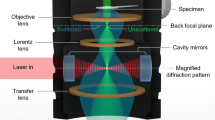
Abstract
Liquid cell electron microscopy possesses a combination of spatial and temporal resolution that provides a unique view of static structures and dynamic processes in liquids. Optimizing the resolution in liquids requires consideration of both the microscope performance and the properties of the sample. In this Review, we survey the competing factors that determine spatial and temporal resolution for transmission electron microscopy and scanning transmission electron microscopy of liquids. We discuss the effects of sample thickness, stability and dose sensitivity on spatial and temporal resolution. We show that for some liquid samples, spatial resolution can be improved by spherical and chromatic aberration correction. However, other benefits offered by aberration correction may be even more useful for liquid samples. We consider the greater image interpretability offered by spherical aberration correction and the improved dose efficiency for thicker samples offered by chromatic aberration correction. Finally, we discuss the importance of detector and sample parameters for higher resolution in future experiments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Ross, F. M. Liquid Cell Electron Microscopy (Cambridge Univ. Press, 2017).
Ross, F. M. Opportunities and challenges in liquid cell electron microscopy. Science 350, aaa9886 (2015). This article discusses the range of problems accessible with closed liquid cell electron microscopy.
de Jonge, N. & Ross, F. M. Electron microscopy of specimens in liquid. Nat. Nanotechnol. 6, 695–704 (2011). This article provides an overview of the breakthroughs in liquid cell electron microscopy in the 2000s that led to the current growth of the field.
de Jonge, N., Peckys, D. B., Kremers, G. J. & Piston, D. W. Electron microscopy of whole cells in liquid with nanometer resolution. Proc. Natl Acad. Sci. USA 106, 2159–2164 (2009). This is the first demonstration of nanometre resolution in micrometre thick liquids containing a mammalian cell.
Yuk, J. M. et al. High-resolution EM of colloidal nanocrystal growth using graphene liquid cells. Science 336, 61–64 (2012). This is the first demonstration of the high resolution possible with a graphene liquid cell.
de Jonge, N. Theory of the spatial resolution of (scanning) transmission electron microscopy in liquid water or ice layers. Ultramicroscopy 187, 113–125 (2018). This article provides the theory needed to calculate the resolution for TEM and STEM of liquid samples.
Nellist, P. D. et al. Direct sub-angstrom imaging of a crystal lattice. Science 305, 1741 (2004).
de Jonge, N., Poirier-Demers, N., Demers, H., Peckys, D. B. & Drouin, D. Nanometer-resolution electron microscopy through micrometers-thick water layers. Ultramicroscopy 110, 1114–1119 (2010).
Zheng, H., Claridge, S. A., Minor, A. M., Alivisatos, A. P. & Dahmen, U. Nanocrystal diffusion in a liquid thin film observed by in situ transmission electron microscopy. Nano Lett. 9, 2460–2465 (2009).
Williamson, M. J., Tromp, R. M., Vereecken, P. M., Hull, R. & Ross, F. M. Dynamic microscopy of nanoscale cluster growth at the solid–liquid interface. Nat. Mater. 2, 532–536 (2003). This is the first demonstration of TEM and electrochemical control in a liquid cell constructed from silicon microchips with silicon nitride windows.
Textor, M. & de Jonge, N. Strategies for preparing graphene liquid cells for transmission electron microscopy. Nano Lett. 18, 3313–3321 (2018).
Liao, H. G., Niu, K. & Zheng, H. Observation of growth of metal nanoparticles. Chem. Commun. 49, 11720–11727 (2013).
Liao, H. G. et al. Facet development during platinum nanocube growth. Science 345, 916–919 (2014).
Lehnert, T. et al. In situ crystallization of the insoluble anhydrite AII phase in graphene pockets. ACS Nano 11, 7967–7973 (2017).
Kelly, D. J. et al. Nanometer resolution elemental mapping in graphene-based TEM liquid cells. Nano Lett. 18, 1168–1174 (2018).
Woehl, T. J. et al. Experimental procedures to mitigate electron beam induced artifacts during in situ fluid imaging of nanomaterials. Ultramicroscopy 127, 53–63 (2013).
Bogner, A., Thollet, G., Basset, D., Jouneau, P. H. & Gauthier, C. Wet STEM: a new development in environmental SEM for imaging nano-objects included in a liquid phase. Ultramicroscopy 104, 290–301 (2005).
