Abstract
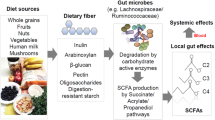
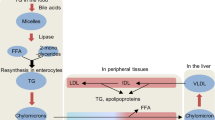
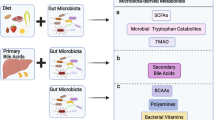
The short-chain fatty acids (SCFAs) butyrate, propionate and acetate are microbial metabolites and their availability in the gut and other organs is determined by environmental factors, such as diet and use of antibiotics, that shape the diversity and metabolism of the microbiota. SCFAs regulate epithelial barrier function as well as mucosal and systemic immunity via evolutionary conserved processes that involve G protein-coupled receptor signalling or histone deacetylase activity. Indicatively, the anti-inflammatory role of butyrate is mediated through direct effects on the differentiation of intestinal epithelial cells, phagocytes, B cells and plasma cells, and regulatory and effector T cells. Intestinally derived SCFAs also directly and indirectly affect immunity at extra-intestinal sites, such as the liver, the lungs, the reproductive tract and the brain, and have been implicated in a range of disorders, including infections, intestinal inflammation, autoimmunity, food allergies, asthma and responses to cancer therapies. An ecological understanding of microbial communities and their interrelated metabolic states, as well as the engineering of butyrogenic bacteria may support SCFA-focused interventions for the prevention and treatment of immune-mediated diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Postler, T. S. & Ghosh, S. Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metab. 26, 110–130 (2017).
GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 5, 17–30 (2020).
Las Heras, V., Melgar, S., MacSharry, J. & Gahan, C. G. M. The influence of the western diet on microbiota and gastrointestinal immunity. Annu. Rev. Food Sci. Technol. 13, 489–512 (2022).
Keeney, K. M., Yurist-Doutsch, S., Arrieta, M. C. & Finlay, B. B. Effects of antibiotics on human microbiota and subsequent disease. Annu. Rev. Microbiol. 68, 217–235 (2014).
Adolph, T. E. & Zhang, J. Diet fuelling inflammatory bowel diseases: preclinical and clinical concepts. Gut 71, 2574–2586 (2022).
Schonfeld, P. & Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J. Lipid Res. 57, 943–954 (2016).
Hu, Y. T. et al. Regulation of genes related to immune signaling and detoxification in Apis mellifera by an inhibitor of histone deacetylation. Sci. Rep. 7, 41255 (2017).
Bortoluzzi, C. et al. Sodium butyrate improved performance while modulating the cecal microbiota and regulating the expression of intestinal immune-related genes of broiler chickens. Poult. Sci. 96, 3981–3993 (2017).
Scott, N. A. et al. Antibiotics induce sustained dysregulation of intestinal T cell immunity by perturbing macrophage homeostasis. Sci. Transl. Med. 10, eaao4755 (2018). This study shows that antibiotic use directly reduced levels of SCFAs in the intestine in mice, leading to hyperresponsive macrophages and increased inflammatory T cell responses in a model system.
Deleu, S., Machiels, K., Raes, J., Verbeke, K. & Vermeire, S. Short chain fatty acids and its producing organisms: an overlooked therapy for IBD? EBioMedicine 66, 103293 (2021).
Park, S. Y. et al. Strain-level fitness in the gut microbiome is an emergent property of glycans and a single metabolite. Cell 185, 513–529.e21 (2022).
Kamal, S. S. et al. Preterm birth has effects on gut colonization in piglets within the first 4 weeks of life. J. Pediatr. Gastroenterol. Nutr. 68, 727–733 (2019).
Backhed, F. et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17, 690–703 (2015).
Loniewska, B. et al. Analysis of fecal short-chain fatty acids (SCFAs) in healthy children during the first two years of life: an observational prospective cohort study. Nutrients 15, 367 (2023).
Li, Y. et al. The effect of breast milk microbiota on the composition of infant gut microbiota: a cohort study. Nutrients 14, 5397 (2022).
Makki, K., Deehan, E. C., Walter, J. & Backhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 23, 705–715 (2018).
Haak, B. W. et al. Long-term impact of oral vancomycin, ciprofloxacin and metronidazole on the gut microbiota in healthy humans. J. Antimicrob. Chemother. 74, 782–786 (2019).
Maltz, R. M. et al. Social stress affects colonic inflammation, the gut microbiome, and short-chain fatty acid levels and receptors. J. Pediatr. Gastroenterol. Nutr. 68, 533–540 (2019).
Ferretti, P. et al. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe 24, 133–145.e5 (2018).
Dominguez-Bello, M. G. et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl Acad. Sci. USA 107, 11971–11975 (2010).
Pannaraj, P. S. et al. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 171, 647–654 (2017).
Fehr, K. et al. Breastmilk feeding practices are associated with the co-occurrence of bacteria in mothers’ milk and the infant gut: the CHILD Cohort Study. Cell Host Microbe 28, 285–297.e4 (2020).
Brodin, P. Immune–microbe interactions early in life: a determinant of health and disease long term. Science 376, 945–950 (2022).
Tsukuda, N. et al. Key bacterial taxa and metabolic pathways affecting gut short-chain fatty acid profiles in early life. ISME J. 15, 2574–2590 (2021).
