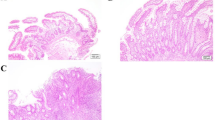
Abstract
Pouchitis is an acute or chronic inflammatory disease of the ileal reservoir. It is common after restorative proctocolectomy with ileal pouch–anal anastomosis, and treatment of chronic antibiotic-refractory pouchitis has proven challenging. Most cases of acute pouchitis evolve into chronic pouchitis. The aetiology of acute pouchitis is likely to be partly related to the gut microbiota, whereas the pathophysiology of chronic pouchitis involves abnormal interactions between genetic disposition, faecal stasis, the gut microbiota, dysregulated host immunity, surgical techniques, ischaemia and mesentery-related factors. Pouchoscopy with biopsy is the most valuable modality for diagnosis, disease monitoring, assessment of treatment response, dysplasia surveillance and delivery of endoscopic therapy. Triggering or risk factors, such as Clostridioides difficile infection and use of non-steroidal anti-inflammatory drugs, should be modified or eradicated. In terms of treatment, acute pouchitis usually responds to oral antibiotics, whereas chronic antibiotic-refractory pouchitis often requires induction and maintenance therapy with integrin, interleukin or tumour necrosis factor inhibitors. Chronic pouchitis with ischaemic features, fistulae or abscesses can be treated with hyperbaric oxygen therapy.
Key points
-
Pouchitis is the most common disorder of ileal pouch–anal anastomosis, and chronic antibiotic-refractory pouchitis has been listed as one of the five difficult-to-treat inflammatory bowel disease conditions.
-
The aetiology and pathogenesis of pouchitis, especially chronic pouchitis, is likely to be multifactorial, involving genetic predisposition, gut microbiota, faecal stasis, disrupted or dysregulated innate and adaptive immunity, mesentery factors, technical factors, tissue ischaemia, and oxidative stress.
-
Accurate diagnosis and classification are important to properly managing and identifying possible aetiopathogenetic factors; known triggering or risk factors should be evaluated and possibly modified.
-
Acute pouchitis usually responds to as-needed oral antibiotic therapy; chronic pouchitis, particularly chronic antibiotic-refractory pouchitis, requires induction and maintenance therapy.
-
Chronic pouchitis can be treated with topical or oral budesonide for induction; chronic antibiotic-refractory pouchitis can be treated with anti-integrin, anti-interleukin or anti-tumour necrosis factor agents for both induction and maintenance; chronic pouchitis with features of ischaemia or fistulas might benefit from hyperbaric oxygen therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Eriksson, C. et al. Changes in medical management and colectomy rates: a population-based cohort study on the epidemiology and natural history of ulcerative colitis in Örebro, Sweden, 1963-2010. Aliment. Pharmacol. Ther. 46, 748–757 (2017).
Cha, J. M. et al. Long-term prognosis of ulcerative colitis and its temporal changes between 1986 and 2015 in a population-based cohort in the Songpa-Kangdong district of Seoul, Korea. Gut 69, 1432–1440 (2020).
Ley, D. et al. New therapeutic strategies are associated with a significant decrease in colectomy rate in pediatric ulcerative colitis. Am. J. Gastroenterol. 118, 1997–2004 (2023).
Ni, A. et al. Trends in colectomies for colorectal neoplasms in ulcerative colitis: a national inpatient sample database analysis over two decades. J. Gastrointest. Surg. 24, 1721–1728 (2020).
Law, C. C. Y. et al. Early biologic treatment decreases risk of surgery in Crohn’s disease but not in ulcerative colitis: systematic review and meta-analysis. Inflamm. Bowel Dis. https://doi.org/10.1093/ibd/izad149 (2023).
Worley, G. et al. Restorative surgery after colectomy for ulcerative colitis in England and Sweden: observations from a comparison of nationwide cohorts. Colorectal Dis. 20, 804–812 (2018).
Holubar, S. D. et al. Long-term direct costs before and after proctocolectomy for ulcerative colitis: a population-based study in Olmsted County, Minnesota. Dis. Colon. Rectum 52, 1815–1823 (2009).
Nordenvall, C. et al. Probability, rate and timing of reconstructive surgery following colectomy for inflammatory bowel disease in Sweden: a population-based cohort study. Colorectal Dis. 17, 882–890 (2015).
Shen, B. et al. Diagnosis and classification of ileal pouch disorders: consensus guidelines from the International Ileal Pouch Consortium. Lancet Gastroenterol. Hepatol. 6, 826–849 (2021).
Fazio, V. W. et al. Ileal pouch anal anastomosis: analysis of outcome and quality of life in 3707 patients. Ann. Surg. 257, 679–685 (2013).
Parigi, T. L. et al. Difficult-to-treat inflammatory bowel disease: results from an international consensus meeting. Lancet Gastroenterol. Hepatol. 8, 853–859 (2023).
James, S. D. et al. The MYTHS of de novo Crohn’s disease after restorative proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis. Jpn J. Gastroenterol. Hepatol. 3, 1166 (2020).
Shen, B. et al. Treatment of pouchitis, Crohn’s disease, cuffitis, and other inflammatory disorders of the pouch: consensus guidelines from the International Ileal Pouch Consortium. Lancet Gastroenterol. Hepatol. 7, 69–95 (2022).
