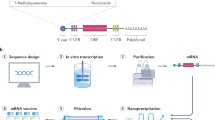
Abstract
The World Health Organization recently declared a global initiative to control arboviral diseases. These are mainly caused by pathogenic flaviviruses (such as dengue, yellow fever and Zika viruses) and alphaviruses (such as chikungunya and Venezuelan equine encephalitis viruses). Vaccines represent key interventions for these viruses, with licensed human and/or veterinary vaccines being available for several members of both genera. However, a hurdle for the licensing of new vaccines is the epidemic nature of many arboviruses, which presents logistical challenges for phase III efficacy trials. Furthermore, our ability to predict or measure the post-vaccination immune responses that are sufficient for subclinical outcomes post-infection is limited. Given that arboviruses are also subject to control by the immune system of their insect vectors, several approaches are now emerging that aim to augment antiviral immunity in mosquitoes, including Wolbachia infection, transgenic mosquitoes, insect-specific viruses and paratransgenesis. In this Review, we discuss recent advances, current challenges and future prospects in exploiting both vertebrate and invertebrate immune systems for the control of flaviviral and alphaviral diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
World Health Organization. Global Arbovirus Initiative https://www.who.int/news-room/events/detail/2022/03/31/default-calendar/global-arbovirus-initiative (2022).
Balakrishnan, V. S. WHO launches global initiative for arboviral diseases. Lancet Microbe 3, e407 (2022).
Roiz, D. et al. The rising global economic costs of Aedes and Aedes-borne diseases. Preprint at Research Square https://doi.org/10.21203/rs.3.rs-2679030/v1 (2023).
Longbottom, J. et al. Aedes albopictus invasion across Africa: the time is now for cross-country collaboration and control. Lancet Glob. Health 11, e623–e628 (2023).
Suhrbier, A. Rheumatic manifestations of chikungunya: emerging concepts and interventions. Nat. Rev. Rheumatol. 15, 597–611 (2019).
Casades-Marti, L. et al. Risk factors for exposure of wild birds to West Nile virus in a gradient of wildlife-livestock interaction. Pathogens 12, 83 (2023).
Read, A. J. et al. Clinical and epidemiological features of West Nile virus equine encephalitis in New South Wales, Australia, 2011. Aust. Vet. J. 97, 133–143 (2019).
Mulvey, P. et al. The ecology and evolution of Japanese encephalitis virus. Pathogens 10, 1534 (2021).
Pham, D. et al. Emergence of Japanese encephalitis in Australia: a diagnostic perspective. Pathology 54, 669–677 (2022).
Pedersen, C. E. Jr., Robinson, D. M. & Cole, F. E. Jr. Isolation of the vaccine strain of Venezuelan equine encephalomyelitis virus from mosquitoes in Louisiana. Am. J. Epidemiol. 95, 490–496 (1972).
Coates, E. E. et al. Safety and immunogenicity of a trivalent virus-like particle vaccine against western, eastern, and Venezuelan equine encephalitis viruses: a phase 1, open-label, dose-escalation, randomised clinical trial. Lancet Infect. Dis. 22, 1210–1220 (2022).
Li, B., Wang, H. & Liang, G. Getah virus (alphavirus): an emerging, spreading zoonotic virus. Pathogens 11, 945 (2022).
Thorarinsson, R. et al. Effects of a DNA and multivalent oil-adjuvanted vaccines against pancreas disease in Atlantic salmon (Salmo salar) challenged with salmonid alphavirus subtype 3. Fish Shellfish Immunol. Rep. 3, 100063 (2022).
Harris, E. FDA approves first chikungunya vaccine. JAMA 330, 2241–2241 (2023).
World Health Organization. Disease Outbreak News: Western Equine Encephalitis in Argentina https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON499 (2023).
Yan, K. et al. A yellow fever virus 17D infection and disease mouse model used to evaluate a chimeric Binjari-yellow fever virus vaccine. Vaccines 8, 368 (2020).
Wu, B., Qi, Z. & Qian, X. Recent advancements in mosquito-borne flavivirus vaccine development. Viruses 15, 813 (2023).
Koopmans, M., de Lamballerie, X., Jaenisch, T. & Consortium, Z. I. Familiar barriers still unresolved — a perspective on the Zika virus outbreak research response. Lancet Infect. Dis. 19, e59–e62 (2019).
Ritchie, S. et al. In: Innovative Strategies for Vector Control (eds Koenraadt, C. J. M., Spitzen, J. & Takken, W.) 58-89 (Wageningen Academic Publishers, 2021).
World Health Organization. Call for Public Consultation-development of Target Product Profiles (TPPs) for Wolbachia infected Aedes aegypti Population Replacement Intervention https://www.who.int/news-room/articles-detail/call-for-public-consultation-development-target-product-profiles-wolbachia-infected-aedes-aegypti-population-replacement-intervention (2021).
Ant, T. H., Mancini, M. V., McNamara, C. J., Rainey, S. M. & Sinkins, S. P. Wolbachia-virus interactions and arbovirus control through population replacement in mosquitoes. Pathog. Glob. Health 117, 245–258 (2023).
Possas, C. et al. Vaccine innovation for dengue, chikungunya, zika and yellow fever: accelerating global development agenda and partnerships in post-COVID era. Vacc. Res. 2, 1–17 (2022).
Pierson, T. C. & Diamond, M. S. The continued threat of emerging flaviviruses. Nat. Microbiol. 5, 796–812 (2020).
Zimmerman, O., Holmes, A. C., Kafai, N. M., Adams, L. J. & Diamond, M. S. Entry receptors — the gateway to alphavirus infection. J. Clin. Invest. 133, e165307 (2023).
