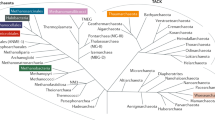
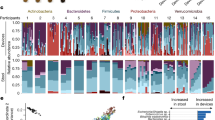
Abstract
The human microbiome is strongly interwoven with human health and disease. Besides bacteria, viruses and eukaryotes, numerous archaea are located in the human gastrointestinal tract and are responsible for methane production, which can be measured in clinical methane breath analyses. Methane is an important readout for various diseases, including intestinal methanogen overgrowth. Notably, the archaea responsible for methane production are largely overlooked in human microbiome studies due to their non-bacterial biology and resulting detection issues. As such, their importance for health and disease remains largely unclear to date, in particular as not a single archaeal representative has been deemed to be pathogenic. In this Perspective, we discuss the current knowledge on the clinical relevance of methanogenic archaea. We explain the archaeal unique response to antibiotics and their negative and positive effects on human physiology, and present the current understanding of the use of methane as a diagnostic marker.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Laforest-Lapointe, I. & Arrieta, M.-C. Asymptomatic intestinal colonization with protist Blastocystis is strongly associated with distinct microbiome ecological patterns. mSystems 3, e00007-18 (2018).
Camarillo-Guerrero, L. F., Almeida, A., Rangel-Pineros, G., Finn, R. D. & Lawley, T. D. Massive expansion of human gut bacteriophage diversity. Cell 184, 1098–1109 (2021).
Geesink, P. & Ettema, T. J. G. The human archaeome in focus. Nat. Microbiol. 7, 10–11 (2022).
Koskinen, K. et al. First insights into the diverse human archaeome: specific detection of archaea in the gastrointestinal tract, lung, and nose and on skin. mBio 8, e00824-17 (2017).
Probst, A. J. et al. Coupling genetic and chemical microbiome profiling reveals heterogeneity of archaeome and bacteriome in subsurface biofilms that are dominated by the same archaeal species. PLoS ONE 9, e99801 (2014).
Miller, T. L., Wolin, M. J., de Macario, E. C. & Macario, A. J. Isolation of Methanobrevibacter smithii from human feces. Appl. Env. Microbiol. 43, 227–232 (1982).
Mahnert, A., Blohs, M., Pausan, M. R. & Moissl-Eichinger, C. The human archaeome: methodological pitfalls and knowledge gaps. Emerg. Top. Life Sci. 2, 469–482 (2018).
Borrel, G., Brugère, J. F., Gribaldo, S., Schmitz, R. A. & Moissl-Eichinger, C. The host-associated archaeome. Nat. Rev. Microbiol. 18, 622–636 (2020).
Gottlieb, K., Wacher, V., Sliman, J. & Pimentel, M. Review article: inhibition of methanogenic archaea by statins as a targeted management strategy for constipation and related disorders. Aliment. Pharmacol. Ther. 43, 197–212 (2015).
Avgerinos, A. et al. Bowel preparation and the risk of explosion during colonoscopic polypectomy. Gut 25, 361–364 (1984).
Ladas, S. D., Karamanolis, G. & Ben-Soussan, E. Colonic gas explosion during therapeutic colonoscopy with electrocautery. World J. Gastroenterol. 13, 5295 (2007).
Bond, J. H., Engel, R. R. & Levitt, M. D. Factors influencing pulmonary methane excretion in man. An indirect method of studying the in situ metabolism of the methane-producing colonic bacteria. J. Exp. Med. 133, 572–588 (1971).
Rezaie, A. et al. Hydrogen and methane-based breath testing in gastrointestinal disorders: The North American Consensus. Am. J. Gastroenterol. 112, 775–784 (2017).
Taffner, J. et al. What is the role of Archaea in plants? New insights from the vegetation of Alpine Bogs. mSphere 3, e00122-18 (2018).
Offre, P., Spang, A. & Schleper, C. Archaea in biogeochemical cycles. Annu. Rev. Microbiol. 67, 437–457 (2013).
Kumpitsch, C. et al. Reduced B12 uptake and increased gastrointestinal formate are associated with archaeome-mediated breath methane emission in humans. Microbiome 9, 193 (2021).
Pausan, M. R., Blohs, M., Mahnert, A. & Moissl-Eichinger, C. The sanitary indoor environment-a potential source for intact human-associated anaerobes. NPJ Biofilms Microbiomes 8, 44 (2022).
Blohs, M. et al. in Encyclopedia of Microbiology (ed. Schmidt, T.) 243–252 (Elsevier, 2019).