Yamamoto, T., Shimoyama, T., Bamba, T. & Matsumoto, K. Consecutive monitoring of fecal calprotectin and lactoferrin for the early diagnosis and prediction of pouchitis after restorative proctocolectomy for ulcerative colitis. Am. J. Gastroenterol. 110, 881–887 (2015).
Gionchetti, P. et al. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology 124, 1202–1209 (2003).
Barnes, E. L., Allin, K. H., Iversen, A. T., Herfarth, H. H. & Jess, T. Increasing incidence of pouchitis between 1996 and 2018: a population-based Danish cohort study. Clin. Gastroenterol. Hepatol. 21, 192–199.e7 (2023).
Sriranganathan, D., Kilic, Y., Nabil Quraishi, M. & Segal, J. P. Prevalence of pouchitis in both ulcerative colitis and familial adenomatous polyposis: a systematic review and meta-analysis. Colorectal Dis. 24, 27–39 (2022).
Pedersen, K. E., Jia, X., Holubar, S. D., Steele, S. R. & Lightner, A. L. Ileal pouch-anal anastomosis in the elderly: a systematic review and meta-analysis. Colorectal Dis. 23, 2062–2074 (2021).
Dubinsky, V. et al. Predominantly antibiotic-resistant intestinal microbiome persists in patients with pouchitis who respond to antibiotic therapy. Gastroenterology 158, 610–624.e13 (2020).
Kayal, M. et al. Inflammatory pouch conditions are common after ileal pouch anal anastomosis in ulcerative colitis patients. Inflamm. Bowel Dis. 26, 1079–1086 (2020).
Tome, J., Raffals, L. E. & Pardi, D. S. Management of acute and chronic pouchitis. Dis. Colon Rectum 65, S69–S76 (2022).
Okita, Y. et al. Characteristics of extremely early-onset pouchitis after proctocolectomy with ileal pouch-anal anastomosis. J. Gastrointest. Surg. 17, 533–539 (2013).
Esckilsen, S., Kochar, B., Weaver, K. N., Herfarth, H. H. & Barnes, E. L. Very early pouchitis is associated with an increased likelihood of chronic inflammatory conditions of the pouch. Dig. Dis. Sci. 68, 3139–3147 (2023).
Hassan, Y., Connell, W. R., Rawal, A. & Wright, E. K. Review of long-term complications and functional outcomes of ileoanal pouch procedures in patients with inflammatory bowel disease. ANZ J. Surg. 93, 1503–1509 (2023).
Cowherd, E. et al. The cumulative incidence of pouchitis in pediatric patients with ulcerative colitis. Inflamm. Bowel Dis. 28, 1332–1337 (2022).
Gajendran, M. et al. A comprehensive review and update on ulcerative colitis. Dis. Mon. 65, 100851 (2019).
Coppell, K. J. et al. Annual incidence and phenotypic presentation of IBD in Southern New Zealand: an 18-year epidemiological analysis. Inflamm. Intest. Dis. 3, 32–39 (2018).
Akiyama, S. et al. Endoscopic phenotype of the J pouch in patients with inflammatory bowel disease: a new classification for pouch outcomes. Clin. Gastroenterol. Hepatol. 20, 293–302.e9 (2022).
Liu, Z. X. et al. Transmural inflammation is not pathognomonic for Crohn’s disease of the pouch. Surg. Endosc. 25, 3509–3517 (2011).
Takakura, W. R. et al. Magnitude of preoperative C-reactive protein elevation is associated with de novo Crohn’s disease after ileal pouch-anal anastomosis in patients with severe colitis. Dis. Colon Rectum 65, 399–405 (2022).
Yang, Y. et al. A literature review and case report of severe and refractory post-colectomy enteritis. BMC Gastroenterol. 19, 61 (2019).
Emile, S. H., Khan, S. M., Silva-Alvarenga, E., Garoufalia, Z. & Wexner, S. D. A systematic review and meta-analysis of the outcome of ileal pouch-anal anastomosis in patients with ulcerative colitis versus patients with familial adenomatous polyposis. Tech. Coloproctol. 26, 691–705 (2022).
Kistangari, G., Lopez, R. & Shen, B. Frequency and risk factors of Clostridium difficile infection in hospitalized patients with pouchitis: a population-based study. Inflamm. Bowel Dis. 23, 661–671 (2017).
Quinn, K. P. et al. Primary sclerosing cholangitis-associated pouchitis: a distinct clinical phenotype. Clin. Gastroenterol. Hepatol. 20, e964–e973 (2022).
Wasmuth, H. H. et al. Primary sclerosing cholangitis and extraintestinal manifestations in patients with ulcerative colitis and ileal pouch-anal anastomosis. J. Gastrointest. Surg. 14, 1099–1104 (2010).
Shen, B. et al. Primary sclerosing cholangitis is associated with endoscopic and histologic inflammation of the distal afferent limb in patients with ileal pouch-anal anastomosis. Inflamm. Bowel Dis. 17, 1890–1900 (2011).
Nakamura, M. et al. Postoperative joint symptoms: a risk factor for pouchitis in ulcerative colitis patients. Dig. Surg. 23, 60–64 (2006).
Hata, K. et al. Meta-analysis of the association of extraintestinal manifestations with the development of pouchitis in patients with ulcerative colitis. BJS Open 3, 436–444 (2019).
