al. Update on treatments for nonmotor symptoms of Parkinson’s disease—an evidence‐based medicine review. Mov. Disord. 34, 180–198 (2019).
Weintraub, D. et al. Association of antipsychotic use with mortality risk in patients with Parkinson disease. JAMA Neurol. 73, 535–541 (2016).
Pollak, P. et al. Clozapine in drug induced psychosis in Parkinson’s disease: a randomised, placebo controlled study with open follow up. J. Neurol. Neurosurg. Psychiatry 75, 689–695 (2004).
Parkinson Study Group. Low-dose clozapine for the treatment of drug-induced psychosis in Parkinson’s disease. N. Engl. J. Med. 340, 757–763 (1999).
Emre, M. et al. Rivastigmine for dementia associated with Parkinson’s disease. N. Engl. J. Med. 351, 2509–2518 (2004).
Burn, D. et al. Effects of rivastigmine in patients with and without visual hallucinations in dementia associated wsith Parkinson’s disease. Mov. Disord. 21, 1899–1907 (2006).
Reading, P. J., Luce, A. K. & McKeith, I. G. Rivastigmine in the treatment of parkinsonian psychosis and cognitive impairment: preliminary findings from an open trial. Mov. Disord. 16, 1171–1174 (2001).
Henderson, E. J. et al. Rivastigmine for gait stability in patients with Parkinson’s disease (ReSPonD): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 15, 249–258 (2016).
d’Angremont, E., Begemann, M. J. H., van Laar, T. & Sommer, I. E. C. Cholinesterase inhibitors for treatment of psychotic symptoms in Alzheimer disease and Parkinson disease. JAMA Neurol. 80, 813–823 (2023).
Vanover, K. E. et al. Pharmacological and behavioral profile of N-(4-fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N-(4-(2-methylpropyloxy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-hydroxytryptamine-2A receptor inverse agonist. J. Pharmacol. Exp. Ther. 317, 910–918 (2006).
Cummings, J. et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet 383, 533–540 (2014).
Meltzer, H. Y. et al. Pimavanserin, a serotonin2A receptor inverse agonist, for the treatment of Parkinson’s disease psychosis. Neuropsychopharmacology 35, 881–892 (2010).
Tariot, P. N. et al. Trial of pimavanserin in dementia-related psychosis. N. Engl. J. Med. 385, 309–319 (2021).
Isaacson, S. H. et al. Efficacy results of pimavanserin from a multi-center, open-label extension study in Parkinson’s disease psychosis patients. Parkinsonism Relat. Disord. 87, 25–31 (2021).
Ballard, C. G. et al. Long-term evaluation of open-label pimavanserin safety and tolerability in Parkinson’s disease psychosis. Parkinsonism Relat. Disord. 77, 100–106 (2020).
Longardner, K. et al. Assessing the risks of treatment in Parkinson disease psychosis: an in-depth analysis. PLoS ONE 18, e0278262 (2023).
Pham Nguyen, T. P., Thibault, D., Hamedani, A. G., Weintraub, D. & Willis, A. W. Atypical antipsychotic use and mortality risk in Parkinson disease. Parkinsonism Relat. Disord. 103, 17–22 (2022).
Wildeboer, K. M., Zheng, L., Choo, K. S. & Stevens, K. E. Ondansetron results in improved auditory gating in DBA/2 mice through a cholinergic mechanism. Brain Res. 1300, 41–50 (2009).
Hashimoto, K., Iyo, M., Freedman, R. & Stevens, K. E. Tropisetron improves deficient inhibitory auditory processing in DBA/2 mice: role of α7 nicotinic acetylcholine receptors. Psychopharmacology 183, 13–19 (2005).
Kishi, T., Mukai, T., Matsuda, Y. & Iwata, N. Selective serotonin 3 receptor antagonist treatment for schizophrenia: meta-analysis and systematic review. Neuromolecular Med. 16, 61–69 (2014).
Zoldan, J., Friedberg, G., Livneh, M. & Melamed, E. Psychosis in advanced Parkinson’s disease: treatment with ondansetron, a 5-HT3 receptor antagonist. Neurology 45, 1305–1308 (1995).
Zoldan, J., Friedberg, G., Goldberg-Stern, H. & Melamed, E. Ondansetron for hallucinosis in advanced Parkinson’s disease. Lancet 341, 562–563 (1993).
Kluger, B., Triolo, P., Jones, W. & Jankovic, J. The therapeutic potential of cannabinoids for movement disorders. Mov. Disord. 30, 313–327 (2015).