Claesson, M. J. et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 488, 178–184 (2012).
Salazar, N. et al. Age-associated changes in gut microbiota and dietary components related with the immune system in adulthood and old age: a cross-sectional study. Nutrients 11, 1765 (2019).
Haskey, N. et al. A Mediterranean diet pattern improves intestinal inflammation concomitant with reshaping of the bacteriome in ulcerative colitis: a randomized controlled trial. J. Crohns Colitis 17, 1569–1578 (2023). This study shows that a Mediterranean diet, high in fibres, increases the SCFA-producing microbiome and total faecal SCFA levels in individuals with quiescent ulcerative colitis, illustrating the feasibility of dietary interventions to potentially maintain remission.
Wastyk, H. C. et al. Gut-microbiota-targeted diets modulate human immune status. Cell 184, 4137–4153.e14 (2021). Using multi-omics profiling of the microbiome and host parameters, this work shows that a plant-based fibre diet promotes microbiota diversity and SCFA production, while simultaneously reducing the presence of inflammatory markers, thereby modulating the immune status of the host.
Desai, M. S. et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167, 1339–1353.e21 (2016).
Corbin, K. D. et al. Host-diet-gut microbiome interactions influence human energy balance: a randomized clinical trial. Nat. Commun. 14, 3161 (2023).
Miranda, P. M. et al. High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. Microbiome 6, 57 (2018).
Ni, J. et al. Early antibiotic exposure and development of asthma and allergic rhinitis in childhood. BMC Pediatr. 19, 225 (2019).
Lu, Y. et al. Early-life antibiotic exposure and childhood asthma trajectories: a national population-based birth cohort. Antibiotics 12, 314 (2023).
Mark-Christensen, A. et al. Early-life exposure to antibiotics and risk for Crohn’s disease: a nationwide danish birth cohort study. Inflamm. Bowel Dis. 28, 415–422 (2022).
Borbet, T. C. et al. Influence of the early-life gut microbiota on the immune responses to an inhaled allergen. Mucosal Immunol. 15, 1000–1011 (2022).
Russell, S. L. et al. Perinatal antibiotic treatment affects murine microbiota, immune responses and allergic asthma. Gut Microbes 4, 158–164 (2013).
Zuo, T. et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology 159, 944–955.e8 (2020).
Yeoh, Y. K. et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 70, 698–706 (2021).
Zhang, F. et al. Prolonged impairment of short-chain fatty acid and l-isoleucine biosynthesis in gut microbiome in patients with COVID-19. Gastroenterology 162, 548–561.e4 (2022). This study uncovers a long-lasting impairment of SCFA production in patients with severe SARS-CoV-2 infection, even after disease resolution.
Shin, J. H. et al. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res. Microbiol. 170, 192–201 (2019).
Gao, H. et al. Antibiotic exposure has sex-dependent effects on the gut microbiota and metabolism of short-chain fatty acids and amino acids in mice. mSystems 4, e00048-19 (2019).
Sanna, S. et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 51, 600–605 (2019). This study provides bidirectional Mendelian randomization of human genetics, microbiome composition, SCFAs, and metabolic and anthropometric traits linking distinct mechanisms with butyrate and propionate levels.
Fiskerstrand, T. et al. Familial diarrhea syndrome caused by an activating GUCY2C mutation. N. Engl. J. Med. 366, 1586–1595 (2012).
Janecke, A. R. et al. Reduced sodium/proton exchanger NHE3 activity causes congenital sodium diarrhea. Hum. Mol. Genet. 24, 6614–6623 (2015).
Canani, R. B. et al. Genotype-dependency of butyrate efficacy in children with congenital chloride diarrhea. Orphanet J. Rare Dis. 8, 194 (2013).
Tronstad, R. R. et al. Guanylate cyclase C activation shapes the intestinal microbiota in patients with familial diarrhea and increased susceptibility for Crohn’s disease. Inflamm. Bowel Dis. 23, 1752–1761 (2017).
Sun, M., Wu, W., Liu, Z. & Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 52, 1–8 (2017).
Zhao, Y. et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 11, 752–762 (2018).
Thomas, S. P. & Denu, J. M. Short-chain fatty acids activate acetyltransferase p300. eLife https://doi.org/10.7554/eLife.72171 (2021).
Lund, P. J. et al. Stable isotope tracing in vivo reveals a metabolic bridge linking the microbiota to host histone acetylation. Cell Rep. 41, 111809 (2022).
Chang, P. V., Hao, L., Offermanns, S. & Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl Acad. Sci. USA 111, 2247–2252 (2014).
Schulthess, J. et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity 50, 432–445.e7 (2019). This study demonstrates that butyrate induces the metabolic reprogramming and antimicrobial activity of macrophages in vitro and protects mice from enteropathogens in vivo.
Furusawa, Y. et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013). A key study showing that SCFAs facilitate the differentiation of colonic regulatory FOXP3+ T cells.
Elce, A. et al. Butyrate modulating effects on pro-inflammatory pathways in human intestinal epithelial cells. Benef. Microbes 8, 841–847 (2017).