Okada, S. et al. A polymorphism in interleukin-1β gene is associated with the development of pouchitis in Japanese patients with ulcerative colitis. Digestion 102, 489–498 (2021).
Achkar, J. P. et al. Differentiating risk factors for acute and chronic pouchitis. Clin. Gastroenterol. Hepatol. 3, 60–66 (2005).
Fleshner, P. et al. A prospective multivariate analysis of clinical factors associated with pouchitis after ileal pouch-anal anastomosis. Clin. Gastroenterol. Hepatol. 5, 952–958 (2007).
Fadel, M. G. et al. Risks factors associated with the development of Crohn’s disease after ileal pouch-anal anastomosis for ulcerative colitis: a systematic review and meta-analysis. J. Crohns Colitis 17, 1537–1548 (2023).
Gonzalez, A., Gupta, K., Rahman, A. U., Wadhwa, V. & Shen, B. Risk factors associated with hospital readmission and costs for pouchitis. Crohns Colitis 360, otab006 (2021).
Kulkarni, G., Liu, X. & Shen, B. Pouchitis associated with pelvic radiation for prostate cancer. ACG Case Rep. J. 3, e129 (2016).
Seril, D. N. & Shen, B. Clostridium difficile infection in the postcolectomy patient. Inflamm. Bowel Dis. 20, 2450–2469 (2014).
Martinez Ugarte, M. L. et al. Clostridium difficile infection after restorative proctocolectomy and ileal pouch anal anastomosis for ulcerative colitis. Colorectal Dis. 18, O154–O157 (2016).
McCurdy, J. D. et al. Cytomegalovirus infection of the ileoanal pouch: clinical characteristics and outcomes. Inflamm. Bowel Dis. 19, 2394–2399 (2013).
Wu, X. R., Zhu, H., Kiran, R. P., Remzi, F. H. & Shen, B. Excessive weight gain is associated with an increased risk for pouch failure in patients with restorative proctocolectomy. Inflamm. Bowel Dis. 19, 2173–2181 (2013).
Tyler, A. D. et al. The NOD2insC polymorphism is associated with worse outcome following ileal pouch-anal anastomosis for ulcerative colitis. Gut 62, 1433–1439 (2013).
Sehgal, R. et al. NOD2/CARD15 mutations correlate with severe pouchitis after ileal pouch-anal anastomosis. Dis. Colon. Rectum 53, 1487–1494 (2010).
Sehgal, R. et al. Genetic risk profiling and gene signature modeling to predict risk of complications after IPAA. Dis. Colon. Rectum 55, 239–248 (2012).
Seril, D. N., Yao, Q. & Shen, B. Auto-inflammatory diseases in ileal pouch patients with NOD2/CARD15 mutations. Gastroenterol. Rep. 4, 73–76 (2016).
Carter, M. J. et al. The interleukin 1 receptor antagonist gene allele 2 as a predictor of pouchitis following colectomy and IPAA in ulcerative colitis. Gastroenterology 121, 805–811 (2001).
Lammers, K. M. et al. Combined carriership of TLR9-1237C and CD14-260T alleles enhances the risk of developing chronic relapsing pouchitis. World J. Gastroenterol. 11, 7323–7329 (2005).
Shen, B. et al. A randomized clinical trial of ciprofloxacin and metronidazole to treat acute pouchitis. Inflamm. Bowel Dis. 7, 301–305 (2001).
Li, K. Y. et al. Fecal microbiota in pouchitis and ulcerative colitis. World J. Gastroenterol. 22, 8929–8939 (2016).
Shen, B. Campylobacter infection in patients with ileal pouches. Am. J. Gastroenterol. 105, 472–473 (2010).
Cohen, G. S. & Schenck, R. J. Cryptosporidium pouchitis. Clin. Gastroenterol. Hepatol. 15, A27–A28 (2017).
Machiels, K. et al. Specific members of the predominant gut microbiota predict pouchitis following colectomy and IPAA in UC. Gut 66, 79–88 (2017).
Palmieri, O. et al. Microbiome analysis of mucosal ileoanal pouch in ulcerative colitis patients revealed impairment of the pouches immunometabolites. Cells 10, 3243 (2021).
Kousgaard, S. J. et al. The microbiota profile in inflamed and non-inflamed ileal pouch-anal anastomosis. Microorganisms 8, 1611 (2020).
Richardson, M. et al. Analysis of 16S rRNA genes reveals reduced Fusobacterial community diversity when translocating from saliva to GI sites. Gut Microbes 12, 1–13 (2020).
Gao, X. et al. Identification of gut microbiome and transcriptome changes in ulcerative colitis and pouchitis. Scand. J. Gastroenterol. 57, 942–952 (2022).
Vineis, J. H. et al. Patient-specific bacteroides genome variants in pouchitis. mBio 7, e01713–e01716 (2016).
Reshef, L. et al. Pouch inflammation is associated with a decrease in specific bacterial taxa. Gastroenterology 149, 718–727 (2015).
Ha, C. W. Y. et al. Translocation of viable gut microbiota to mesenteric adipose drives formation of creeping fat in humans. Cell 183, 666–683.e17 (2020).