Pan, Y. et al. Flaviviruses: innate immunity, inflammasome activation, inflammatory cell death, and cytokines. Front. Immunol. 13, 829433 (2022).
Hobson-Peters, J. et al. A recombinant platform for flavivirus vaccines and diagnostics using chimeras of a new insect-specific virus. Sci. Transl. Med. 11, eaax7888 (2019).
Abbo, S. R. et al. Comparative efficacy of Mayaro virus-like particle vaccines produced in insect or mammalian cells. J. Virol. 97, e0160122 (2023).
Castilho, L. R., Mattos, N. R., Abreu, W. S. & Gutarra, M. L. E. Virus-like particles (VLPs) as important tools for flavivirus vaccine development. Biologics 2, 226–242 (2022).
Bennett, S. R. et al. Safety and immunogenicity of PXVX0317, an aluminium hydroxide-adjuvanted chikungunya virus-like particle vaccine: a randomised, double-blind, parallel-group, phase 2 trial. Lancet Infect. Dis. 22, 1343–1355 (2022).
Adams, C. et al. Structure and neutralization mechanism of a human antibody targeting a complex epitope on Zika virus. PLoS Pathog. 19, e1010814 (2023).
Salem, G. M. et al. Antibodies from dengue patients with prior exposure to Japanese encephalitis virus are broadly neutralizing against Zika virus. Commun. Biol. 7, 15 (2024).
Tsuji, I. et al. Somatic hypermutation and framework mutations of variable region contribute to anti-Zika virus-specific monoclonal antibody binding and function. J. Virol. 96, e0007122 (2022).
Kim, A. S. & Diamond, M. S. A molecular understanding of alphavirus entry and antibody protection. Nat. Rev. Microbiol. 21, 396–407 (2023).
Raju, S. et al. A chikungunya virus-like particle vaccine induces broadly neutralizing and protective antibodies against alphaviruses in humans. Sci. Transl. Med. 15, eade8273 (2023).
Hasan, S. S. et al. A human antibody against Zika virus crosslinks the E protein to prevent infection. Nat. Commun. 8, 14722 (2017).
Zhang, S. et al. A human antibody neutralizes different flaviviruses by using different mechanisms. Cell Rep. 31, 107584 (2020).
Fox, J. M. et al. Broadly neutralizing alphavirus antibodies bind an epitope on E2 and inhibit entry and egress. Cell 163, 1095–1107 (2015).
Sharma, A. et al. The epitope arrangement on flavivirus particles contributes to Mab C10’s extraordinary neutralization breadth across Zika and dengue viruses. Cell 184, 6052–6066 (2021). An excellent example illustrating how antibodies neutralize flaviviruses.
Roux, K. H., Strelets, L. & Michaelsen, T. E. Flexibility of human IgG subclasses. J. Immunol. 159, 3372–3382 (1997).
Wrigley, N. G., Brown, E. B. & Skehel, J. J. Electron microscopic evidence for the axial rotation and inter-domain flexibility of the Fab regions of immunoglobulin G. J. Mol. Biol. 169, 771–774 (1983).
Chu, T. H., Patz, E. F. Jr. & Ackerman, M. E. Coming together at the hinges: therapeutic prospects of IgG3. mAbs 13, 1882028 (2021).
Kam, Y. W. et al. Early appearance of neutralizing immunoglobulin G3 antibodies is associated with chikungunya virus clearance and long-term clinical protection. J. Infect. Dis. 205, 1147–1154 (2012).
Zepeda, O. et al. Antibody immunity to Zika virus among young children in a flavivirus-endemic area in Nicaragua. Viruses 15, 796 (2023).
Lim, X. X. et al. Human antibody C10 neutralizes by diminishing Zika but enhancing dengue virus dynamics. Cell 184, 6067–6080.e13 (2021).
Stiasny, K., Medits, I., Rossbacher, L. & Heinz, F. X. Impact of structural dynamics on biological functions of flaviviruses. FEBS J. 290, 1973–1985 (2023).
Gould, C. V. et al. Transmission of yellow fever vaccine virus through blood transfusion and organ transplantation in the USA in 2021: report of an investigation. Lancet Microbe 4, e711–e721 (2023). A cautionary tale for the transmission of live-attenuated virus vaccines, from an example of the use of metagenomic next-generation sequencing for diagnosis.
Strauss, J. H. & Strauss, E. G. Recombination in alphaviruses. Semin. Virol. 8, 85–94 (1997).
Seligman, S. J. & Gould, E. A. Live flavivirus vaccines: reasons for caution. Lancet 363, 2073–2075 (2004).
Brault, A. C. et al. A single positively selected West Nile viral mutation confers increased virogenesis in American crows. Nat. Genet. 39, 1162–1166 (2007).
Dietrich, E. A., Ong, Y. T., Stovall, J. L., Dean, H. & Huang, C. Y. Limited transmission potential of Takeda’s tetravalent dengue vaccine candidate by Aedes albopictus. Am. J. Trop. Med. Hyg. 97, 1423–1427 (2017).
de Miranda, R. M. et al. Neotropical sylvatic mosquitoes and Aedes aegypti are not competent to transmit 17DD attenuated yellow fever virus from vaccinated viremic new world non-human primates. Viruses 14, 2231 (2022).
Wang, X., Usama, A., Huanchun, C., Cao, S. & Ye, J. Biological determinants perpetuating the transmission dynamics of mosquito-borne flaviviruses. Emerg. Microbes Infect. 12, 2212812 (2023).
Querec, T. D. et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat. Immunol. 10, 116–125 (2009).
Prow, N. A. et al. A vaccinia-based single vector construct multi-pathogen vaccine protects against both Zika and chikungunya viruses. Nat. Commun. 9, 1230 (2018).
Zhang, T. et al. Research progress of aluminum phosphate adjuvants and their action mechanisms. Pharmaceutics 15, 1756 (2023).