Grouls, M. et al. Differential gene expression in iPSC-derived human intestinal epithelial cell layers following exposure to two concentrations of butyrate, propionate and acetate. Sci. Rep. 12, 13988 (2022).
Goudarzi, A. et al. Dynamic competing histone H4 K5K8 acetylation and butyrylation are hallmarks of highly active gene promoters. Mol. Cell 62, 169–180 (2016).
Fellows, R. et al. Microbiota derived short chain fatty acids promote histone crotonylation in the colon through histone deacetylases. Nat. Commun. 9, 105 (2018). This study shows that butyrate promotes histone crotonylation in intestinal cells and in intestinal organoids by inhibiting the decrotonylase activity of HDACs and by acting as a substrate for corotonyl-CoA generation.
Fellows, R. & Varga-Weisz, P. Chromatin dynamics and histone modifications in intestinal microbiota–host crosstalk. Mol. Metab. 38, 100925 (2020).
Sarkar, A. et al. Butyrate limits inflammatory macrophage niche in NASH. Cell Death Dis. 14, 332 (2023).
Byndloss, M. X. et al. Microbiota-activated PPAR-gamma signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 357, 570–575 (2017).
Chen, Y., Wang, Y., Fu, Y., Yin, Y. & Xu, K. Modulating AHR function offers exciting therapeutic potential in gut immunity and inflammation. Cell Biosci. 13, 85 (2023).
Willemsen, L. E., Koetsier, M. A., van Deventer, S. J. & van Tol, E. A. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut 52, 1442–1447 (2003).
Gaudier, E. et al. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G1168–G1174 (2004).
Barcelo, A. et al. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut 46, 218–224 (2000).
Jiang, W. et al. Differential regulation of human cathelicidin LL-37 by free fatty acids and their analogs. Peptides 50, 129–138 (2013).
Kaiko, G. E. et al. The colonic crypt protects stem cell microbiota-derived metabolites. Cell 167, 1137 (2016).
Hinrichsen, F. et al. Microbial regulation of hexokinase 2 links mitochondrial metabolism and cell death in colitis. Cell Metab. 33, 2355–2366.e8 (2021).
Yan, H. & Ajuwon, K. M. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS ONE 12, e0179586 (2017).
Fachi, J. L. et al. Butyrate protects mice from Clostridium difficile-induced colitis through an HIF-1-dependent mechanism. Cell Rep. 27, 750–761.e7 (2019).
Feng, Y., Wang, Y., Wang, P., Huang, Y. & Wang, F. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy. Cell Physiol. Biochem. 49, 190–205 (2018).
Kelly, C. J. et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17, 662–671 (2015).
Pral, L. P., Fachi, J. L., Correa, R. O., Colonna, M. & Vinolo, M. A. R. Hypoxia and HIF-1 as key regulators of gut microbiota and host interactions. Trends Immunol. 42, 604–621 (2021).
Ferrer-Picon, E. et al. Intestinal inflammation modulates the epithelial response to butyrate in patients with inflammatory bowel disease. Inflamm. Bowel Dis. https://doi.org/10.1093/ibd/izz119 (2019).
Nastasi, C. et al. Butyrate and propionate inhibit antigen-specific CD8+ T cell activation by suppressing IL-12 production by antigen-presenting cells. Sci. Rep. 7, 14516 (2017).
Huang, C., Du, W., Ni, Y., Lan, G. & Shi, G. The effect of short-chain fatty acids on M2 macrophages polarization in vitro and in vivo. Clin. Exp. Immunol. 207, 53–64 (2022).
Zhao, C. et al. A fiber-enriched diet alleviates Staphylococcus aureus-induced mastitis by activating the HDAC3-mediated antimicrobial program in macrophages via butyrate production in mice. PLoS Pathog. 19, e1011108 (2023).
Singh, N. et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40, 128–139 (2014).
Vinolo, M. A. et al. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 22, 849–855 (2011).
Fachi, J. L. et al. Acetate coordinates neutrophil and ILC3 responses against C. difficile through FFAR2. J. Exp. Med. 217, jem.20190489 (2020).
Li, G. et al. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes 13, 1968257 (2021).
Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013).
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013). A landmark study showing that administration of SCFAs to mice via drinking water increases the number of Treg cells and protects against colitis in a GPR43-dependent manner.
Hao, F. et al. Butyrate enhances CPT1A activity to promote fatty acid oxidation and iTreg differentiation. Proc. Natl Acad. Sci. USA 118, e2014681118 (2021).
Schilderink, R. et al. The SCFA butyrate stimulates the epithelial production of retinoic acid via inhibition of epithelial HDAC. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G1138–G1146 (2016).
Goverse, G. et al. Diet-derived short chain fatty acids stimulate intestinal epithelial cells to induce mucosal tolerogenic dendritic cells. J. Immunol. 198, 2172–2181 (2017).
Wu, W. et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 10, 946–956 (2017).
Gurav, A. et al. Slc5a8, a Na+-coupled high-affinity transporter for short-chain fatty acids, is a conditional tumour suppressor in colon that protects against colitis and colon cancer under low-fibre dietary conditions. Biochem. J. 469, 267–278 (2015).