Sugi, H. et al. Dynamic electron microscopy of ATP-induced myosin head movement in living muscle filaments. Proc. Natl Acad. Sci. USA 94, 4378–4392 (1997).
Huang, J. Y. et al. In situ observation of the electrochemical lithiation of a single SnO2 nanowire electrode. Science 330, 1515–1520 (2010).
Kolmakov, A. in Liquid Cell Electron Microscopy (ed. Ross, F. M.) 78–105 (Cambridge Univ. Press, 2016).
Reimer, L. & Kohl, H. Transmission Electron Microscopy: Physics of Image Formation. (Springer, New York, 2008).
Egerton, R. F., Li, P. & Malac, M. Radiation damage in the TEM and SEM. Micron 35, 399–409 (2004).
Schneider, N. M. in Liquid Cell Electron Microscopy (ed. Ross, F. M.) 140–163 (Cambridge Univ. Press, 2016).
Jiang, N. & Spence, J. C. H. On the dose-rate threshold of beam damage in TEM. Ultramicroscopy 113, 77–82 (2012).
Frank, J. Three-dimensional Electron Microscopy of Macromolecular Assemblies: Visualization of Biological Molecules in Their Native State (Oxford Univ. Press, 2006).
Pierson, J., Sani, M., Tomova, C., Godsave, S. & Peters, P. J. Toward visualization of nanomachines in their native cellular environment. Histochem. Cell Biol. 132, 253–262 (2009).
Hoenger, A. & McIntosh, J. R. Probing the macromolecular organization of cells by electron tomography. Curr. Opin. Cell Biol. 21, 89–96 (2009).
Matricardi, V. R., Moretz, R. C. & Parsons, D. F. Electron diffraction of wet proteins: catalase. Science 177, 268–270 (1972).
Mirsaidov, U. M., Zheng, H., Casana, Y. & Matsudaira, P. Imaging protein structure in water at 2.7 nm resolution by transmission electron microscopy. Biophys. J. 102, L15–L17 (2012).
Keskin, S. et al. Visualization of multimerization and self-assembly of DNA-functionalized gold nanoparticles using in-liquid transmission electron microscopy. J. Phys. Chem. Lett. 6, 4487–4492 (2015).
Hermannsdörfer, J., Tinnemann, V., Peckys, D. B. & de Jonge, N. The effect of electron beam irradiation in environmental scanning transmission electron microscopy of whole cells in liquid. Microsc. Microanal. 20, 656–665 (2016).
de Jonge, N. & Peckys, D. B. Live cell electron microscopy is probably impossible. ACS Nano 10, 9061–9063 (2016).
Peckys, D. B. & de Jonge, N. in Liquid Cell Electron Microscopy (ed. Ross, F. M.) 334–355 (Cambridge Univ. Press, 2016).
Peckys, D. B., Mazur, P., Gould, K. L. & de Jonge, N. Fully hydrated yeast cells imaged with electron microscopy. Biophys. J. 100, 2522–2529 (2011).
Liv, N. et al. Electron microscopy of living cells during in situ fluorescence microscopy. ACS Nano 10, 265–273 (2016).
Abellan, P. et al. Factors influencing quantitative liquid (scanning) transmission electron microscopy. Chem. Commun. 50, 4873–4880 (2014).
Yamazaki, T. et al. Two types of amorphous protein particles facilitate crystal nucleation. Proc. Natl Acad. Sci. USA 114, 2154–2159 (2017).
Schneider, N. M. et al. Electron–water interactions and implications for liquid cell electron microscopy. J. Phys. Chem. C 118, 22373–22382 (2014). This study reports the modelling of radiolysis effects in water upon electron-beam irradiation.
Hermannsdörfer, J., de Jonge, N. & Verch, A. Electron beam induced chemistry of gold nanoparticles in saline solution. Chem. Comm. 51, 16393–16396 (2015).
Woehl, T. J., Evans, J. E., Arslan, L., Ristenpart, W. D. & Browning, N. D. Direct in situ determination of the mechanisms controlling nanoparticle nucleation and growth. ACS Nano 6, 8599–8610 (2012).
Zheng, H. et al. Observation of single colloidal platinum nanocrystal growth trajectories. Science 324, 1309–1312 (2009). This is the first observation of nanoparticle growth using liquid cell electron microscopy.
Contarato, D., Denes, P., Doering, D., Joseph, J. & Krieger, B. High speed, radiation hard CMOS pixel sensors for transmission electron microscopy. Phys. Procedia 37, 1504–1510 (2013).