Balash, Y. et al. Medical cannabis in Parkinson disease: real-life patients’ experience. Clin. Neuropharmacol. 40, 268–272 (2017).
Chesney, E., Oliver, D. & McGuire, P. Cannabidiol (CBD) as a novel treatment in the early phases of psychosis. Psychopharmacology 239, 1179–1190 (2022).
McGuire, P. et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am. J. Psychiatry 175, 225–231 (2018).
Zuardi, A. et al. Cannabidiol for the treatment of psychosis in Parkinson’s disease. J. Psychopharmacol. 23, 979–983 (2009).
Chagas, M. H. N. et al. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: an exploratory double-blind trial. J. Psychopharmacol. 28, 1088–1098 (2014).
Koblan, K. S. et al. A non-D2-receptor-binding drug for the treatment of schizophrenia. N. Engl. J. Med. 382, 1497–1506 (2020).
Isaacson, S. H. & Citrome, L. Hallucinations and delusions associated with Parkinson’s disease psychosis: safety of current treatments and future directions. Expert Opin. Drug Saf. 21, 873–879 (2022).
Acknowledgements
This work was partially supported by funding from Centres de Recerca de Catalunya (CERCA) and Centro de Investigación Biomédica en Red, Enfermedades Neurodegenerativas (CIBERNED).
Author information
Authors and Affiliations
Contributions
J.P., H.B.-K. and S.M.-H. researched data for the article. All authors contributed substantially to discussion of the content, wrote the manuscript and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neurology thanks J. Friedman, S. Lewis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Bottom-up processing
-
Processing of information on the basis of incoming data from the environment to form a perception.
- Capgras syndrome
-
The belief that another person, often a friend or relative, has been replaced by an identical or near-identical impostor.
- Complex visual illusions
-
Illusions in which a real object is perceived as another entity, for example, a lamp in the living room is perceived as a standing person.
- Controllability ranking
-
Quantification of the influence that a brain region has across the rest of the network.
- Delusional jealousy
-
Recurrent suspicions, without justification, regarding the fidelity of one’s spouse or sexual partner.
- Delusions of control
-
The experience that one’s feelings, impulses, thoughts or actions are not one’s own but are being imposed by some external force.
- Delusions of reference
-
The false belief that innocuous events or mere coincidences have strong personal significance.
- Delusions of theft
-
The false belief that someone is stealing one’s belongings.
- Dysmorphopsia
-
A visual illusion in which the shape of an object appears distorted.
- Effective connectivity
-
The influence that a node exerts over another under a network model of causal dynamics, which defines the mechanisms of neuronal coupling.
- Endogenous systems
-
Neuronal networks subserving the processing of internal mentations independently from environmental stimuli.
- Exogenous attentional systems
-
Neuronal networks and engrams involved in directing mental processes towards environmental stimuli.
- Fregoli syndrome
-
The belief that another person, often a friend or relative, is able to disguise themself as an unfamiliar person to influence the behaviour of the patient.
- Functional coactivation
-
Functional interactions among different brain regions.
- Intermetamorphosis
-
The belief that another person, often a friend or relative, has been transformed both physically and psychologically into another person.
- Kinetopsia
-
A visual illusion in which stationary objects seem to be moving.
- Metachromatopsia
-
A visual illusion in which colours of an object appear different from those in reality.
- Mirrored-self misidentification
-
Misidentification and reduplication of oneself in the mirror.
- Pareidolias
-
Visual illusions in which formless visual stimuli, such as clouds, tree bark or patterns in carpets or wallpaper, are perceived as human faces or animals.
- Persecutory delusions
-
Pervasive distrust and suspicion of others such that their motives are interpreted as malevolent (exploiting, harming, threatening or deceiving).
- Reduplication of a person
-
The belief that a double of another person exists. Also known as the syndrome of subjective doubles.
- Reduplicative paramnesia
-
The belief that oneself has been relocated to an identical or near-identical duplicated place.
- Sensorimotor delay
-
Time delay between a perception and movements previously associated with that perception.
- Visual hallucinations
-
Visual perceptions of an animate being, object or event in the absence of any external stimulus.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pagonabarraga, J., Bejr-Kasem, H., Martinez-Horta, S. et al. Parkinson disease psychosis: from phenomenology to neurobiological mechanisms. Nat Rev Neurol 20, 135–150 (2024). https://doi.org/10.1038/s41582-023-00918-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-023-00918-8