Chen, L. et al. Microbiota metabolite butyrate differentially regulates Th1 and Th17 cells’ differentiation and function in induction of colitis. Inflamm. Bowel Dis. 25, 1450–1461 (2019).
Sun, M. et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat. Commun. 9, 3555 (2018).
Park, J. et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR–S6K pathway. Mucosal Immunol. 8, 80–93 (2015).
Yang, W. et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 11, 4457 (2020).
Dupraz, L. et al. Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal γδ T cells. Cell Rep. 36, 109332 (2021).
Trompette, A. et al. Dietary fiber confers protection against flu by shaping Ly6c− patrolling monocyte hematopoiesis and CD8+ T cell metabolism. Immunity 48, 992–1005.e8 (2018).
Bachem, A. et al. Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8+ T cells. Immunity 51, 285–297.e5 (2019).
Qiu, J. et al. Acetate promotes T cell effector function during glucose restriction. Cell Rep. 27, 2063–2074.e5 (2019).
Luu, M. et al. Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 12, 4077 (2021). This study shows that butyrate promotes the antitumour activities of cytotoxic T cells in mouse models of melanoma and pancreatic cancer.
Kim, M., Qie, Y., Park, J. & Kim, C. H. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe 20, 202–214 (2016).
Sanchez, H. N. et al. B cell-intrinsic epigenetic modulation of antibody responses by dietary fiber-derived short-chain fatty acids. Nat. Commun. 11, 60 (2020).
Rosser, E. C. et al. Microbiota-derived metabolites suppress arthritis by amplifying aryl-hydrocarbon receptor activation in regulatory B cells. Cell Metab. 31, 837–851.e10 (2020).
Sepahi, A., Liu, Q., Friesen, L. & Kim, C. H. Dietary fiber metabolites regulate innate lymphoid cell responses. Mucosal Immunol. 14, 317–330 (2021).
Chun, E. et al. Metabolite-sensing receptor Ffar2 regulates colonic group 3 innate lymphoid cells and gut immunity. Immunity 51, 871–884.e6 (2019).
Thio, C. L., Chi, P. Y., Lai, A. C. & Chang, Y. J. Regulation of type 2 innate lymphoid cell-dependent airway hyperreactivity by butyrate. J. Allergy Clin. Immunol. 142, 1867–1883.e12 (2018).
Uhlig, H. H. & Powrie, F. Translating immunology into therapeutic concepts for inflammatory bowel disease. Annu. Rev. Immunol. 36, 755–781 (2018).
Machiels, K. et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 63, 1275–1283 (2014).
Yilmaz, B. et al. Microbial network disturbances in relapsing refractory Crohn’s disease. Nat. Med. 25, 323–336 (2019). This multi-omics study provides longitudinal molecular profiles of individuals with IBD, reporting changes in microbial composition, microbial transcription and microbial production of metabolites such as SCFAs.
Lloyd-Price, J. et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 569, 655–662 (2019).
Gueimonde, M., Ouwehand, A., Huhtinen, H., Salminen, E. & Salminen, S. Qualitative and quantitative analyses of the bifidobacterial microbiota in the colonic mucosa of patients with colorectal cancer, diverticulitis and inflammatory bowel disease. World J. Gastroenterol. 13, 3985–3989 (2007).
Zhuang, X. et al. Systematic review and meta-analysis: short-chain fatty acid characterization in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 25, 1751–1763 (2019).
Imhann, F. et al. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut 67, 108–119 (2018).
Zhou, L. et al. Faecalibacterium prausnitzii produces butyrate to maintain Th17/Treg balance and to ameliorate colorectal colitis by inhibiting histone deacetylase 1. Inflamm. Bowel Dis. https://doi.org/10.1093/ibd/izy182 (2018).
Ortqvist, A. K., Lundholm, C., Halfvarson, J., Ludvigsson, J. F. & Almqvist, C. Fetal and early life antibiotics exposure and very early onset inflammatory bowel disease: a population-based study. Gut 68, 218–225 (2019).
Knoop, K. A. et al. Microbial antigen encounter during a preweaning interval is critical for tolerance to gut bacteria. Sci. Immunol. 2, eaao1314 (2017).
Al Nabhani, Z. et al. A weaning reaction to microbiota is required for resistance to immunopathologies in the adult. Immunity 50, 1276–1288.e5 (2019).
Breuer, R. I. et al. Short chain fatty acid rectal irrigation for left-sided ulcerative colitis: a randomised, placebo controlled trial. Gut 40, 485–491 (1997).
Hamer, H. M. et al. Effect of butyrate enemas on inflammation and antioxidant status in the colonic mucosa of patients with ulcerative colitis in remission. Clin. Nutr. 29, 738–744 (2010).
Facchin, S. et al. Microbiota changes induced by microencapsulated sodium butyrate in patients with inflammatory bowel disease. Neurogastroenterol. Motil. 32, e13914 (2020).
Gerasimidis, K. et al. Decline in presumptively protective gut bacterial species and metabolites are paradoxically associated with disease improvement in pediatric Crohn’s disease during enteral nutrition. Inflamm. Bowel Dis. 20, 861–871 (2014).