Dridi, B., Fardeau, M.-L., Ollivier, B., Raoult, D. & Drancourt, M. The antimicrobial resistance pattern of cultured human methanogens reflects the unique phylogenetic position of archaea. J. Antimicrob. Chemother. 66, 2038–2044 (2011).
Mohammadzadeh, R., Mahnert, A., Duller, S. & Moissl-Eichinger, C. Archaeal key-residents within the human microbiome: characteristics, interactions and involvement in health and disease. Curr. Opin. Microbiol. 67, 102146 (2022).
Oxley, A. et al. Halophilic archaea in the human intestinal mucosa. Environ. Microbiol. 12, 2398–2410 (2010).
Lepp, P. W. et al. Methanogenic Archaea and human periodontal disease. Proc. Natl Acad. Sci. USA 101, 6176–6181 (2004).
Belkacemi, S. et al. Peri-implantitis-associated methanogens: a preliminary report. Sci. Rep. 8, 9447 (2018).
Morris, B. E. L., Henneberger, R., Huber, H. & Moissl-Eichinger, C. Microbial syntrophy: interaction for the common good. FEMS Microbiol. Rev. 37, 384–406 (2013).
Vierbuchen, T., Bang, C., Rosigkeit, H., Schmitz, R. A. & Heine, H. The human-associated archaeon Methanosphaera stadtmanae is recognized through Its RNA and induces TLR8-dependent NLRP3 inflammasome activation. Front. Immunol. 8, 1535 (2017).
Hoffmann, C. et al. Archaea and fungi of the human gut microbiome: correlations with diet and bacterial residents. PLoS ONE 8, e66019 (2013).
Chibani, C. M. et al. A catalogue of 1167 genomes from the human gut archaeome. Nat. Microbiol. 7, 48–61 (2022).
Ruaud, A. et al. Syntrophy via interspecies H2 transfer between Christensenella and Methanobrevibacter underlies their global cooccurrence in the human gut. mBio 11, e03235-19 (2020).
Wang, C. & Sahay, P. Breath analysis using laser spectroscopic techniques: breath biomarkers, spectral fingerprints, and detection limits. Sensors 9, 8230–8262 (2009).
Polag, D. & Keppler, F. Global methane emissions from the human body: past, present and future. Atmos. Environ. 214, 116823 (2019).
Triantafyllou, K., Chang, C. & Pimentel, M. Methanogens, methane and gastrointestinal motility. J. Neurogastroenterol. Motil. 20, 31–40 (2013).
Pimentel, M. et al. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G1089–G1095 (2006).
Park, Y. M., Lee, Y. J., Hussain, Z., Lee, Y. H. & Park, H. The effects and mechanism of action of methane on ileal motor function. Neurogastroenterol. Motil. 29, e13077 (2017).
Singh, P. et al. Breath methane does not correlate with constipation severity or bloating in patients with constipation. J. Clin. Gastroenterol. 54, 365–369 (2020).
Hammer, H. F., Petritsch, W., Pristautz, H. & Krejs, G. Assessment of the influence of hydrogen nonexcretion on the usefulness of the hydrogen breath test and lactose tolerance test. Wien. Klin. Wochenschr. 108, 137–141 (1996).
Bjorneklett, A. & Jenssen, E. Relationships between hydrogen (H2) and methane (CH4) production in man. Scand. J. Gastroenterol. 17, 985–992 (1982).
Houben, E., De Preter, V., Billen, J., Van Ranst, M. & Verbeke, K. Additional value of CH4 measurement in a combined (13)C/H2 lactose malabsorption breath test: a retrospective analysis. Nutrients 7, 7469–7485 (2015).
Oliphant, K. & Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome 7, 91 (2019).
Gibson, G. R., Macfarlane, G. T. & Cummings, J. H. Sulphate reducing bacteria and hydrogen metabolism in the human large intestine. Gut 34, 437–439 (1993).
Hansen, E. E. et al. Pan-genome of the dominant human gut-associated archaeon, Methanobrevibacter smithii, studied in twins. Proc. Natl Acad. Sci. USA 108, 4599–4606 (2011).
Hammer, K., Hasanagic, H., Memaran, N., Huber, W.-D. & Hammer, J. Relevance of methane and carbon dioxide evaluation in breath tests for carbohydrate malabsorption in a paediatric cohort. J. Pediatr. Gastroenterol. Nutr. 72, e71–e77 (2021).