Zhao, L. et al. Microbiota DNA translocation into mesentery lymph nodes is associated with early development of pouchitis after IPAA for ulcerative colitis. Dis. Colon Rectum 66, e1107–e1118 (2023).
Kühbacher, T. et al. Bacterial and fungal microbiota in relation to probiotic therapy (VSL#3) in pouchitis. Gut 55, 833–841 (2006).
Karjalainen, E. K. et al. Fecal microbiota transplantation in chronic pouchitis: a randomized, parallel, double-blinded clinical trial. Inflamm. Bowel Dis. 27, 1766–1772 (2021).
Kayal, M., Lambin, T., Pinotti, R., Dubinsky, M. C. & Grinspan, A. A systematic review of fecal microbiota transplant for the management of pouchitis. Crohns Colitis 360, otaa034 (2020).
Shen, B. Acute and chronic pouchitis — pathogenesis, diagnosis and treatment. Nat. Rev. Gastroenterol. Hepatol. 9, 323–333 (2012).
Landy, J. et al. Innate immune factors in the development and maintenance of pouchitis. Inflamm. Bowel Dis. 20, 1942–1949 (2014).
Knowles, J. & Church, J. Normal ileal mucus is inadequate for epithelial protection in ileal pouch mucosa. Dis. Colon Rectum https://doi.org/10.1097/DCR.0000000000003163 (2024).
Kiehne, K. et al. Defensin expression in chronic pouchitis in patients with ulcerative colitis or familial adenomatous polyposis coli. World J. Gastroenterol. 12, 1056–1062 (2006).
Scarpa, M. et al. Innate immune environment in ileal pouch mucosa: α5 defensin up-regulation as predictor of chronic/relapsing pouchitis. J. Gastrointest. Surg. 16, 188–202 (2012).
Leal, R. F. et al. Differential expression of pro-inflammatory cytokines and a pro-apoptotic protein in pelvic ileal pouches for ulcerative colitis and familial adenomatous polyposis. Tech. Coloproctol. 12, 33–38 (2008).
Verstockt, B. et al. Outcome of biological therapies in chronic antibiotic-refractory pouchitis: a retrospective single-centre experience. U. Eur. Gastroenterol. J. 7, 1215–1225 (2019).
Huguet, M. et al. Systematic review with meta-analysis: anti-TNF therapy in refractory pouchitis and Crohn’s disease-like complications of the pouch after ileal pouch-anal anastomosis following colectomy for ulcerative colitis. Inflamm. Bowel Dis. 24, 261–268 (2018).
Uchino, M. et al. Association between serum tumor necrosis factor-alpha level and the efficacy of infliximab for refractory pouchitis after restorative proctocolectomy in patients with ulcerative colitis. J. Anus Rectum Colon. 1, 106–111 (2018).
Morgan, X. C. et al. Associations between host gene expression, the mucosal microbiome, and clinical outcome in the pelvic pouch of patients with inflammatory bowel disease. Genome Biol. 16, 67 (2015).
Inoue, E. et al. Altered expression of microRNAs in patients with pouchitis after restorative proctocolectomy. Surg. Today 47, 1484–1491 (2017).
Boniface, K., Blom, B., Liu, Y. J. & de Waal Malefyt, R. From interleukin-23 to T-helper 17 cells: human T-helper cell differentiation revisited. Immunol. Rev. 226, 132–146 (2008).
Lyakh, L., Trinchieri, G., Provezza, L., Carra, G. & Gerosa, F. Regulation of interleukin-12/interleukin-23 production and the T-helper 17 response in humans. Immunol. Rev. 226, 112–131 (2008).
Rocchi, C. et al. Is ustekinumab effective in refractory Crohn’s disease of the pouch and chronic pouchitis? A systematic review. Dig. Dis. Sci. 67, 1948–1955 (2022).
Travis, S. et al. EARNEST Study Group. Vedolizumab for the treatment of chronic pouchitis. N. Engl. J. Med. 388, 1191–1200 (2023).
de Krijger, M. et al. Expression of MAdCAM-1 and gut-homing T cells in inflamed pouch mucosa. J. Crohns Colitis 15, 1491–1499 (2021).
Melde, M. et al. α4β7 integrin-dependent adhesion of T cells to MAdCAM-1 is blocked by vedolizumab in patients with chronic refractory pouchitis. Ther. Adv. Gastroenterol. 14, 17562848211054707 (2021).
Miner, P., Wedel, M., Bane, B. & Bradley, J. An enema formulation of alicaforsen, an antisense inhibitor of intercellular adhesion molecule-1, in the treatment of chronic, unremitting pouchitis. Aliment. Pharmacol. Ther. 19, 281–286 (2004).
Feagan, B., Lindsay, J., Gerhard, R., Moran, G. & Varawalla, N. Alicaforsen enema in chronic pouchitis: results of a phase 3 randomized, double-blind, placebo-controlled trial. Am. J. Gastroenterol. 116, S365 (2021).
Leal, R. F. et al. Activation of signal transducer and activator of transcription-1 (STAT-1) and differential expression of interferon-gamma and anti-inflammatory proteins in pelvic ileal pouches for ulcerative colitis and familial adenomatous polyposis. Clin. Exp. Immunol. 160, 380–385 (2010).