Verma, S. K. et al. New-age vaccine adjuvants, their development, and future perspective. Front. Immunol. 14, 1043109 (2023).
Strezova, A. et al. Long-term protection against herpes zoster by the adjuvanted recombinant zoster vaccine: interim efficacy, immunogenicity, and safety results up to 10 years after initial vaccination. Open Forum Infect. Dis. 9, ofac485 (2022).
Weaver, S. C., Pfeffer, M., Marriott, K., Kang, W. & Kinney, R. M. Genetic evidence for the origins of Venezuelan equine encephalitis virus subtype IAB outbreaks. Am. J. Trop. Med. Hyg. 60, 441–448 (1999).
Nguyen, W. et al. Arthritogenic alphavirus vaccines: serogrouping versus cross-protection in mouse models. Vaccines 8, 209 (2020).
Wijewardana, V., Ulbert, S. & Cattoli, G. Editorial: Irradiation technologies for vaccine development. Front. Immunol. 13, 1075335 (2023).
Sundaram, A. K. et al. Comparison of purified psoralen-inactivated and formalin-inactivated dengue vaccines in mice and nonhuman primates. Vaccine 38, 3313–3320 (2020).
Adam, A. et al. Optimized production and immunogenicity of an insect virus-based chikungunya virus candidate vaccine in cell culture and animal models. Emerg. Microbes Infect. 10, 305–316 (2021).
The University of Queensland Australia. Vaccine to Protect Pigs from Japanese Encephalitis Virus https://www.uq.edu.au/news/article/2023/02/vaccine-protect-pigs-japanese-encephalitis-virus (2023).
Shaw, C. A. et al. A phase 1, randomized, placebo-controlled, dose-ranging study to evaluate the safety and immunogenicity of an mRNA-based chikungunya virus vaccine in healthy adults. Vaccine 41, 3898–3906 (2023). One of the first mRNA vaccine trials for an alphavirus, showing that 79% of vaccine recipients had measurable neutralizing antibody responses to CHIKV 1 year after a two-dose vaccine regimen.
Essink, B. et al. The safety and immunogenicity of two Zika virus mRNA vaccine candidates in healthy flavivirus baseline seropositive and seronegative adults: the results of two randomised, placebo-controlled, dose-ranging, phase 1 clinical trials. Lancet Infect. Dis. 23, 621–633 (2023). Some of the first mRNA vaccine trials for a flavivirus showing that responses to ZIKV persisted for up to a year; collection of data beyond 13 months is planned.
Prow, N. A. et al. The vaccinia virus based sementis copenhagen vector vaccine against Zika and chikungunya is immunogenic in non-human primates. NPJ Vaccines 5, 44 (2020).
Konishi, E. et al. Mice immunized with a subviral particle containing the Japanese encephalitis virus prM/M and E proteins are protected from lethal JEV infection. Virology 188, 714–720 (1992).
Bollman, B. et al. An optimized messenger RNA vaccine candidate protects non-human primates from Zika virus infection. NPJ Vaccines 8, 58 (2023).
Rzymski, P., Szuster-Ciesielska, A., Dzieciatkowski, T., Gwenzi, W. & Fal, A. mRNA vaccines: the future of prevention of viral infections? J. Med. Virol. 95, e28572 (2023).
Evans, J. P. et al. Neutralizing antibody responses elicited by SARS-CoV-2 mRNA vaccination wane over time and are boosted by breakthrough infection. Sci. Transl. Med. 14, eabn8057 (2022). This study describes in detail a key potential limitation for mRNA vaccine technology in terms of waning antibody titres over a relatively short timeframe.
Wieten, R. W. et al. A single 17D yellow fever vaccination provides lifelong immunity; characterization of yellow-fever-specific neutralizing antibody and T-cell responses after vaccination. PLoS One 11, e0149871 (2016).
O’Connor, M. A. et al. A replicon RNA vaccine can induce durable protective immunity from SARS-CoV-2 in nonhuman primates after neutralizing antibodies have waned. PLoS Pathog. 19, e1011298 (2023).
Comes, J. D. G., Pijlman, G. P. & Hick, T. A. H. Rise of the RNA machines — self-amplification in mRNA vaccine design. Trends Biotechnol. 41, 1417–1429 (2023).
McGee, J. E. et al. Complete substitution with modified nucleotides suppresses the early interferon response and increases the potency of self-amplifying RNA. Preprint at bioRxiv https://doi.org/10.1101/2023.09.15.557994 (2023). For current mRNA vaccines, N-methyl pseudouridine is used in place of uridine to reduce type I interferon responses that inhibit translation; this modification does not support self-amplifying RNA but this study identifies other modifications that do.
Piras-Douce, F. et al. Evaluation of safety and immuno-efficacy of a next generation live-attenuated yellow fever vaccine in cynomolgus macaques. Vaccine 41, 1457–1470 (2023).
Schneider, M. et al. Safety and immunogenicity of a single-shot live-attenuated chikungunya vaccine: a double-blind, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 401, 2138–2147 (2023). A pivotal phase III trial for IXCHIQ, leading the way to approval via the accelerated approval pathway; the first approved CHIKV vaccine.
Matangkasombut, P. et al. Dengue viremia kinetics in asymptomatic and symptomatic infection. Int. J. Infect. Dis. 101, 90–97 (2020).
Wressnigg, N. et al. Single-shot live-attenuated chikungunya vaccine in healthy adults: a phase 1, randomised controlled trial. Lancet Infect. Dis. 20, 1193–1203 (2020).
Roques, P. et al. Effectiveness of CHIKV vaccine VLA1553 demonstrated by passive transfer of human sera. JCI Insight 7, e160173 (2022). This study describes a novel approach to demonstrate the protective capacity of vaccine-induced antibodies via passive transfer of vaccine-recipient sera to non-human primates that were then challenged with CHIKV.