Hammer, H. F. et al. European guideline on indications, performance, and clinical impact of hydrogen and methane breath tests in adult and pediatric patients: European Association for gastroenterology, endoscopy and nutrition, European Society of Neurogastroenterology and Motility, and European Society for Paediatric Gastroenterology Hepatology and Nutrition consensus. U. Eur. Gastroenterol. J. 10, 15–40 (2022).
De Lacy Costello, B. P. J., Ledochowski, M. & Ratcliffe, N. M. The importance of methane breath testing: a review. J. Breath Res. 7, 024001 (2013).
Quigley, E. M. M., Murray, J. A. & Pimentel, M. AGA clinical practice update on small intestinal bacterial overgrowth: expert review. Gastroenterology 159, 1526–1532 (2020).
Rezaie, A., Pimentel, M. & Rao, S. S. How to test and treat small intestinal bacterial overgrowth: an evidence-based approach. Curr. Gastroenterol. Rep. 18, 8 (2016).
Madigan, K. E., Bundy, R. & Weinberg, R. B. Distinctive clinical correlates of small intestinal bacterial overgrowth with methanogens. Clin. Gastroenterol. Hepatol. https://doi.org/10.1016/j.cgh.2021.09.035 (2021).
Pimentel, M., Saad, R. J., Long, M. D. & Rao, S. S. C. ACG clinical guideline: small intestinal bacterial overgrowth. Am. J. Gastroenterol. 115, 165–178 (2020).
Gandhi, A. et al. Methane positive small intestinal bacterial overgrowth in inflammatory bowel disease and irritable bowel syndrome: a systematic review and meta-analysis. Gut Microbes 13, 1933313 (2021).
Ford, A. C., Spiegel, B. M. R., Talley, N. J. & Moayyedi, P. Small intestinal bacterial overgrowth in irritable bowel syndrome: systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 7, 1279–1286 (2009).
Takakura, W. et al. A single fasting exhaled methane level correlates with fecal methanogen load, clinical symptoms and accurately detects intestinal methanogen overgrowth. Am. J. Gastroenterol. 117, 470–477 (2022).
Donaldson, R. M. Studies on the pathogenesis of steatorrhea in the blind loop syndrome. J. Clin. Invest. 44, 1815–1825 (1965).
Shah, A. et al. Small intestinal bacterial overgrowth in irritable bowel syndrome: a systematic review and meta-analysis of case-control studies. Am. J. Gastroenterol. 115, 190–201 (2020).
Chatterjee, S., Park, S., Low, K., Kong, Y. & Pimentel, M. The degree of breath methane production in IBS correlates with the severity of constipation. Am. J. Gastroenterol. 102, 837 (2007).
Soares, A. C. F., Lederman, H. M., Fagundes-Neto, U. & de Morais, M. B. Breath methane associated with slow colonic transit time in children with chronic constipation. J. Clin. Gastroenterol. 39, 512–515 (2005).
Attaluri, A., Jackson, M., Valestin, J. & Rao, S. S. C. Breath methane associated with slow colonic transit time in children with chronic constipation. Am. J. Gastroenterol. 105, 1407 (2010).
Khelaifia, S., Raoult, D. & Drancourt, M. A versatile medium for cultivating methanogenic archaea. PLoS ONE 8, e61563 (2013).
Ghoshal, U. C., Srivastava, D. & Misra, A. A randomized double-blind placebo-controlled trial showing rifaximin to improve constipation by reducing methane production and accelerating colon transit: a pilot study. Indian J. Gastroenterol. 37, 416–423 (2018).
Pimentel, M., Chatterjee, S., Chow, E. J., Park, S. & Kong, Y. Neomycin improves constipation-predominant irritable bowel syndrome in a fashion that is dependent on the presence of methane gas: subanalysis of a double-blind randomized controlled study. Dig. Dis. Sci. 51, 1297–1301 (2006).
World Cancer Research Fund International. Colorectal Cancer Statistics https://www.wcrf.org/cancer-trends/colorectal-cancer-statistics/ (2020).
Cullin, N., Azevedo Antunes, C., Straussman, R., Stein-Thoeringer, C. K. & Elinav, E. Microbiome and cancer. Cancer Cell 39, 1317–1341 (2021).
Poore, G. D. et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature 579, 567–574 (2020).
Chattopadhyay, I. et al. Exploring the role of gut microbiome in colon cancer. Appl. Biochem. Biotechnol. 193, 1780–1799 (2021).