Uzzan, M. et al. & GETAID TOFA-POUCH Study Group. Effectiveness and safety of tofacitinib in patients with chronic pouchitis multirefractory to biologics. Dig. Liver Dis. 55, 1158–1160 (2023).
Akiyama, S. et al. Treatment of chronic inflammatory pouch conditions with tofacitinib: a case series from 2 tertiary IBD centers in the United States. Inflamm. Bowel Dis. 29, 1504–1507 (2023).
Devlin, J. C. et al. Single-cell transcriptional survey of ileal-anal pouch immune cells from ulcerative colitis patients. Gastroenterology 160, 1679–1693 (2021).
Yanai, H. et al. Gene expression alterations in ulcerative colitis patients after restorative proctocolectomy extend to the small bowel proximal to the pouch. Gut 64, 756–764 (2015).
Sherman Horev, H. et al. Increase in processing factors is involved in skewed microRNA expression in patients with ulcerative colitis who develop small intestine inflammation after pouch surgery. Inflamm. Bowel Dis. 24, 1045–1054 (2018).
Burns, E. M. et al. Volume analysis of outcome following restorative proctocolectomy. Br. J. Surg. 98, 408–417 (2011).
Wu, X. R. et al. The impact of mesenteric tension on pouch outcome and quality of life in patients undergoing restorative proctocolectomy. Colorectal Dis. 16, 986–994 (2014).
Ray, J. J. et al. Association of malnutrition with postoperative outcomes after ileal pouch-anal anastomosis. J. Gastrointest. Surg. 25, 1562–1564 (2021).
Syed, A. et al. Association between portal vein thrombosis and pouchitis in patients with ulcerative colitis. Dig. Dis. Sci. 67, 1303–1310 (2022).
Kayal, M. et al. Early pouchitis is associated with Crohn’s disease-like pouch inflammation in patients with ulcerative colitis. Inflamm. Bowel Dis. 28, 1821–1825 (2022).
Shen, B. Endoscopic evaluation of the ileal pouch. Dis. Colon Rectum https://doi.org/10.1097/DCR.0000000000003269 (2024).
Ma, H. Y., Kim, H. W., Khurana, S., Bentley-Hibbert, S. & Shen, B. Fluoroscopic defecography characterization of floppy pouch complex with clinical and endoscopic correlations. J. Radiol. Clin. Imaging https://doi.org/10.26502/jrci.2809090 (2024).
Mukewar, S., Wu, X., Lopez, R. & Shen, B. Comparison of long-term outcomes of S and J pouches and continent ileostomies in ulcerative colitis patients with restorative proctocolectomy-experience in subspecialty pouch center. J. Crohns Colitis 8, 1227–1236 (2014).
Aoki, T. et al. Abdominal fat accumulation, as measured by computed tomography, increases the risk of ischemic colitis: a retrospective case-control study. Dig. Dis. Sci. 60, 2104–2111 (2015).
Rowan, C. R., McManus, J., Boland, K. & O’Toole, A. Visceral adiposity and inflammatory bowel disease. Int. J. Colorectal Dis. 36, 2305–2319 (2021).
Emile, S. H., Khan, S. M. & Wexner, S. D. A systematic review and meta-analysis of the outcome of ileal pouch anal anastomosis in patients with obesity. Surgery 170, 1629–1636 (2021).
Pandrangi, V. et al. Abdominal visceral fat area and chronic pouchitis after ileal pouch-anal anastomosis. Am. Surg. 83, 1029–1032 (2017).
Gao, X. H. et al. Greater peripouch fat area on CT image is associated with chronic pouchitis and pouch failure in inflammatory bowel diseases patients. Dig. Dis. Sci. 65, 3660–3671 (2020).
Ginocchio, L. A. et al. Structured versus non-structured reporting of pelvic MRI for ileal pouch evaluation: clarity and effectiveness. Abdom. Radiol. 48, 2978–2985 (2023).
Broder, J. C., Tkacz, J. N., Anderson, S. W., Soto, J. A. & Gupta, A. Ileal pouch-anal anastomosis surgery: imaging and intervention for post-operative complications. Radiographics 30, 221–233 (2010).
Viscido, A., Kohn, A., Papi, C. & Caprilli, R. Management of refractory fistulizing pouchitis with infliximab. Eur. Rev. Med. Pharmacol. Sci. 8, 239–246 (2004).
Luglio, G. et al. Surgical prevention of anastomotic recurrence by excluding mesentery in Crohn’s disease: the SuPREMe-CD study — a randomized clinical trial. Ann. Surg. 272, 210–217 (2020).
Shen, B. et al. Asymmetric endoscopic inflammation of the ileal pouch: a sign of ischemic pouchitis? Inflamm. Bowel Dis. 16, 836–846 (2010).
Eltzschig, H. K., Bratton, D. L. & Colgan, S. P. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat. Rev. Drug Discov. 13, 852–869 (2014).
Vavricka, S. R. et al. High altitude journeys and flights are associated with an increased risk of flares in inflammatory bowel disease patients. J. Crohns Colitis 8, 191–199 (2014).
Kienle, P. et al. Association of decreased perfusion of the ileoanal pouch mucosa with early postoperative pouchitis and local septic complications. Arch. Surg. 136, 1124–1130 (2001).