Svolos, V. et al. Treatment of active Crohn’s disease with an ordinary food-based diet that replicates exclusive enteral nutrition. Gastroenterology 156, 1354–1367.e6 (2019).
Fukuda, S. et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469, 543–547 (2011).
Benor, S. et al. Probiotic supplementation in mothers of very low birth weight infants. Am. J. Perinatol. 31, 497–504 (2014).
De Vuyst, L. & Leroy, F. Cross-feeding between bifidobacteria and butyrate-producing colon bacteria explains bifdobacterial competitiveness, butyrate production, and gas production. Int. J. Food Microbiol. 149, 73–80 (2011).
Raqib, R. et al. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc. Natl Acad. Sci. USA 103, 9178–9183 (2006).
Jacobson, A. et al. A gut commensal-produced metabolite mediates colonization resistance to Salmonella infection. Cell Host Microbe 24, 296–307.e7 (2018).
Hryckowian, A. J. et al. Microbiota-accessible carbohydrates suppress Clostridium difficile infection in a murine model. Nat. Microbiol. 3, 662–669 (2018).
Pensinger, D. A. et al. Butyrate differentiates permissiveness to Clostridioides difficile infection and influences growth of diverse C. difficile isolates. Infect. Immun. 91, e0057022 (2023).
Vonaesch, P. et al. Stunted childhood growth is associated with decompartmentalization of the gastrointestinal tract and overgrowth of oropharyngeal taxa. Proc. Natl Acad. Sci. USA 115, E8489–E8498 (2018).
Farras, M. et al. Characterizing the metabolic phenotype of intestinal villus blunting in Zambian children with severe acute malnutrition and persistent diarrhea. PLoS ONE 13, e0192092 (2018).
Panigrahi, P. et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature 548, 407–412 (2017).
Mirzaei, R. et al. Microbiota metabolites in the female reproductive system: focused on the short-chain fatty acids. Heliyon 9, e14562 (2023).
Guo, J. et al. Sodium butyrate alleviates lipopolysaccharide-induced endometritis in mice through inhibiting inflammatory response. Microb. Pathog. 137, 103792 (2019).
Chadchan, S. B. et al. Gut microbiota-derived short-chain fatty acids protect against the progression of endometriosis. Life Sci. Alliance 4, e202101224 (2021).
Ye, Q. et al. Maternal short and medium chain fatty acids supply during early pregnancy improves embryo survival through enhancing progesterone synthesis in rats. J. Nutr. Biochem. 69, 98–107 (2019).
Nakajima, A. et al. Maternal high fiber diet during pregnancy and lactation influences regulatory T cell differentiation in offspring in mice. J. Immunol. 199, 3516–3524 (2017).
Kimura, I. et al. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science 367, eaaw8429 (2020). This study reports that SCFAs from the colonic lumen of pregnant mice reach developing embryos to regulate embryonic energy metabolism, generating resistance to obesity and metabolic syndrome in offspring via activation of GPR41 and GPR43.
Perez, R. et al. Sodium butyrate upregulates Kupffer cell PGE2 production and modulates immune function. J. Surg. Res. 78, 1–6 (1998).
Ye, J. et al. Butyrate protects mice against methionine-choline-deficient diet-induced non-alcoholic steatohepatitis by improving gut barrier function, attenuating inflammation and reducing endotoxin levels. Front. Microbiol. 9, 1967 (2018).
Sun, B. et al. Sodium butyrate ameliorates high-fat-diet-induced non-alcoholic fatty liver disease through peroxisome proliferator-activated receptor α-mediated activation of β oxidation and suppression of inflammation. J. Agric. Food Chem. 66, 7633–7642 (2018).
Tian, P. et al. Early life gut microbiota sustains liver-resident natural killer cells maturation via the butyrate–IL-18 axis. Nat. Commun. 14, 1710 (2023).
Dalile, B., Van Oudenhove, L., Vervliet, B. & Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 16, 461–478 (2019).
Erny, D. et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977 (2015).
Erny, D. et al. Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metab. 33, 2260–2276.e7 (2021). This study shows that SCFAs drive microglia maturation by modulating their metabolic pathways.
Braniste, V. et al. The gut microbiota influences blood–brain barrier permeability in mice. Sci. Transl. Med. 6, 263ra158 (2014).
Wang, P. et al. Sodium butyrate triggers a functional elongation of microglial process via Akt-small RhoGTPase activation and HDACs inhibition. Neurobiol. Dis. 111, 12–25 (2018).
Chen, T., Noto, D., Hoshino, Y., Mizuno, M. & Miyake, S. Butyrate suppresses demyelination and enhances remyelination. J. Neuroinflammation 16, 165 (2019).
Mirzaei, R. et al. Role of microbiota-derived short-chain fatty acids in nervous system disorders. Biomed. Pharmacother. 139, 111661 (2021).
Unger, M. M. et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat. Disord. 32, 66–72 (2016).
Aho, V. T. E. et al. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol. Neurodegener. 16, 6 (2021).
Trompette, A. et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 20, 159–166 (2014).
Cait, A. et al. Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal Immunol. 11, 785–795 (2018).