Li, X. et al. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nat. Methods 10, 584–590 (2013).
Stevens, A., Yang, H., Carin, L., Arslan, I. & Browning, N. D. The potential for Bayesian compressive sensing to significantly reduce electron dose in high-resolution STEM images. Microscopy 63, 41–51 (2014).
Masiel, D. J., Bloom, R. S., Park, S. T. & Reed, B. W. Temporal compressive sensing instrumentation for TEM. Microsc. Microanal. 23, S20–S21 (2017).
Rose, A. Television pickup tubes and the problem of noise. Adv. Electron. 1, 131–166 (1948).
Egerton, R. F. Control of radiation damage in the TEM. Ultramicroscopy 127, 100–108 (2013).
Rez, P. Comparison of phase contrast transmission electron microscopy with optimized scanning transmission annular dark field imaging for protein imaging. Ultramicroscopy 96, 117–124 (2003).
Joy, D. C. & Joy, C. S. Scanning electron microscope imaging in liquids — some data on electron interactions in water. J. Microsc. 221, 84–99 (2005).
Colliex, C., Mory, C., Olins, A. L., Olins, D. E. & Tencé, D. E. Energy filtered STEM imaging of thick biological sections. J. Microsc. 153, 1–21 (1989).
Park, J. et al. Direct observation of wet biological samples by graphene liquid cell transmission electron microscopy. Nano Lett. 15, 4737–4744 (2015).
Liao, H.-G., Cui, L., Whitelam, S. & Zheng, H. Real-time imaging of Pt3Fe nanorod growth in solution. Science 336, 1011–1014 (2012).
Peckys, D. B., Veith, G. M., Joy, D. C. & de Jonge, N. Nanoscale imaging of whole cells using a liquid enclosure and a scanning transmission electron microscope. PLOS ONE 4, e8214 (2009).
Klein, K. L., Anderson, I. M. & de Jonge, N. Transmission electron microscopy with a liquid flow cell. J. Microsc. 242, 117–123 (2011).
Ring, E. A. & de Jonge, N. Video-frequency scanning transmission electron microscopy of moving gold nanoparticles in liquid. Micron 43, 1078–1084 (2012).
Thust, A., Coene, W. M. J., Op de Beeck, M. & Van Dyck, D. Focal-series reconstruction in HRTEM: simulation studies on non-periodic objects. Ultramicroscopy 64, 211–230 (1996).
Kisielowski, C. et al. Imaging columns of the light elements carbon, nitrogen and oxygen with sub angstrom resolution. Ultramicroscopy 89, 243–263 (2001).
Haider, M. et al. A spherical-aberration-corrected 200 kV transmission electron microscope. Ultramicroscopy 75, 53–60 (1998).
Lentzen, M. et al. High-resolution imaging with an aberration-corrected transmission electron microscope. Ultramicroscopy 92, 233–242 (2002).
Coene, W. & Jansen, A. J. Image delocalisation and high resolution transmission electron microscopic imaging with a field emission gun. Scan. Microsc. 6, S379–S403 (1992).
Cervera Gontard, L., Dunin-Borkowski, R. E., Hytch, M. J. & Ozkaya, D. Delocalisation in images of Pt nanoparticles. J. Phys. Conf. Ser. 26, 292–295 (2006).
Borisevich, A. Y., Lupini, A. R. & Pennycook, S. J. Depth sectioning with the aberration-corrected scanning transmission electron microscope. Proc. Natl Acad. Sci. USA 103, 3044–3048 (2006).
de Jonge, N., Sougrat, R., Northan, B. M. & Pennycook, S. J. Three-dimensional scanning transmission electron microscopy of biological specimens. Microsc. Microanal. 16, 54–63 (2010).
Nellist, P. D., Cosgriff, E. C., Behan, G. & Kirkland, A. I. Imaging modes for scanning confocal electron microscopy in a double aberration-corrected transmission electron microscope. Microsc. Microanal. 14, 82–88 (2008).
Jia, C. L., Lentzen, M. & Urban, K. Atomic-resolution imaging of oxygen in perovskite ceramics. Science 299, 870–873 (2003).
Jia, C. L. et al. Atomic-scale study of electric dipoles near charged and uncharged domain walls in ferroelectric films. Nat. Mater. 7, 57–61 (2008).
Jia, C. L., Houben, L., Thust, A. & Barthel, J. On the benefit of the negative-spherical-aberration imaging technique for quantitative HRTEM. Ultramicroscopy 110, 500–505 (2010).