El Muhtaseb, M. S. et al. Free radical activity and lipid soluble anti-oxidant vitamin status in patients with long-term ileal pouch-anal anastomosis. Colorectal Dis. 11, 67–72 (2009).
Shebani, K. O. et al. Role of stasis and oxidative stress in ileal pouch inflammation. J. Surg. Res. 90, 67–75 (2000).
Hasan, B. et al. Hyperbaric oxygen therapy in chronic inflammatory conditions of the pouch. Inflamm. Bowel Dis. 27, 965–970 (2021).
Fahad, H., Dulai, P. S., Shen, B. & Kochhar, G. S. Hyperbaric oxygen therapy is effective in the treatment of inflammatory and fistulizing pouch complications. Clin. Gastroenterol. Hepatol. 19, 1288–1291 (2021).
Mehta, M., Ahmed, S. & Dryden, G. Refractory pouchitis improves after administration of the green tea polyphenol EGCG: a retrospective review. Int. J. Colorectal Dis. 33, 83–86 (2018).
Abbass, M. A. et al. Nonspecific, acute pouchitis in patients with familial adenomatous polyposis: less common than we think. Dis. Colon Rectum 65, 846–850 (2022).
Ward, M. A. et al. Insights into the pathogenesis of ulcerative colitis from a murine model of stasis-induced dysbiosis, colonic metaplasia, and genetic susceptibility. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G973–G988 (2016).
Quinn, K. P. et al. Nonrelaxing pelvic floor dysfunction is an underestimated complication of ileal pouch-anal anastomosis. Clin. Gastroenterol. Hepatol. 15, 1242–1247 (2017).
Nugent, E. & Church, J. M. When pouches cannot empty: a cohort study of the symptoms this causes, the reasons it’s happening, and the treatments needed. ANZ J. Surg. 92, 3237–3241 (2022).
Lan, N. et al. Endoscopic treatment of pouch inlet and afferent limb strictures: stricturotomy vs. balloon dilation. Surg. Endosc. 35, 1722–1733 (2021).
Sandborn, W. J., Tremaine, W. J., Batts, K. P., Pemberton, J. H. & Phillips, S. F. Pouchitis after ileal pouch-anal anastomosis: a Pouchitis Disease Activity Index. Mayo Clin. Proc. 69, 409–415 (1994).
Shen, B. et al. Modified pouchitis disease activity index: a simplified approach to the diagnosis of pouchitis. Dis. Colon Rectum 46, 748–753 (2003).
Sedano, R. et al. An expert consensus to standardise clinical, endoscopic and histologic items and inclusion and outcome criteria for evaluation of pouchitis disease activity in clinical trials. Aliment. Pharmacol. Ther. 53, 1108–1117 (2021).
Shen, B. et al. Comprehensive evaluation of inflammatory and noninflammatory sequelae of ileal pouch-anal anastomoses. Am. J. Gastroenterol. 100, 93–101 (2005).
Freeha, K., Gao, X. H., Hull, T. L. & Shen, B. Characterization of risk factors for floppy pouch complex in ulcerative colitis. Int. J. Colorectal Dis. 34, 1061–1067 (2019).
Khan, F., Hull, T. L. & Shen, B. Diagnosis and management of floppy pouch complex. Gastroenterol. Rep. 6, 246–256 (2018).
Roussel, B. N. & Shah, S. A. Diagnosis and management of functional pouch disorders: a systematic review. Dis. Colon Rectum 65, S113–S118 (2022).
Haveran, L. A. et al. Infliximab and/or azathioprine in the treatment of Crohn’s disease-like complications after IPAA. Dis. Colon Rectum 54, 15–20 (2011).
Akiyama, S., Dyer, E. C. & Rubin, D. T. Diagnostic and management considerations for the IPAA with Crohn’s disease-like features. Dis. Colon Rectum 65, S77–S84 (2022).
Ardalan, Z. S. et al. Perceived dietary intolerances, habitual intake and diet quality of patients with an ileoanal pouch: associations with pouch phenotype (and behaviour). Clin. Nutr. 42, 2095–2108 (2023).
Ardalan, Z. S., Yao, C. K., Sparrow, M. P. & Gibson, P. R. Review article: the impact of diet on ileoanal pouch function and on the pathogenesis of pouchitis. Aliment. Pharmacol. Ther. 52, 1323–1340 (2020).
Godny, L. et al. Fruit consumption is associated with alterations in microbial composition and lower rates of pouchitis. J. Crohns Colitis 13, 1265–1272 (2019).
Godny, L. et al. Adherence to the Mediterranean diet is associated with decreased fecal calprotectin in patients with ulcerative colitis after pouch surgery. Eur. J. Nutr. 59, 3183–3190 (2020).
McLaughlin, S. D. et al. Exclusive elemental diet impacts on the gastrointestinal microbiota and improves symptoms in patients with chronic pouchitis. J. Crohns Colitis 7, 460–466 (2013).
Shen, B. et al. Effect of withdrawal of nonsteroidal anti-inflammatory drug use on ileal pouch disorders. Dig. Dis. Sci. 52, 3321–3328 (2007).
Liu, Z. X. et al. Chronic pouchitis is associated with pouch polyp formation in patients with underlying ulcerative colitis. J. Crohns Colitis 8, 363–369 (2014).