Di Simone, S. K., Rudloff, I., Nold-Petry, C. A., Forster, S. C. & Nold, M. F. Understanding respiratory microbiome-immune system interactions in health and disease. Sci. Transl. Med. 15, eabq5126 (2023).
Yip, W. et al. Butyrate shapes immune cell fate and function in allergic asthma. Front. Immunol. 12, 628453 (2021).
Bottcher, M. F., Nordin, E. K., Sandin, A., Midtvedt, T. & Bjorksten, B. Microflora-associated characteristics in faeces from allergic and nonallergic infants. Clin. Exp. Allergy 30, 1590–1596 (2000).
Roduit, C. et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy 74, 799–809 (2019).
Cait, A. et al. Reduced genetic potential for butyrate fermentation in the gut microbiome of infants who develop allergic sensitization. J. Allergy Clin. Immunol. 144, 1638–1647.e3 (2019).
Depner, M. et al. Maturation of the gut microbiome during the first year of life contributes to the protective farm effect on childhood asthma. Nat. Med. 26, 1766–1775 (2020). This study reports that children raised in an ‘asthma-protective’ farm-related environment exhibit a lower incidence of asthma, atopic sensitization and food allergy, a trend linked to increased faecal levels of butyrate.
Sencio, V. et al. Gut dysbiosis during influenza contributes to pulmonary pneumococcal superinfection through altered short-chain fatty acid production. Cell Rep. 30, 2934–2947.e6 (2020).
Ichinohe, T. et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl Acad. Sci. USA 108, 5354–5359 (2011).
Haak, B. W. et al. Impact of gut colonization with butyrate-producing microbiota on respiratory viral infection following allo-HCT. Blood 131, 2978–2986 (2018).
Zhao, Y., Dong, B. R. & Hao, Q. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst. Rev. 8, CD006895 (2022).
d’Ettorre, G. et al. Challenges in the management of SARS-CoV2 infection: the role of oral bacteriotherapy as complementary therapeutic strategy to avoid the progression of COVID-19. Front. Med. 7, 389 (2020).
van der Beek, C. M. et al. Hepatic uptake of rectally administered butyrate prevents an increase in systemic butyrate concentrations in humans. J. Nutr. 145, 2019–2024 (2015).
Wang, R. et al. Treatment of peanut allergy and colitis in mice via the intestinal release of butyrate from polymeric micelles. Nat. Biomed. Eng. 7, 38–55 (2023).
Suez, J., Zmora, N., Segal, E. & Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 25, 716–729 (2019).
Zhang, H. et al. Next-generation probiotics: microflora intervention to human diseases. Biomed. Res. Int. 2022, 5633403 (2022).
Mallick, H. et al. Predictive metabolomic profiling of microbial communities using amplicon or metagenomic sequences. Nat. Commun. 10, 3136 (2019).
Vital, M., Karch, A. & Pieper, D. H. Colonic butyrate-producing communities in humans: an overview using omics data. mSystems 2, e00130-17 (2017).
Park, Y. T. et al. Physiological activity of E. coli engineered to produce butyric acid. Microb. Biotechnol. 15, 832–843 (2022).
Zhang, T., Zhang, J. & Duan, L. The role of genetically engineered probiotics for treatment of inflammatory bowel disease: a systematic review. Nutrients 15, 1566 (2023).
Juul, F. E. et al. Fecal microbiota transplantation for primary Clostridium difficile infection. N. Engl. J. Med. 378, 2535–2536 (2018).
Baunwall, S. M. D. et al. Faecal microbiota transplantation for first or second Clostridioides difficile infection (EarlyFMT): a randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 7, 1083–1091 (2022).
Fuentes, S. et al. Microbial shifts and signatures of long-term remission in ulcerative colitis after faecal microbiota transplantation. ISME J. 11, 1877–1889 (2017).
De Filippis, F. et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 65, 1812–1821 (2016).
Sobh, M. et al. Tolerability and SCFA production after resistant starch supplementation in humans: a systematic review of randomized controlled studies. Am. J. Clin. Nutr. 115, 608–618 (2022).
Liu, X. et al. High-fiber diet mitigates maternal obesity-induced cognitive and social dysfunction in the offspring via gut–brain axis. Cell Metab. 33, 923–938.e6 (2021).
Hodgkinson, K. et al. Butyrate’s role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin. Nutr. 42, 61–75 (2023).
Henrick, B. M. et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 184, 3884–3898.e11 (2021).
Akay, H. K. et al. The relationship between bifidobacteria and allergic asthma and/or allergic dermatitis: a prospective study of 0–3 years-old children in Turkey. Anaerobe 28, 98–103 (2014).
Miraglia Del Giudice, M. et al. Bifidobacterium mixture (B longum BB536, B infantis M-63, B breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma. Ital. J. Pediatr. 43, 25 (2017).
Metsala, J. et al. Prenatal and post-natal exposure to antibiotics and risk of asthma in childhood. Clin. Exp. Allergy 45, 137–145 (2015).
Al-Qadami, G. H., Secombe, K. R., Subramaniam, C. B., Wardill, H. R. & Bowen, J. M. Gut microbiota-derived short-chain fatty acids: impact on cancer treatment response and toxicities. Microorganisms 10, 2048 (2022).