Jia, C. L. et al. Atomic-scale measurement of structure and chemistry of a single-unit-cell layer of LaAlO3 embedded in SrTiO3. Microsc. Microanal. 19, 310–318 (2013).
Takeda, S., Kuwauchi, Y. & Yoshida, H. Environmental transmission electron microscopy for catalyst materials using a spherical aberration corrector. Ultramicroscopy 151, 178–190 (2015).
Hansen, T. W. & Wagner, J. B. Environmental transmission electron microscopy in an aberration-corrected environment. Microsc. Microanal. 18, 684–690 (2012).
Barthel, J. & Thust, A. On the optical stability of high-resolution transmission electron microscopes. Ultramicroscopy 134, 6–17 (2013).
Tromp, R. M. & Schramm, S. M. Optimization and stability of the contrast transfer function in aberration-corrected electron microscopy. Ultramicroscopy 125, 72–80 (2013).
Haider, M., Hartel, P., Muller, H., Uhlemann, S. & Zach, J. Information transfer in a TEM corrected for spherical and chromatic aberration. Microsc. Microanal. 16, 393–408 (2010).
Zach, J. Chromatic correction: a revolution in electron microscopy? Phil. Trans. R. Soc. A 367, 3699–3707 (2009).
Rose, H. H. Future trends in aberration-corrected electron microscopy. Phil. Trans. R. Soc. A 367, 3809–3823 (2009).
Kabius, B. et al. First application of CC-corrected imaging for high-resolution and energy-filtered TEM. J. Electron. Microsc. 58, 147–155 (2009).
Leary, R. & Brydson, R. Chromatic aberration correction: the next step in electron microscopy. Adv. Imag. Electron. Phys. 165, 73–130 (2011).
Forbes, B. D., Houben, L., Mayer, J., Dunin-Borkowski, R. E. & Allen, L. J. Elemental mapping in achromatic atomic-resolution energy-filtered transmission electron microscopy. Ultramicroscopy 147, 98–105 (2014).
Urban, K. W. et al. Achromatic elemental mapping beyond the nanoscale in the transmission electron microscope. Phys. Rev. Lett. 110, 185507 (2013).
Reimer, L. & Ross-Messemer, M. Top-bottom effect in energy-selecting transmission electron microscopy. Ultramicroscopy 21, 385–387 (1987).
Baudoin, J. P., Jinschek, J. R., Boothroyd, C. B., Dunin-Borkowski, R. E. & de Jonge, N. Chromatic aberration-corrected tilt series transmission electron microscopy of nanoparticles in a whole mount macrophage cell. Microsc. Microanal. 19, 814–820 (2013).
Danev, R. & Nagayama, K. Transmission electron microscopy with Zernike phase plate. Ultramicroscopy 88, 243–252 (2001).
Danev, R., Buijsse, B., Khoshouei, M., Plitzko, J. M. & Baumeister, W. Volta potential phase plate for in-focus phase contrast transmission electron microscopy. Proc. Natl Acad. Sci. USA 111, 15635–15640 (2014).
Prozorov, T., Almeida, T. P., Kovacs, A. & Dunin-Borkowski, R. E. Off-axis electron holography of bacterial cells and magnetic nanoparticles in liquid. J. R. Soc. Interface 14, 20170464 (2017).
Shirai, M. et al. In situ electron holographic study of ionic liquid. Ultramicroscopy 146, 125–129 (2014).
Dunin-Borkowski, R. E. & Houben, L. in Liquid Cell Electron Microscopy (ed. Ross, F. M.) 408–433 (Cambridge Univ. Press, 2016).
Tanigaki, T. et al. Magnetic field observations in CoFeB/Ta layers with 0.67-nm resolution by electron holography. Sci. Rep. 7, 16598 (2017).
Linck, M. et al. Chromatic aberration correction for atomic resolution TEM imaging from 20 to 80 kV. Phys. Rev. Lett. 117, 076101 (2016).
Demers, H., Poirier-Demers, N., Drouin, D. & de Jonge, N. Simulating STEM imaging of nanoparticles in micrometers-thick substrates. Microsc. Microanal. 16, 795–804 (2010).
Besztejan, S. et al. Visualization of cellular components in a mammalian cell with liquid-cell transmission electron microscopy. Microsc. Microanal. 23, 46–55 (2017).
de Jonge, N., Verch, A. & Demers, H. The influence of beam broadening on the spatial resolution of annular dark field scanning transmission electron microscopy. Microsc. Microanal. 24, 8–16 (2018).