Konishi, E. et al. The anamnestic neutralizing antibody response is critical for protection of mice from challenge following vaccination with a plasmid encoding the Japanese encephalitis virus premembrane and envelope genes. J. Virol. 73, 5527–5534 (1999).
Hugo, L. E., Prow, N. A., Tang, B., Devine, G. & Suhrbier, A. Chikungunya virus transmission between Aedes albopictus and laboratory mice. Parasit. Vectors 9, 555 (2016).
Lambrechts, L. et al. Direct mosquito feedings on dengue-2 virus-infected people reveal dynamics of human infectiousness. PLoS Negl. Trop. Dis. 17, e0011593 (2023).
Hou, J., Ye, W. & Chen, J. Current development and challenges of tetravalent live-attenuated dengue vaccines. Front. Immunol. 13, 840104 (2022).
de Castro Ferreira, C. et al. The 17D-204 and 17DD yellow fever vaccines: an overview of major similarities and subtle differences. Expert Rev. Vaccines 17, 79–90 (2018).
Laera, D., HogenEsch, H. & O’Hagan, D. T. Aluminum adjuvants — ‘back to the future’. Pharmaceutics 15, 1884 (2023).
Burrack, K. S., Montgomery, S. A., Homann, D. & Morrison, T. E. CD8+ T cells control Ross River virus infection in musculoskeletal tissues of infected mice. J. Immunol. 194, 678–689 (2015).
Grubor-Bauk, B. et al. NS1 DNA vaccination protects against Zika infection through T cell-mediated immunity in immunocompetent mice. Sci. Adv. 5, eaax2388 (2019).
Castilow, E. M. & Varga, S. M. Overcoming T cell-mediated immunopathology to achieve safe RSV vaccination. Future Virol. 3, 445–454 (2008).
Iwata-Yoshikawa, N. et al. A lethal mouse model for evaluating vaccine-associated enhanced respiratory disease during SARS-CoV-2 infection. Sci. Adv. 8, eabh3827 (2022).
Doherty, T. M. & Rook, G. Progress and hindrances in tuberculosis vaccine development. Lancet 367, 947–949 (2006).
Stone, E. T. & Pinto, A. K. T cells in tick-borne flavivirus encephalitis: a review of current paradigms in protection and disease pathology. Viruses 15, 958 (2023).
Garber, C. et al. T cells promote microglia-mediated synaptic elimination and cognitive dysfunction during recovery from neuropathogenic flaviviruses. Nat. Neurosci. 22, 1276–1288 (2019).
Kehn-Hall, K. & Bradfute, S. B. Understanding host responses to equine encephalitis virus infection: implications for therapeutic development. Expert Rev. Anti Infect. Ther. 20, 1551–1566 (2022).
Poo, Y. S. et al. Multiple immune factors are involved in controlling acute and chronic chikungunya virus infection. PLoS Negl. Trop. Dis. 8, e3354 (2014).
Reagin, K. L. & Funk, K. E. The role of antiviral CD8+ T cells in cognitive impairment. Curr. Opin. Neurobiol. 76, 102603 (2022).
Saxena, V., Mathur, A., Krishnani, N. & Dhole, T. N. An insufficient anti-inflammatory cytokine response in mouse brain is associated with increased tissue pathology and viral load during Japanese encephalitis virus infection. Arch. Virol. 153, 283–292 (2008).
Lee, J., Woodruff, M. C., Kim, E. H. & Nam, J. H. Knife’s edge: balancing immunogenicity and reactogenicity in mRNA vaccines. Exp. Mol. Med. 55, 1305–1313 (2023).
Sahin, U. et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 586, 594–599 (2020).
Farmer, J. F. & Suhrbier, A. Interpreting paired serology for Ross River virus and Barmah Forest virus diseases. Aust. J. Gen. Pract. 48, 645–649 (2019).
Wressnigg, N. et al. An inactivated Ross River virus vaccine is well tolerated and immunogenic in an adult population in a randomized phase 3 trial. Clin. Vaccin. Immunol. 22, 267–273 (2015).
Nasveld, P. E. et al. Long term immunity to live attenuated Japanese encephalitis chimeric virus vaccine: randomized, double-blind, 5-year phase II study in healthy adults. Hum. Vaccin. 6, 1038–1046 (2010).
Salje, H. et al. Evaluation of the extended efficacy of the Dengvaxia vaccine against symptomatic and subclinical dengue infection. Nat. Med. 27, 1395–1400 (2021).
Amanna, I. J., Carlson, N. E. & Slifka, M. K. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 357, 1903–1915 (2007).
Ernst, D. Long-Term Immunogenicity, Effectiveness Observed with HPV Vaccine Gardasil 9 https://www.empr.com/home/news/long-term-immunogenicity-effectiveness-observed-with-hpv-vaccine-gardasil-9/ (2023).
Yu, D., Walker, L. S. K., Liu, Z., Linterman, M. A. & Li, Z. Targeting T(FH) cells in human diseases and vaccination: rationale and practice. Nat. Immunol. 23, 1157–1168 (2022).
Corrado, M. & Pearce, E. L. Targeting memory T cell metabolism to improve immunity. J. Clin. Invest. 132, e148546 (2022).
Tran, Q. M. et al. Expected endpoints from future chikungunya vaccine trial sites informed by serological data and modeling. Vaccine 41, 182–192 (2023).
Chen, G. L. et al. Effect of a chikungunya virus-like particle vaccine on safety and tolerability outcomes: a randomized clinical trial. JAMA 323, 1369–1377 (2020).