He, Y. et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8+ T cell immunity. Cell Metab. 33, 988–1000.e7 (2021).
Nomura, M. et al. Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA Netw. Open 3, e202895 (2020).
Falony, G., Vlachou, A., Verbrugghe, K. & De Vuyst, L. Cross-feeding between Bifidobacterium longum BB536 and acetate-converting, butyrate-producing colon bacteria during growth on oligofructose. Appl. Env. Microbiol. 72, 7835–7841 (2006).
Duncan, S. H., Louis, P. & Flint, H. J. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl. Env. Microbiol. 70, 5810–5817 (2004).
Rooks, M. G. & Garrett, W. S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 16, 341–352 (2016).
Singh, V. et al. Butyrate producers, “The Sentinel of Gut”: their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 13, 1103836 (2022).
Belenguer, A. et al. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl. Env. Microbiol. 72, 3593–3599 (2006).
Heinken, A. & Thiele, I. Anoxic conditions promote species-specific mutualism between gut microbes in silico. Appl. Env. Microbiol. 81, 4049–4061 (2015).
Clark, R. L. et al. Design of synthetic human gut microbiome assembly and butyrate production. Nat. Commun. 12, 3254 (2021).
Riviere, A., Selak, M., Lantin, D., Leroy, F. & De Vuyst, L. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front. Microbiol. 7, 979 (2016).
Ordonez-Rodriguez, A., Roman, P., Rueda-Ruzafa, L., Campos-Rios, A. & Cardona, D. Changes in gut microbiota and multiple sclerosis: a systematic review. Int. J. Environ. Res. Public Health 20, 4624 (2023).
Mizuno, M., Noto, D., Kaga, N., Chiba, A. & Miyake, S. The dual role of short fatty acid chains in the pathogenesis of autoimmune disease models. PLoS ONE 12, e0173032 (2017).
Vatanen, T. et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature 562, 589–594 (2018).
Yuan, X. et al. Functional and metabolic alterations of gut microbiota in children with new-onset type 1 diabetes. Nat. Commun. 13, 6356 (2022).
Marino, E. et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat. Immunol. 18, 552–562 (2017).
Jia, L. et al. Butyrate ameliorates antibiotic-driven type 1 diabetes in the female offspring of nonobese diabetic mice. J. Agric. Food Chem. 68, 3112–3120 (2020).
Huang, J. et al. Gut microbial metabolites alter IgA immunity in type 1 diabetes. JCI Insight https://doi.org/10.1172/jci.insight.135718 (2020).
de Groot, P. F. et al. Oral butyrate does not affect innate immunity and islet autoimmunity in individuals with longstanding type 1 diabetes: a randomised controlled trial. Diabetologia 63, 597–610 (2020).
Fujiwara, H. et al. Microbial metabolite sensor GPR43 controls severity of experimental GVHD. Nat. Commun. 9, 3674 (2018).
Mathewson, N. D. et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat. Immunol. 17, 505–513 (2016).
Tanaka, J. S. et al. Anaerobic antibiotics and the risk of graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transpl. 26, 2053–2060 (2020).
Markey, K. A. et al. The microbe-derived short-chain fatty acids butyrate and propionate are associated with protection from chronic GVHD. Blood 136, 130–136 (2020).
van Lier, Y. F. et al. Donor fecal microbiota transplantation ameliorates intestinal graft-versus-host disease in allogeneic hematopoietic cell transplant recipients. Sci. Transl. Med. 12, eaaz8926 (2020).
Burgos da Silva, M. et al. Preservation of the fecal microbiome is associated with reduced severity of graft-versus-host disease. Blood 140, 2385–2397 (2022).
Golob, J. L. et al. Butyrogenic bacteria after acute graft-versus-host disease (GVHD) are associated with the development of steroid-refractory GVHD. Blood Adv. 3, 2866–2869 (2019).
De Filippis, F. et al. Specific gut microbiome signatures and the associated pro-inflammatory functions are linked to pediatric allergy and acquisition of immune tolerance. Nat. Commun. 12, 5958 (2021).
Di Costanzo, M., De Paulis, N. & Biasucci, G. Butyrate: a link between early life nutrition and gut microbiome in the development of food allergy. Life 11, 384 (2021).
Paparo, L. et al. Butyrate as a bioactive human milk protective component against food allergy. Allergy 76, 1398–1415 (2021). This study shows that butyrate and propionate are depleted in the gut of children with allergies.
Berni Canani, R. et al. Extensively hydrolyzed casein formula containing Lactobacillus rhamnosus GG reduces the occurrence of other allergic manifestations in children with cow’s milk allergy: 3-year randomized controlled trial. J. Allergy Clin. Immunol. 139, 1906–1913.e4 (2017).
Tan, J. et al. Dietary fiber and bacterial SCFA enhance oral tolerance and protect against food allergy through diverse cellular pathways. Cell Rep. 15, 2809–2824 (2016).
Song, H., Yoo, Y., Hwang, J., Na, Y. C. & Kim, H. S. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J. Allergy Clin. Immunol. 137, 852–860 (2016).