Wong, S. H. & Yu, J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 16, 690–704 (2019).
Coker, O. O., Wu, W. K. K., Wong, S. H., Sung, J. J. Y. & Yu, J. Altered gut archaea composition and interaction with bacteria are associated with colorectal cancer. Gastroenterology 159, 1459–1470.e5 (2020).
Altermann, E., Schofield, L. R., Ronimus, R. S., Beattie, A. K. & Reilly, K. Inhibition of Rumen methanogens by a novel archaeal lytic enzyme displayed on tailored bionanoparticles. Front. Microbiol. 9, 2378 (2018).
Goodrich, J. K. et al. Genetic determinants of the gut microbiome in UK twins. Cell Host Microbe 19, 731–743 (2016).
Goodrich, J. K. et al. Human genetics shape the gut microbiome. Cell 159, 789–799 (2014).
Boros, M. et al. The anti-inflammatory effects of methane. Crit. Care Med. 40, 1269–1278 (2012).
Xin, L., Sun, X. & Lou, S. Effects of methane-rich saline on the capability of one-time exhaustive exercise in male SD rats. PLoS ONE 11, e0150925 (2016).
Laverdure, R., Mezouari, A., Carson, M. A., Basiliko, N. & Gagnon, J. A role for methanogens and methane in the regulation of GLP-1. Endocrinol. Diabetes Metab. 1, e00006 (2018).
Boros, M. & Keppler, F. Methane production and bioactivity-a link to oxido-reductive stress. Front. Physiol. 10, 1244 (2019).
Geng, J. et al. Trimethylamine N-oxide promotes atherosclerosis via CD36-dependent MAPK/JNK pathway. Biomed. Pharmacother. 97, 941–947 (2018).
Borrel, G. et al. Comparative genomics highlights the unique biology of Methanomassiliicoccales, a Thermoplasmatales-related seventh order of methanogenic archaea that encodes pyrrolysine. BMC Genomics 15, 679 (2014).
Ramezani, A. et al. Gut colonization with methanogenic archaea lowers plasma trimethylamine N-oxide concentrations in Apolipoprotein e–/– mice. Sci. Rep. 8, 14752 (2018).
Brugère, J. F. et al. Archaebiotics: proposed therapeutic use of archaea to prevent trimethylaminuria and cardiovascular disease. Gut Microbes 5, 5–10 (2013).
Nguyen, R., Khanna, N. R., Safadi, A. O. & Sun, Y. Bacitracin Topical. StatPearls [online] https://www.ncbi.nlm.nih.gov/books/NBK536993/ (updated 5 Nov 2021).
Khelaifia, S. & Drancourt, M. Susceptibility of archaea to antimicrobial agents: applications to clinical microbiology. Clin. Microbiol. Infect. 18, 841–848 (2012).
Werth, B. J. et al. Overview of Antibiotics. MSD Manual https://www.msdmanuals.com (2022).
Londei, P. et al. Unique antibiotic sensitivity of archaebacterial polypeptide elongation factors. J. Bacteriol. 167, 265–271 (1986).
Sanz, J. L., Rodríguez, N. & Amils, R. The action of antibiotics on the anaerobic digestion process. Appl. Microbiol. Biotechnol. 46, 587–592 (1996).
Moore, K. S. et al. Squalamine: an aminosterol antibiotic from the shark. Proc. Natl Acad. Sci. USA 90, 1354–1358 (1993).
Low, K. et al. A combination of rifaximin and neomycin is most effective in treating irritable bowel syndrome patients with methane on lactulose breath test. J. Clin. Gastroenterol. 44, 547–550 (2010).
Ramos-Morales, E. et al. Antiprotozoal effect of saponins in the rumen can be enhanced by chemical modifications in their structure. Front. Microbiol. 8, 399 (2017).
Kates, M., Kushner, D. & Metheson, A. The Biochemistry of Archae (Archaebacteria) (Elsevier, 1993).
Friend, T. The Third Domain: The Untold Stoy of Archaea and the Future of Biotechnology (National Academies Press, 2007).
Nkamga, V. D., Henrissat, B. & Drancourt, M. Archaea: essential inhabitants of the human digestive microbiota. Hum. Microbiome J. 3, 1–8 (2017).
Broucek, J. Options to methane production abatement in ruminants: a review. J. Anim. Plant Sci. 28, 348–364 (2018).
Hook, S. E., Wright, A.-D. G. & McBride, B. W. Methanogens: methane producers of the rumen and mitigation strategies. Archaea 2010, 945785 (2010).