Zaluzec, N. J. The influence of CS/CC correction in analytical imaging and spectroscopy in scanning and transmission electron microscopy. Ultramicroscopy 151, 240–249 (2015).
Hohmann-Marriott, M. F. et al. Nanoscale 3D cellular imaging by axial scanning transmission electron tomography. Nat. Methods 6, 729–731 (2009).
Hyun, J. K., Ercius, P. & Muller, D. A. Beam spreading and spatial resolution in thick organic specimens. Ultramicroscopy 109, 1–7 (2008).
Wolf, S. G., Houben, L. & Elbaum, M. Cryo-scanning transmission electron tomography of vitrified cells. Nat. Methods 11, 423–428 (2014).
Lazic, I. & Bosch, E. G. T. Analytical review of direct STEM imaging techniques for thin samples. Adv. Imag. Electron. Phys. 199, 75–184 (2018).
Jungjohann, K. L., Evans, J. E., Aguiar, J. A., Arslan, I. & Browning, N. D. Atomic-scale imaging and spectroscopy for in situ liquid scanning transmission electron microscopy. Microsc. Microanal. 18, 621–627 (2012).
Unocic, R. R. et al. Direct-write liquid phase transformations with a scanning transmission electron microscope. Nanoscale 8, 15581–15588 (2016).
Wang, C., Qiao, Q., Shokuhfar, T. & Klie, R. F. High-resolution electron microscopy and spectroscopy of ferritin in biocompatible graphene liquid cells and graphene sandwiches. Adv. Mater. 26, 3410–3414 (2014).
Barth, J. E. & Kruit, P. Addition of different contributions to the charged particle probe size. Optik 101, 101–109 (1996).
van Benthem, K. et al. Three-dimensional ADF imaging of individual atoms by through-focal series stem. Ultramicroscopy 106, 1062–1068 (2006).
Dahmen, T., Trampert, P., de Jonge, N. & Slusallek, P. Advanced recording schemes for electron tomography. MRS Bull. 41, 537–541 (2016).
Einstein, A. On the motion — required by the molecular kinetic theory of heat — of small particles suspended in a stationary liquid. Ann. Phys. 17, 549–560 (1905).
Browning, N. D. & Evans, J. E. in Liquid Cell Electron Microscopy (ed. Ross, F. M.) 456–475 (Cambridge Univ. Press, 2016).
Chee, S. W., Baraissov, Z., Loh, N. D., Matsudaira, P. T. & Mirsaidov, U. Desorption-mediated motion of nanoparticles at the liquid-solid interface. J. Phys. Chem. C 120, 20462–20470 (2016).
Chee, S. W., Loh, D., Mirsaidov, U. & Matsudaira, P. Probing nanoparticle dynamics in 200 nm thick liquid layers at millisecond time resolution. Microsc. Microanal. 21(Suppl. 3), 267–268 (2015).
Tian, X., Zheng, H. & Mirsaidov, U. Aggregation dynamics of nanoparticles at solid–liquid interfaces. Nanoscale 9, 10044–10050 (2017).
Chen, X. & Wen, J. In situ wet-cell TEM observation of gold nanoparticle motion in an aqueous solution. Nanoscale Res. Lett. 7, 598 (2012).
White, E. R., Mecklenburg, M., Shevitski, B., Singer, S. B. & Regan, B. C. Charged nanoparticle dynamics in water induced by scanning transmission electron microscopy. Langmuir 28, 3695–3698 (2012).
Liu, Y., Lin, X.-M., Sun, Y. & Rajh, T. In situ visualization of self-assembly of charged gold nanoparticles. J. Am. Chem. Soc. 135, 3764–3767 (2013).
Tan, S. F., Chee, S. W., Lin, G. H. & Mirsaidov, U. Direct observation of interactions between nanoparticles and nanoparticle self-assembly in solution. Acc. Chem. Res. 50, 1303–1312 (2017).
Verch, A., Pfaff, M. & De Jonge, N. Exceptionally slow movement of gold nanoparticles at a solid:liquid interface investigated by scanning transmission electron microscopy. Langmuir 31, 6956–6964 (2015).
Jiang, N. Note on in situ (scanning) transmission electron microscopy study of liquid samples. Ultramicroscopy 179, 81–83 (2017).