Ta, L. D. H. et al. A compromised developmental trajectory of the infant gut microbiome and metabolome in atopic eczema. Gut Microbes 12, 1–22 (2020).
Wopereis, H. et al. Intestinal microbiota in infants at high risk for allergy: effects of prebiotics and role in eczema development. J. Allergy Clin. Immunol. 141, 1334–1342.e5 (2018).
Nylund, L. et al. Severity of atopic disease inversely correlates with intestinal microbiota diversity and butyrate-producing bacteria. Allergy 70, 241–244 (2015).
Trompette, A. et al. Gut-derived short-chain fatty acids modulate skin barrier integrity by promoting keratinocyte metabolism and differentiation. Mucosal Immunol. 15, 908–926 (2022).
Luo, C. H., Lai, A. C. & Chang, Y. J. Butyrate inhibits Staphylococcus aureus-aggravated dermal IL-33 expression and skin inflammation through histone deacetylase inhibition. Front. Immunol. 14, 1114699 (2023).
Sanford, J. A. et al. Inhibition of HDAC8 and HDAC9 by microbial short-chain fatty acids breaks immune tolerance of the epidermis to TLR ligands. Sci. Immunol. 1, eaah4609 (2016).
Sawada, Y. et al. Cutaneous innate immune tolerance is mediated by epigenetic control of MAP2K3 by HDAC8/9. Sci. Immunol. 6, eabe1935 (2021).
Schwarz, A., Bruhs, A. & Schwarz, T. The short-chain fatty acid sodium butyrate functions as a regulator of the skin immune system. J. Invest. Dermatol. 137, 855–864 (2017).
Chen, C. et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 8, 875 (2017).
Ravel, J. et al. Vaginal microbiome of reproductive-age women. Proc. Natl Acad. Sci. USA 108, 4680–4687 (2011).
Fredricks, D. N., Fiedler, T. L. & Marrazzo, J. M. Molecular identification of bacteria associated with bacterial vaginosis. N. Engl. J. Med. 353, 1899–1911 (2005).
Aldunate, M. et al. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front. Physiol. 6, 164 (2015).
Delgado-Diaz, D. J. et al. Distinct immune responses elicited from cervicovaginal epithelial cells by lactic acid and short chain fatty acids associated with optimal and non-optimal vaginal microbiota. Front. Cell Infect. Microbiol. 9, 446 (2019).
Acknowledgements
E.R.M. is supported by the Wellcome Trust and the Royal Society (206206/Z/17/Z). H.H.U. is supported by the National Institute for Health Research Biomedical Research Centre Oxford, and The Leona M. and Harry B. Helmsley Charitable Trust. The authors thank A. Mowat and A. Cavounidis for critical comments on the manuscript. In light of the large field covered, the authors express their sincere apologies for any literature omissions in the Review due to limitations in the number of references.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
H.H.U. has received research support or consultancy fees from Janssen, UCB Pharma, Eli Lilly, GSK, Celgene/BMS and AbbVie. There is no direct link between any funding and the subject reviewed. All other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks all anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Antimicrobial peptides
-
Peptides that are essential for the defence of intestinal barrier surfaces against invading bacteria.
- Colonization resistance
-
The ability of the intestinal microbiota to prevent expansion of new microorganisms.
- Cross-feeding
-
An exchange of metabolites among different species or strains of microorganisms as energy or metabolic source.
- Faecal microbiota transplantation
-
The transfer of microorganisms from the stool of one individual to another.
- First-pass effect
-
The reduction of the concentration of a metabolite owing to metabolism at a specific location in the body (such as the liver) before reaching the systemic circulation.
- Genetically modified bacteria
-
Bacteria that have been engineered to express specific genes to modify the bacterial ecosystem, to produce metabolites or proteins, and to modify host function.
- Histone deacetylase
-
(HDAC). An enzyme that mediates protein deacetylation, in particular histone deacetylation, to modify histone function, DNA accessibility and gene transcription.
- Holobiont
-
An ecological unit of a host and other species including commensal bacteria, viruses and fungi residing in the intestine or skin.
- Kupffer cells
-
Liver-resident specialized mononuclear phagocytes that line the hepatic sinusoids.
- Microglia
-
Brain-resident phagocytes that protect the cerebral microenvironment.
- Prebiotics
-
Substances such as carbohydrates that stimulate the growth of probiotic bacteria.
- Probiotic bacteria
-
Bacteria that provide health benefits when consumed.
- Resistant starch
-
Starch that is resistant to digestion in the small intestine.
- Short-chain fatty acids
-
(SCFAs). Comprise fatty acids with a carbon backbone of 1–6 carbons: formate (C1), acetate (C2), propionate (C3), butyrate (C4) and valerate (C5).
- T follicular helper cells
-
Specialized CD4+ T cells that support B cell responses.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mann, E.R., Lam, Y.K. & Uhlig, H.H. Short-chain fatty acids: linking diet, the microbiome and immunity. Nat Rev Immunol (2024). https://doi.org/10.1038/s41577-024-01014-8
Accepted:
Published:
DOI: https://doi.org/10.1038/s41577-024-01014-8