Pereira, S. S. et al. NS1-based ELISA test efficiently detects dengue infections without cross-reactivity with Zika virus. Int. J. Infect. Dis. 112, 202–204 (2021).
Edelman, R. et al. Phase II safety and immunogenicity study of live chikungunya virus vaccine TSI-GSD-218. Am. J. Trop. Med. Hyg. 62, 681–685 (2000).
Hills, S. Evidence to Recommendations for Chikungunya Vaccine use Among Adult Travelers https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-10-25-26/02-Chikungunya-Hills-508.pdf (2023).
Waickman, A. T. et al. Evolution of inflammation and immunity in a dengue virus 1 human infection model. Sci. Transl. Med. 14, eabo5019 (2022).
Barnes, M. V. C., Mandla, A., Smith, E., Maskuniitty, M. & Openshaw, P. J. M. Human infection challenge in the pandemic era and beyond, HIC-Vac annual meeting report, 2022. Immunother. Adv. 3, ltad024 (2023).
Cooper, M. M., Loiseau, C., McCarthy, J. S. & Doolan, D. L. Human challenge models: tools to accelerate the development of malaria vaccines. Expert Rev. Vaccines 18, 241–251 (2019).
Paixao, E. S. et al. Mortality from congenital Zika syndrome — nationwide cohort study in Brazil. N. Engl. J. Med. 386, 757–767 (2022).
Yin, P. et al. Chikungunya virus cell-to-cell transmission is mediated by intercellular extensions in vitro and in vivo. Nat. Microbiol. 8, 1653–1667 (2023).
Bruhns, P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood 119, 5640–5649 (2012).
Zhang, A. et al. Beyond neutralization: Fc-dependent antibody effector functions in SARS-CoV-2 infection. Nat. Rev. Immunol. 23, 381–396 (2023).
European Medicines Agency. Assessment Report: Qdenga https://www.ema.europa.eu/en/documents/assessment-report/qdenga-epar-public-assessment-report_en.pdf (2022).
Dias, A. G. Jr. et al. Antibody Fc characteristics and effector functions correlate with protection from symptomatic dengue virus type 3 infection. Sci. Transl. Med. 14, eabm3151 (2022). This study describes a systems serology approach illustrating a role for Fc receptor engagement in protection against symptomatic DENV infection.
Loos, C. et al. Systems serology-based comparison of antibody effector functions induced by adjuvanted vaccines to guide vaccine design. NPJ Vaccines 8, 34 (2023).
O’Connor, D. The omics strategy: the use of systems vaccinology to characterize immune responses to childhood immunization. Expert Rev. Vaccines 21, 1205–1214 (2022).
Hagan, T. et al. Transcriptional atlas of the human immune response to 13 vaccines reveals a common predictor of vaccine-induced antibody responses. Nat. Immunol. 23, 1788–1798 (2022).
Hazlewood, J. E. et al. Injection site vaccinology of a recombinant vaccinia-based vector reveals diverse innate immune signatures. PLoS Pathog. 17, e1009215 (2021).
Kosoy, G. & Miller, B. L. Two decades of arrayed imaging reflectometry for sensitive, high-throughput biosensing. Biosensors 13, 870 (2023).
Liu, X. et al. Metabolomics acts as a powerful tool for comprehensively evaluating vaccines approved under emergency: a CoronaVac retrospective study. Front. Immunol. 14, 1168308 (2023).
Kannan, S., Subbaram, K. & Faiyazuddin, M. In A Handbook of Artificial Intelligence in Drug Delivery (eds Philip, A., Shahiwala, A., Rashid, M. & Faiyazuddin, M.) 467-486 (Academic Press, 2023).
Carvalho, D. O. et al. Transgene-induced cell death following dengue-2 virus infection in Aedes aegypti. Sci. Rep. 13, 5958 (2023).
Spinner, S. A. M. et al. New self-sexing Aedes aegypti strain eliminates barriers to scalable and sustainable vector control for governments and communities in dengue-prone environments. Front. Bioeng. Biotechnol. 10, 975786 (2022).
Waltz, E. Biotech firm announces results from first US trial of genetically modified mosquitoes. Nature 604, 608–609 (2022).
Lee, W. S., Webster, J. A., Madzokere, E. T., Stephenson, E. B. & Herrero, L. J. Mosquito antiviral defense mechanisms: a delicate balance between innate immunity and persistent viral infection. Parasit. Vectors 12, 165 (2019).
Samuel, G. H., Adelman, Z. N. & Myles, K. M. Antiviral immunity and virus-mediated antagonism in disease vector mosquitoes. Trends Microbiol. 26, 447–461 (2018).
Perveen, N. et al. Host-pathogen interaction in arthropod vectors: lessons from viral infections. Front. Immunol. 14, 1061899 (2023).
Schuster, S., Miesen, P. & van Rij, R. P. Antiviral RNAi in insects and mammals: parallels and differences. Viruses 11, 448 (2019).
Utarini, A. et al. Efficacy of Wolbachia-infected mosquito deployments for the control of dengue. N. Engl. J. Med. 384, 2177–2186 (2021). This study reports an impressive result for Wolbachia intervention, having a protective efficacy of 77.1% against dengue.
World Health Organization Vector Control Advisory Group. Eighteenth Meeting of the WHO Vector Control Advisory Group, Meeting report, 24–26 April 2023 https://apps.who.int/iris/rest/bitstreams/1523233/retrieve (2023).
Zug, R. & Hammerstein, P. Wolbachia and the insect immune system: what reactive oxygen species can tell us about the mechanisms of Wolbachia-host interactions. Front. Microbiol. 6, 1201 (2015).
Wang, Z., Yong, H., Zhang, S., Liu, Z. & Zhao, Y. Colonization resistance of symbionts in their insect hosts. Insects 14, 594 (2023).