Parent, L. R. et al. Tackling the challenges of dynamic experiments using liquid-cell transmission electron microscopy. Acc. Chem. Res. 51, 3–11 (2018).
LaGrange, T. et al. Nanosecond time-resolved investigations using the in situ of dynamic transmission electron microscope (DTEM). Ultramicroscopy 108, 1441–1449 (2008).
Fu, X., Chen, B., Tang, J., Hassan, M. T. & Zewail, A. H. Imaging rotational dynamics of nanoparticles in liquid by 4D electron microscopy. Science 355, 494–498 (2017).
Evans, J. E., Jungjohann, K. L., Browning, N. D. & Arslan, I. Controlled growth of nanoparticles from solution with in situ liquid transmission electron microscopy. Nano Lett. 11, 2809–2813 (2011).
Alloyeau, D. et al. Unravelling kinetic and thermodynamic effects on the growth of gold nanoplates by liquid transmission electron microscopy. Nano Lett. 15, 2574–2581 (2015).
Grogan, J. M., Schneider, N. M., Ross, F. M. & Bau, H. H. Bubble and pattern formation in liquid induced by an electron beam. Nano Lett. 14, 359–364 (2014).
White, E. R. et al. In situ transmission electron microscopy of lead dendrites and lead ions in aqueous solution. ACS Nano 6, 6308–6317 (2012).
Sang, X. H. et al. Dynamic scan control in STEM: spiral scans. Adv. Struct. Chem. Imaging 2, 6 (2016).
De Yoreo, J. J. & Sommerdijk, N. A. J. M. Investigating materials formation with liquid-phase and cryogenic TEM. Nat. Rev. Mater. 1, 16035 (2016).
Park, J. H. et al. Control of electron beam-induced Au nanocrystal growth kinetics through solution chemistry. Nano Lett. 15, 5314–5320 (2015).
de Jonge, N., Browning, N., Evans, J. E., Chee, S. W. & Ross, F. M. in Liquid Cell Electron Microscopy (ed. Ross, F. M.) 164–188 (Cambridge Univ. Press, 2016).
Freitag, B., Kujawa, S., Mul, P. M., Ringnalda, J. & Tiemeijer, P. C. Breaking the spherical and chromatic aberration barrier in transmission electron microscopy. Ultramicroscopy 102, 209–214 (2005).
Krivanek, O. L., Kundmann, M. K. & Kimoto, K. Spatial resolution in EFTEM elemental maps. J. Microsc. 180, 277–287 (1995).
Acknowledgements
N.dJ. acknowledges H. Demers, T. Dahmen, M. Elbaum, D. Peckys and S. Wolf for discussions, a fellowship of the Visiting Faculty Program of the Weizmann Institute and E. Arzt for his support through the Leibniz Institute for New Materials (INM). L.H. and R.E.D.-B. are grateful to M. Luysberg, J. Barthel, A. Thust, K. Urban, S. Mi, C. Boothroyd, A. Kovács, J. Mayer, L. Allen, B. Forbes, J. Jinschek, J.-P. Baudoin, L. Cervera Gontard, D. Ozkaya, T. Hansen and M. Bar Sadan for discussions. F.M.R. acknowledges J.H. Park, N. Browning, S.W. Chee, J. Evans, D. Muller, R. Tromp and J. Hannon for helpful discussions.
Author information
Authors and Affiliations
Contributions
All authors contributed to the discussion of content and researched data for the article. R.E.D.-B. and L.H. wrote the section on aberration correction. N.dJ. and F.M.R. wrote the sections on spatial and temporal resolution. All authors edited the article prior to submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
de Jonge, N., Houben, L., Dunin-Borkowski, R.E. et al. Resolution and aberration correction in liquid cell transmission electron microscopy. Nat Rev Mater 4, 61–78 (2019). https://doi.org/10.1038/s41578-018-0071-2
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41578-018-0071-2
This article is cited by
-
Visualizing the multi-level assembly structures of conjugated molecular systems with chain-length dependent behavior
Nature Communications (2023)
-
High-resolution cryo-electron microscopy structure of block copolymer nanofibres with a crystalline core
Nature Materials (2023)
-
Tracking single adatoms in liquid in a transmission electron microscope
Nature (2022)
-
Fabrication of liquid cell for in situ transmission electron microscopy of electrochemical processes
Nature Protocols (2022)
-
Dynamic transformation of cubic copper catalysts during CO2 electroreduction and its impact on catalytic selectivity
Nature Communications (2021)