Navaneethan, U. et al. Impact of budesonide on liver function tests and gut inflammation in patients with primary sclerosing cholangitis and ileal pouch anal anastomosis. J. Crohns Colitis 6, 536–542 (2012).
Shen, B. et al. Endoscopic and histologic evaluation together with symptom assessment are required to diagnose pouchitis. Gastroenterology 121, 261–267 (2001).
Samaan, M. A. et al. Reliability among central readers in the evaluation of endoscopic disease activity in pouchitis. Gastrointest. Endosc. 88, 360–369.e2 (2018).
Jairath, V. et al. Travis mucosal healing with vedolizumab in inflammatory bowel disease patients with chronic pouchitis: evidence from EARNEST, a randomized, double-blind, placebo-controlled trial. J. Crohns Colitis 17, i13–i14 (2023).
Ollech, J. E. et al. Fecal calprotectin is increased in pouchitis and progressively increases with more severe endoscopic and histologic disease. Clin. Gastroenterol. Hepatol. 20, 1839–1846.e2 (2022).
Barreiro-de Acosta, M. et al. Recommendations of the Spanish Working Group on Crohn’s Disease and Ulcerative Colitis (GETECCU) on pouchitis in ulcerative colitis. Part 2: treatment. Recomendaciones del Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa (GETECCU) sobre la reservoritis en la colitis ulcerosa. Parte 2: Tratamiento. Gastroenterol. Hepatol. 43, 649–658 (2020).
Lamb, C. A. et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 68, s1–s106 (2019).
Magro, F. et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J. Crohns Colitis 11, 649–670 (2017).
Pardi, D. S., D’Haens, G., Shen, B., Campbell, S. & Gionchetti, P. Clinical guidelines for the management of pouchitis. Inflamm. Bowel Dis. 15, 1424–1431 (2009).
Barnes, E. L. et al. AGA clinical practice guideline on the management of pouchitis and inflammatory pouch disorders. Gastroenterology 166, 59–85 (2024).
Nguyen, N., Zhang, B., Holubar, S. D., Pardi, D. S. & Singh, S. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. Cochrane Database Syst. Rev. 5, CD001176 (2019).
Gionchetti, P. et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology 119, 305–309 (2000).
Mimura, T. et al. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut 53, 108–114 (2004).
Shen, B. et al. Maintenance therapy with a probiotic in antibiotic-dependent pouchitis: experience in clinical practice. Aliment. Pharmacol. Ther. 22, 721–728 (2005).
Yasueda, A. et al. The effect of Clostridium butyricum MIYAIRI on the prevention of pouchitis and alteration of the microbiota profile in patients with ulcerative colitis. Surg. Today 46, 939–949 (2016).
Gosselink, M. P. et al. Delay of the first onset of pouchitis by oral intake of the probiotic strain Lactobacillus rhamnosus GG. Dis. Colon Rectum 47, 876–884 (2004).
Brown, S. J. et al. Bifidobacterium longum BB-536 and prevention of pouchitis. Gastroenterology 126, S465 (2004).
Ha, C. Y. et al. Early institution of tinidazole may prevent pouchitis following ileal pouch-anal anastomosis (IPAA) surgery in ulcerative colitis (UC) patients. Gastroenterology 138, S69 (2010).
Isaacs, K. L. et al. Rifaximin for the treatment of active pouchitis: a randomized, double-blind, placebo-controlled pilot study. Inflamm. Bowel Dis. 13, 1250–1255 (2007).
Shen, B., Remzi, F. H., Lopez, A. R. & Queener, E. Rifaximin for maintenance therapy in antibiotic-dependent pouchitis. BMC Gastroenterol. 8, 26 (2008).
Abdelrazeq, A. S., Kelly, S. M., Lund, J. N. & Leveson, S. H. Rifaximin-ciprofloxacin combination therapy is effective in chronic active refractory pouchitis. Colorectal Dis. 7, 182–186 (2005).
Mimura, T. et al. Four-week open-label trial of metronidazole and ciprofloxacin for the treatment of recurrent or refractory pouchitis. Aliment. Pharmacol. Ther. 16, 909–917 (2002).
Shen, B. et al. Combined ciprofloxacin and tinidazole therapy in the treatment of chronic refractory pouchitis. Dis. Colon Rectum 50, 498–508 (2007).
Shen, B. Oral vancomycin in the treatment of primary sclerosing cholangitis-associated pouchitis. Gastroenterol. Rep. 9, 274–275 (2021).
Seril, D. N., Ashburn, J. H., Lian, L. & Shen, B. Risk factors and management of refractory or recurrent Clostridium difficile infection in ileal pouch patients. Inflamm. Bowel Dis. 20, 2226–2233 (2014).
Bar, N. et al. Long-term antibiotic treatment in pouchitis-patterns of use and safety. Inflamm. Bowel Dis. 28, 1027–1033 (2022).
Fukushima, K., Saito, T., Kohyama, A. & Watanabe, K. Increased quinolone-resistant mutations of gyrA and parC genes after pouchitis treatment with ciprofloxacin. Dig. Surg. 37, 321–330 (2020).
Scaioli, E. et al. Sulfasalazine in prevention of pouchitis after proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis. Dig. Dis. Sci. 62, 1016–1024 (2017).