Fattouh, N., Cazevieille, C. & Landmann, F. Wolbachia endosymbionts subvert the endoplasmic reticulum to acquire host membranes without triggering ER stress. PLoS Negl. Trop. Dis. 13, e0007218 (2019).
Kaur, R. et al. Living in the endosymbiotic world of Wolbachia: a centennial review. Cell Host Microbe 29, 879–893 (2021).
Shropshire, J. D., Leigh, B. & Bordenstein, S. R. Symbiont-mediated cytoplasmic incompatibility: what have we learned in 50 years? eLife 9, e61989 (2020).
Zheng, R. et al. Holobiont perspectives on tripartite interactions among microbiota, mosquitoes, and pathogens. ISME J. 17, 1143–1152 (2023).
Zhang, G., Hussain, M., O’Neill, S. L. & Asgari, S. Wolbachia uses a host microRNA to regulate transcripts of a methyltransferase, contributing to dengue virus inhibition in Aedes aegypti. Proc. Natl Acad. Sci. USA 110, 10276–10281 (2013).
Shin, S. W., Kokoza, V., Lobkov, I. & Raikhel, A. S. Relish-mediated immune deficiency in the transgenic mosquito Aedes aegypti. Proc. Natl Acad. Sci. USA 100, 2616–2621 (2003).
Kokoza, V. et al. Engineering blood meal-activated systemic immunity in the yellow fever mosquito, Aedes aegypti. Proc. Natl Acad. Sci. USA 97, 9144–9149 (2000).
Bian, G., Shin, S. W., Cheon, H. M., Kokoza, V. & Raikhel, A. S. Transgenic alteration of Toll immune pathway in the female mosquito Aedes aegypti. Proc. Natl Acad. Sci. USA 102, 13568–13573 (2005).
Franz, A. W. et al. Engineering RNA interference-based resistance to dengue virus type 2 in genetically modified Aedes aegypti. Proc. Natl Acad. Sci. USA 103, 4198–4203 (2006).
Reid, W. R., Olson, K. E. & Franz, A. W. E. Current effector and gene-drive developments to engineer arbovirus-resistant Aedes aegypti (Diptera: Culicidae) for a sustainable population replacement strategy in the field. J. Med. Entomol. 58, 1987–1996 (2021).
Buchman, A. et al. Engineered resistance to Zika virus in transgenic Aedes aegypti expressing a polycistronic cluster of synthetic small RNAs. Proc. Natl Acad. Sci. USA 116, 3656–3661 (2019).
Buchman, A. et al. Broad dengue neutralization in mosquitoes expressing an engineered antibody. PLoS Pathog. 16, e1008103 (2020).
Mishra, P., Furey, C., Balaraman, V. & Fraser, M. J. Antiviral hammerhead ribozymes are effective for developing transgenic suppression of chikungunya virus in Aedes aegypti mosquitoes. Viruses 8, 163 (2016).
Williams, A. E., Franz, A. W. E., Reid, W. R. & Olson, K. E. Antiviral effectors and gene drive strategies for mosquito population suppression or replacement to mitigate arbovirus transmission by Aedes aegypti. Insects 11, 52 (2020).
Bier, E. Gene drives gaining speed. Nat. Rev. Genet. 23, 5–22 (2022).
Cobb, M. Gene drives could fight malaria and other global killers but might have unintended consequences. Scientific American https://go.nature.com/3Ty3JFY (13 January 2023).
National Gene Technology Scheme. Draft National Gene Drive Policy Guide https://go.nature.com/3xcbOIK (2023).
Riabinina, O., Quinn, M. & Whitehead, J. P. Genetic toolbox approaches in mosquitoes. Cold Spring Harb. Protoc. https://doi.org/10.1101/pdb.top107691 (2022).
World Health Organization. Guidance Framework for Testing Genetically Modified Mosquitoes https://iris.who.int/handle/10665/341370 (2021).
James, S. L., Dass, B. & Quemada, H. Regulatory and policy considerations for the implementation of gene drive-modified mosquitoes to prevent malaria transmission. Transgenic Res. 32, 17–32 (2023).
Patterson, E. I., Villinger, J., Muthoni, J. N., Dobel-Ober, L. & Hughes, G. L. Exploiting insect-specific viruses as a novel strategy to control vector-borne disease. Curr. Opin. Insect Sci. 39, 50–56 (2020).
Hall, R. A. et al. Commensal viruses of mosquitoes: host restriction, transmission, and interaction with arboviral pathogens. Evol. Bioinform. Online 12, 35–44 (2016).
Olmo, R. P. et al. Mosquito vector competence for dengue is modulated by insect-specific viruses. Nat. Microbiol. 8, 135–149 (2023). This study provides a mechanistic explanation for how insect-specific viruses can increase the transmission of dengue.
Zakrzewski, M. et al. Mapping the virome in wild-caught Aedes aegypti from Cairns and Bangkok. Sci. Rep. 8, 4690 (2018).
Feng, Y. et al. A time-series meta-transcriptomic analysis reveals the seasonal, host, and gender structure of mosquito viromes. Virus Evol. 8, veac006 (2022).
Romo, H., Kenney, J. L., Blitvich, B. J. & Brault, A. C. Restriction of Zika virus infection and transmission in Aedes aegypti mediated by an insect-specific flavivirus. Emerg. Microbes Infect. 7, 181 (2018).
Goenaga, S. et al. Potential for co-infection of a mosquito-specific flavivirus, Nhumirim virus, to block West Nile virus transmission in mosquitoes. Viruses 7, 5801–5812 (2015).
Baidaliuk, A. et al. Cell-fusing agent virus reduces arbovirus dissemination in Aedes aegypti mosquitoes in vivo. J. Virol. 93, e00705-19 (2019).