Nguyen-Hieu, T., Khelaifia, S., Aboudharam, G. & Drancourt, M. Methanogenic archaea in subgingival sites: a review. APMIS 121, 467–477 (2013).
Lurie-Weinberger, M. N. & Gophna, U. Archaea in and on the human body: health implications and future directions. PLoS Pathog. 11, e1004833 (2015).
Wishart, D. S. et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 46, D1074–D1082 (2018).
Moissl-Eichinger, C. Association of methanogens and different human host phenotypes_Table. Mendeley Data https://doi.org/10.17632/njn6x2kjhg.1 (2022).
Leonel, A. J. & Alvarez-Leite, J. I. Butyrate: implications for intestinal function. Curr. Opin. Clin. Nutr. Metab. Care 15, 474–479 (2012).
Cushing, K., Alvarado, D. M. & Ciorba, M. A. Butyrate and mucosal inflammation: new scientific evidence supports clinical observation. Clin. Transl. Gastroenterol. 6, e108 (2015).
Mortensen, P. B. & Clausen, M. R. Short-chain fatty acids in the human colon: relation to gastrointestinal health and disease. Scand. J. Gastroenterol. 31, 132–148 (1996).
Gaci, N., Borrel, G., Tottey, W., O’Toole, P. W. & Brugère, J.-F. Archaea and the human gut: new beginning of an old story. World J. Gastroenterol. 20, 16062 (2014).
Gottlieb, K., Wacher, V., Sliman, J. & Pimentel, M. Review article: inhibition of methanogenic archaea by statins as a targeted management strategy for constipation and related disorders. Aliment. Pharmacol. Ther. 43, 197–212 (2016).
Marsh, E. et al. Lovastatin lactone inhibits methane production in human stool homogenates [abstract]. Presented at ACG 2016 (2016).
Muskal, S. M. et al. Lovastatin lactone may improve irritable bowel syndrome with constipation (IBS-C) by inhibiting enzymes in the archaeal methanogenesis pathway. F1000Res 5, 606 (2016).
Garcia, P. S., Gribaldo, S. & Borrel, G. Diversity and evolution of methane-related pathways in archaea. Annu. Rev. Microbiol. https://doi.org/10.1146/annurev-micro-041020-024935 (2022).
Lyu, Z. & Lu, Y. Metabolic shift at the class level sheds light on adaptation of methanogens to oxidative environments. ISME J. 12, 411–423 (2018).
Roccarina, D. et al. The role of methane in intestinal diseases. Am. J. Gastroenterol. 105, 1250–1256 (2010).
Wibowo, M. C. et al. Reconstruction of ancient microbial genomes from the human gut. Nature 594, 234–239 (2021).
Youngblut, N. D. et al. Vertebrate host phylogeny influences gut archaeal diversity. Nat. Microbiol. 6, 1443–1454 (2021).
Miquel, S. et al. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 16, 255–261 (2013).
Lecours, P. B. et al. Increased prevalence of Methanosphaera stadtmanae in inflammatory bowel diseases. PLoS ONE 9, e87734 (2014).
Barnett, D. J. M., Mommers, M., Penders, J., Arts, I. C. W. & Thijs, C. Intestinal archaea inversely associated with childhood asthma. J. Allergy Clin. Immunol. 143, 2305–2307 (2019).
Acknowledgements
The authors thank R. Mohammadzadeh (Diagnostic and Research Department of Microbiology, Hygiene and Environmental Medicine, Medical University of Graz) for the correction of and discussion on the manuscript. They gratefully acknowledge research funding by the Austrian Science Fund FWF (P 32697) given to C.M.-E.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Cancer Microbiome: http://cancermicrobiome.ucsd.edu/
DrugBank: https://go.drugbank.com/
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hoegenauer, C., Hammer, H.F., Mahnert, A. et al. Methanogenic archaea in the human gastrointestinal tract. Nat Rev Gastroenterol Hepatol 19, 805–813 (2022). https://doi.org/10.1038/s41575-022-00673-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-022-00673-z
This article is cited by
-
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases
Signal Transduction and Targeted Therapy (2024)
-
Composition of the colon microbiota in the individuals with inflammatory bowel disease and colon cancer
Folia Microbiologica (2024)
-
Gut microbiota in colorectal cancer development and therapy
Nature Reviews Clinical Oncology (2023)
-
Symbiotic Interactions of Archaea in Animal and Human Microbiomes
Current Clinical Microbiology Reports (2023)