Balbir-Gurman, A., Schapira, D. & Nahir, M. Arthritis related to ileal pouchitis following total proctocolectomy for ulcerative colitis. Semin. Arthritis Rheum. 30, 242–248 (2001).
Miner, P. B. Jr, Wedel, M. K., Xia, S. & Baker, B. F. Safety and efficacy of two dose formulations of alicaforsen enema compared with mesalazine enema for treatment of mild to moderate left-sided ulcerative colitis: a randomized, double-blind, active-controlled trial. Aliment. Pharmacol. Ther. 23, 1403–1413 (2006).
Gionchetti, P. et al. Oral budesonide in the treatment of chronic refractory pouchitis. Aliment. Pharmacol. Ther. 25, 1231–1236 (2007).
Viazis, N. et al. Long term benefit of one year infliximab administration for the treatment of chronic refractory pouchitis. J. Crohns Colitis 7, e457–e460 (2013).
Winter, T. A., Dalton, H. R., Merrett, M. N., Campbell, A. & Jewell, D. P. Cyclosporin A retention enemas in refractory distal ulcerative colitis and ‘pouchitis’. Scand. J. Gastroenterol. 28, 701–704 (1993).
Daniels, Beynon & Carr. The use of cyclosporin retention enemas for pouchitis. Colorectal Dis. 1, 49 (1999).
Uchino, M. et al. Topical tacrolimus therapy for antibiotic-refractory pouchitis. Dis. Colon Rectum 56, 1166–1173 (2013).
Barreiro-de Acosta, M. et al. Efficacy of infliximab rescue therapy in patients with chronic refractory pouchitis: a multicenter study. Inflamm. Bowel Dis. 18, 812–817 (2012).
Kjær, M. D., Qvist, N., Nordgaard-Lassen, I., Christensen, L. A. & Kjeldsen, J. Adalimumab in the treatment of chronic pouchitis. A randomized double-blind, placebo-controlled trial. Scand. J. Gastroenterol. 54, 188–193 (2019).
Lindh, S., Bengtsson, J. & Kaczynski, J. Is biologic therapy effective for antibiotic-refractory pouchitis? Scand. J. Gastroenterol. 58, 148–150 (2023).
Ribaldone, D. G. et al. Treatment of antibiotic refractory chronic pouchitis with JAK inhibitors and S1P receptor modulators: an ECCO CONFER Multicentre Case Series. J. Crohns Colitis https://doi.org/10.1093/ecco-jcc/jjad194 (2023).
Lan, N. & Shen, B. Efficacy and safety of upadacitinib in the treatment of chronic pouchitis, cuffitis, and Crohn’s disease of the pouch. ACG Case Rep. J. 11, e01245 (2024).
Lan, N., Ashburn, J. & Shen, B. Fecal microbiota transplantation for Clostridium difficile infection in patients with ileal pouches. Gastroenterol. Rep. 5, 200–207 (2017).
Nyabanga, C. T., Kulkarni, G. & Shen, B. Hyperbaric oxygen therapy for chronic antibiotic-refractory ischemic pouchitis. Gastroenterol. Rep. 5, 320–321 (2017).
Remzi, F. H. et al. Transabdominal redo ileal pouch surgery for failed restorative proctocolectomy: lessons learned over 500 patients. Ann. Surg. 262, 675–682 (2015).
Novello, M., Shen, B. & Stocchi, L. Pouchitis as an indication for ileal pouch surgical revision. Inflamm. Bowel Dis. 25, e25 (2019).
Maspero, M. et al. Ileal pouch-anal anastomosis in primary sclerosing cholangitis-inflammatory bowel disease (PSC-IBD): long-term pouch and liver transplant outcomes. Ann. Surg. https://doi.org/10.1097/SLA.0000000000006041 (2023).
Freeman, K. et al. Impact of orthotopic liver transplant for primary sclerosing cholangitis on chronic antibiotic refractory pouchitis. Clin. Gastroenterol. Hepatol. 6, 62–68 (2008).
Lee, K. E. & Shen, B. Endoscopic therapy for pouch leaks and strictures: a systematic review. Dis. Colon Rectum 65, S92–S104 (2022).
Acknowledgements
The author would like to thank S. Edelman, S. Jarislowsky, E. Story, J. Story, D. Quint, S. Quint, B. Donaghy, L. Donaghy, J. Hyman and R. Hyman for their philanthropy support to the Ileal Pouch Program at Columbia University Irving Medical Center/New York Presbyterian Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
B.S. is a former consultant for Abbvie, Takeda and Janssen.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Ailsa Hart and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria A literature search was performed using PubMed for articles published from January 2001 to August 2023. Additionally, relevant abstracts from professional society meetings were searched. The following keywords were used: “ulcerative colitis”, “ileal pouch”, “pouchitis”, “restorative proctocolectomy”, “antibiotics”, “primary sclerosing cholangitis”, “extraintestinal manifestations”, “bacteria”, “gut microbiota”, “gut microflora”, “ischemia”, “mesentery”, “pathogenesis”, “treatment”. Only relevant, English language articles were included.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shen, B. Pouchitis: pathophysiology and management. Nat Rev Gastroenterol Hepatol (2024). https://doi.org/10.1038/s41575-024-00920-5
Accepted:
Published:
DOI: https://doi.org/10.1038/s41575-024-00920-5