Joseph, R. E., Bozic, J., Werling, K. L., Urakova, N. & Rasgon, J. L. Eilat virus (EILV) causes superinfection exclusion against West NILE virus (WNV) in a strain specific manner in Culex tarsalis mosquitoes. Preprint at bioRxiv https://doi.org/10.1101/2023.05.25.542294 (2023).
Koh, C., Henrion-Lacritick, A., Frangeul, L. & Saleh, M. C. Interactions of the insect-specific palm Creek virus with Zika and Chikungunya viruses in Aedes mosquitoes. Microorganisms 9, 1652 (2021).
Tan, L., Zhang, Y., Kim, D. Y. & Li, R. Insect-specific chimeric viruses potentiated antiviral responses and inhibited pathogenic alphavirus growth in mosquito cells. Microbiol. Spectr. 11, e0361322 (2023).
Prince, B. C., Walsh, E., Torres, T. Z. B. & Rückert, C. Recognition of arboviruses by the mosquito immune system. Biomolecules 13, 1159 (2023).
McLean, B. J. et al. A novel insect-specific flavivirus replicates only in Aedes-derived cells and persists at high prevalence in wild Aedes vigilax populations in Sydney, Australia. Virology 486, 272–283 (2015).
Ratcliffe, N. A. et al. Overview of paratransgenesis as a strategy to control pathogen transmission by insect vectors. Parasit. Vectors 15, 112 (2022).
Wang, S. & Jacobs-Lorena, M. Genetic approaches to interfere with malaria transmission by vector mosquitoes. Trends Biotechnol. 31, 185–193 (2013).
Fofana, A. et al. The strategy of paratransgenesis for the control of malaria transmission. Front. Trop. Dis. 3, 867104 (2022).
Wang, S. et al. Fighting malaria with engineered symbiotic bacteria from vector mosquitoes. Proc. Natl Acad. Sci. USA 109, 12734–12739 (2012).
Wang, S. et al. Driving mosquito refractoriness to Plasmodium falciparum with engineered symbiotic bacteria. Science 357, 1399–1402 (2017).
Patterson, E. I. et al. Negeviruses reduce replication of alphaviruses during coinfection. J. Virol. 95, e0043321 (2021).
Liu, P. et al. Development of non-defective recombinant densovirus vectors for microRNA delivery in the invasive vector mosquito, Aedes albopictus. Sci. Rep. 6, 20979 (2016).
World Mosquito Program. Annual Review https://www.worldmosquitoprogram.org/sites/default/files/2023-06/WMP-AnnualReview-2022.pdf (2022).
US Food & Drug Administration. FDA Approves First Vaccine to Prevent Disease Caused by Chikungunya Virus https://www.fda.gov/news-events/press-announcements/fda-approves-first-vaccine-prevent-disease-caused-chikungunya-virus (2023).
Götte, B., Liu, L. & McInerney, G. M. The enigmatic alphavirus non-structural protein 3 (nsP3) revealing its secrets at last. Viruses 10, 105 (2018).
Roques, P. et al. Attenuated and vectored vaccines protect nonhuman primates against Chikungunya virus. JCI Insight 2, e83527 (2017).
Burton, D. R. Antiviral neutralizing antibodies: from in vitro to in vivo activity. Nat. Rev. Immunol. 23, 720–734 (2023).
Powell, L. A. et al. Human mAbs broadly protect against arthritogenic alphaviruses by recognizing conserved elements of the Mxra8 receptor-binding site. Cell Host Microbe 28, 699–711 (2020).
Basore, K. et al. Cryo-EM structure of Chikungunya virus in complex with the Mxra8 receptor. Cell 177, 1725–1737 (2019).
Ma, H. et al. The low-density lipoprotein receptor promotes infection of multiple encephalitic alphaviruses. Nat. Commun. 15, 246 (2024).
Xie, S., Zhang, H., Liang, Z., Yang, X. & Cao, R. AXL, an important host factor for DENV and ZIKV replication. Front. Cell Infect. Microbiol. 11, 575346 (2021).
Zhou, Q. F. et al. Structural basis of Chikungunya virus inhibition by monoclonal antibodies. Proc. Natl Acad. Sci. USA 117, 27637–27645 (2020).
Williamson, L. E. et al. Structural constraints link differences in neutralization potency of human anti-Eastern equine encephalitis virus monoclonal antibodies. Proc. Natl Acad. Sci. USA 120, e2213690120 (2023).
Kafai, N. M. et al. Neutralizing antibodies protect mice against Venezuelan equine encephalitis virus aerosol challenge. J. Exp. Med. 219, e20212532 (2022).
Ramjag, A. et al. A high-throughput screening assay to identify inhibitory antibodies targeting alphavirus release. Virol. J. 19, 170 (2022).
Williamson, L. E. et al. Therapeutic alphavirus cross-reactive E1 human antibodies inhibit viral egress. Cell 184, 4430–4446 (2021).
Malekshahi, Z. et al. Interference of the Zika virus E-protein with the membrane attack complex of the complement system. Front. Immunol. 11, 569549 (2020).
Earnest, J. T. et al. The mechanistic basis of protection by non-neutralizing anti-alphavirus antibodies. Cell Rep. 35, 108962 (2021).
Chen, X. et al. Development and optimization of a Zika virus antibody-dependent cell-mediated cytotoxicity (ADCC) assay. J. Immunol. Methods 488, 112900 (2021).
Piliponsky, A. M., Acharya, M. & Shubin, N. J. Mast cells in viral, bacterial, and fungal infection immunity. Int. J. Mol. Sci. 20, 2851 (2019).
Schwedler, J. L. et al. Therapeutic efficacy of a potent anti-Venezuelan equine encephalitis virus antibody is contingent on Fc effector function. mAbs 16, 2297451 (2024).
Corti, D., Purcell, L. A., Snell, G. & Veesler, D. Tackling COVID-19 with neutralizing monoclonal antibodies. Cell 184, 4593–4595 (2021).
Mangalmurti, N. & Hunter, C. A. Cytokine storms: understanding COVID-19. Immunity 53, 19–25 (2020). This article introduces the concept of protective inflammation, an area that is increasingly being characterized for COVID-19 but remains understudied for arbovirus infections.
Long, K. M. & Heise, M. T. Protective and pathogenic responses to Chikungunya virus. Infect. Curr. Trop. Med. Rep. 2, 13–21 (2015).
Foy, B. H., Sundt, T. M., Carlson, J. C. T., Aguirre, A. D. & Higgins, J. M. Human acute inflammatory recovery is defined by co-regulatory dynamics of white blood cell and platelet populations. Nat. Comm. 13, 4705 (2022).
Yang, X. et al. Antibody-dependent enhancement: “Evil” antibodies favorable for viral infections. Viruses 14, 1739 (2022).
Martinez-Vega, R. A., Carrasquila, G., Luna, E. & Ramos-Castaneda, J. ADE and dengue vaccination. Vaccine 35, 3910–3912 (2017).
Mallapaty, S. Dengue vaccine poised for roll-out but safety concerns linger. Nature 611, 434–435 (2022).
Thomas, S. J. Is new dengue vaccine efficacy data a relief or cause for concern? NPJ Vaccines 8, 55 (2023).
Katzelnick, L. C. et al. Zika virus infection enhances future risk of severe dengue disease. Science 369, 1123–1128 (2020).
Modhiran, N. et al. A broadly protective antibody that targets the flavivirus NS1 protein. Science 371, 190–194 (2021).
Guirakhoo, F., Domi, A. & Paul, N. Methods for generating a ZIKV immune response utilizing a recombinant modified vaccina Ankara vector encoding the NS1 protein. US Patent 11638750 B2 (2020).
Suhrbier, A. & La Linn, M. Suppression of antiviral responses by antibody-dependent enhancement of macrophage infection. Trends Immunol. 24, 165–168 (2003).
Taylor, A. et al. Fc receptors in antibody-dependent enhancement of viral infections. Immunol. Rev. 268, 340–364 (2015).
Garcia-Nicolas, O. et al. A Japanese encephalitis virus vaccine inducing antibodies strongly enhancing in vitro infection is protective in pigs. Viruses 9, 124 (2017).
Kubinski, M. et al. Tick-borne encephalitis virus: a quest for better vaccines against a virus on the rise. Vaccines 8, 451 (2020).
Simon-Loriere, E. & Schwartz, O. Towards SARS-CoV-2 serotypes? Nat. Rev. Microbiol. 20, 187–188 (2022).
Nakayama, E. E. & Shioda, T. SARS-CoV-2 related antibody-dependent enhancement phenomena in vitro and in vivo. Microorganisms 11, 1015 (2023).
Gan, L., Chen, Y., Tan, J., Wang, X. & Zhang, D. Does potential antibody-dependent enhancement occur during SARS-CoV-2 infection after natural infection or vaccination? A meta-analysis. BMC Infect. Dis. 22, 742 (2022).
Powers, J. M. et al. Infection with chikungunya virus confers heterotypic cross-neutralizing antibodies and memory B-cells against other arthritogenic alphaviruses predominantly through the B domain of the E2 glycoprotein. PLoS Negl. Trop. Dis. 17, e0011154 (2023).
Salgado, R. et al. West Nile virus vaccination protects against Usutu virus disease in mice. Viruses 13, 2352 (2021).
Prow, N. A., Jimenez Martinez, R., Hayball, J. D., Howley, P. M. & Suhrbier, A. Poxvirus-based vector systems and the potential for multi-valent and multi-pathogen vaccines. Expert Rev. Vaccines 17, 925–934 (2018).
Acknowledgements
A.S. is funded by an investigator grant from the National Health and Medical Research Council (NHMRC) of Australia (APP1173880) ‘Chikungunya and Zika viruses; understanding disease and developing interventions’. G.J.D. is a chief investigator on an NHMRC grant (APP2012404) ‘Removing mosquito populations by releasing incompatible males’. L.E.H. is a principal investigator on an NHMRC grant (APP2012500) ‘Mosquito-specific viruses: novel agents to control transmission of arboviral pathogens’. The authors thank the Brazil Family Foundation for their generous philanthropic donations to support PC3-based virus research at QIMR Berghofer Medical Research Institute. The funders had no role in the preparation of the manuscript or in the decision to publish. The authors thank N. Beebe for his helpful input.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. D.J.R., L.E.H., G.J.D. and A.S. contributed substantially to the discussion of the content. A.S., G.J.D. and L.E.H. wrote the article. D.J.R. and A.S. reviewed and/or edited the manuscript before submission. A.L.C. designed the original concepts for the figures.
Corresponding author
Ethics declarations
Competing interests
A.S. is a chief investigator on a Medical Research Future Fund grant (Australia) and will receive research grant funding from that grant for preclinical evaluation of mRNA vaccines; the principal unvestigator is Southern RNA. A.S. and D.J.R. are chief investigators on an NHMRC development grant application and may receive research grant funding from that grant for preclinical evaluation of chimeric insect-specific virus vaccines; the principal investigator and patent holders are from the University of Queensland. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks G. Christophides and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rawle, D.J., Hugo, L.E., Cox, A.L. et al. Generating prophylactic immunity against arboviruses in vertebrates and invertebrates. Nat Rev Immunol (2024). https://doi.org/10.1038/s41577-024-01016-6
Accepted:
Published:
DOI: https://doi.org/10.1038/s41577-024-01016-6