Abstract
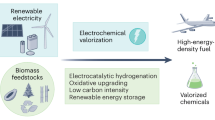
The transition from petroleum to biorenewable sources of carbon to meet our energy and chemical feedstock needs is difficult, in part because these sources are so different, with petroleum being under-functionalized and biomass being over-functionalized relative to commercial chemicals. However, target lists such as the US Department of Energy’s Top 10 have converged efforts to develop the technologies needed to manufacture the most important feedstocks accessible from biorenewables. Less well defined but equally important to the economic viability of an integrated biorefinery are low-volume, high-value product streams, which would help offset the capital costs of a biorefinery. In this Review, we attempt to bring together some of the advances that could fill these niche areas, with a focus on the conversion of cellulosics into chemicals using homogeneous catalysis. The products range from high-value jet fuels to monomers for high-performance polymers and materials to pharmaceutical intermediates and cover a broad range of structural complexities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Bozell, J. J. & Petersen, G. R. Technology development for the production of biobased products from biorefinery carbohydrates-the US Department of Energy’s ‘Top 10’ revisited. Green Chem. 12, 539–554 (2010).
Werpy, T. & Peterson, G. Top Value Added Chemicals from Biomass. Volume I: Results of Screening for Potential Candidates from Sugars and Synthesis Gas (U.S. Department of Energy, 2004).
Deuss, P. J., Barta, K. & de Vries, J. G. Homogeneous catalysis for the conversion of biomass and biomass-derived platform chemicals. Catal. Sci. Technol. 4, 1174–1196 (2014).
Corma, A., Iborra, S. & Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 107, 2411 (2007).
Gilkey, M. J. & Xu, B. Heterogeneous catalytic transfer hydrogenation as an effective pathway in biomass upgrading. ACS Catal. 6, 1420–1436 (2016).
Chatterjee, C., Pong, F. & Sen, A. Chemical conversion pathways for carbohydrates. Green Chem. 17, 40–71 (2015).
Robinson, A. M., Hensley, J. E. & Medlin, J. W. Bifunctional catalysts for upgrading of biomass-derived oxygenates: a review. ACS Catal. 6, 5026–5043 (2016).
Young, R. A. The chemistry of solid wood. Wood Sci. Technol. 19, 17–18 (1985).
Román-Leshkov, Y., Chheda, J. N. & Dumesic, J. A. Phase modifiers promote efficient production of hydroxymethylfurfural from fructose. Science 312, 1933–1937 (2006).
Tollefson, J. Not your father’s biofuels: if biofuels are to help the fight against climate change, they have to be made from more appropriate materials and in better ways. Jeff Tollefson asks what innovation can do to improve the outlook. Nature 451, 880–884 (2008).
Xiong, M., Schneiderman, D. K., Bates, F. S., Hillmyer, M. A. & Zhang, K. Scalable production of mechanically tunable block polymers from sugar. Proc. Natl Acad. Sci. USA 111, 8357–8362 (2014).
Chheda, J. N., Huber, G. W. & Dumesic, J. A. Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew. Chem. Int. Ed. 46, 7164–7183 (2007).
Huber, G. W., Chheda, J. N., Barrett, C. J. & Dumesic, J. A. Production of liquid alkanes by aqueous-phase processing of biomass-derived carbohydrates. Science 308, 1446–1450 (2005).
Kunkes, E. L. et al. Catalytic conversion of biomass to monofunctional hydrocarbons and targeted liquid-fuel classes. Science 322, 417–421 (2008).
Metzger, J. O. Production of liquid hydrocarbons from biomass. Angew. Chem. Int. Ed. 45, 696–698 (2006).
Adduci, L. L., McLaughlin, M. P., Bender, T. A., Becker, J. J. & Gagné, M. R. Metal-free deoxygenation of carbohydrates. Angew. Chem. Int. Ed. 53, 1646–1649 (2014).
McLaughlin, M. P., Adduci, L. L., Becker, J. J. & Gagne, M. R. Iridium-catalyzed hydrosilylative reduction of glucose to hexane(s). J. Am. Chem. Soc. 135, 1225 (2013).
Mahdi, T. & Stephan, D. W. Enabling catalytic ketone hydrogenation by frustrated Lewis pairs. J. Am. Chem. Soc. 136, 15809–15812 (2014).
Scott, D. J., Fuchter, M. J. & Ashley, A. E. Nonmetal catalyzed hydrogenation of carbonyl compounds. J. Am. Chem. Soc. 136, 15813–15816 (2014).
Kamm, B., Kamm, M., Gruber, P. R. & Kromus, S. in Biorefineries — Industrial Processes and Products: Status Quo and Future Directions 1–40 (Wiley-VCH, 2008).
van Putten, R.-J. et al. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 113, 1499–1597 (2013).
Zakrzewska, M. E., Bogel-Łukasik, E. & Bogel-Łukasik, R. Ionic liquid-mediated formation of 5-hydroxymethylfurfural — a promising biomass-derived building block. Chem. Rev. 111, 397–417 (2011).
Ståhlberg, T., Fu, W., Woodley, J. M. & Riisager, A. Synthesis of 5-(hydroxymethyl)furfural in ionic liquids: paving the way to renewable chemicals. ChemSusChem 4, 451–458 (2011).
Rosatella, A. A., Simeonov, S. P., Frade, R. F. M. & Afonso, C. A. M. 5-Hydroxymethylfurfural (HMF) as a building block platform: biological properties, synthesis and synthetic applications. Green Chem. 13, 754–793 (2011).
Liu, D. & Chen, E. Y.-X. Integrated catalytic process for biomass conversion and upgrading to C12 furoin and alkane fuel. ACS Catal. 4, 1302–1310 (2014).
Liu, D. & Chen, E. Y.-X. Diesel and alkane fuels from biomass by organocatalysis and metal–acid tandem catalysis. ChemSusChem 6, 2236–2239 (2013).
Mou, Z. & Chen, E. Y.-X. Polyesters and poly(ester-urethane)s from biobased difuranic polyols. ACS Sustain. Chem. Eng. 4, 7118–7129 (2016).
Cook, G. K. & Andrews, M. A. Toward nonoxidative routes to oxygenated organics: stereospecific deoxydehydration of diols and polyols to alkenes and allylic alcohols catalyzed by the metal oxo complex (C5Me5)ReO3. J. Am. Chem. Soc. 118, 9448–9449 (1996).
Dauth, A. & Love, J. A. Reactivity by design — metallaoxetanes as centerpieces in reaction development. Chem. Rev. 111, 2010–2047 (2011).
Ziegler, J. E., Zdilla, M. J., Evans, A. J. & Abu-Omar, M. M. H2-driven deoxygenation of epoxides and diols to alkenes catalyzed by methyltrioxorhenium. Inorg. Chem. 48, 9998–10000 (2009).
Vkuturi, S., Chapman, G., Ahmad, I. & Nicholas, K. M. Rhenium-catalyzed deoxydehydration of glycols by sulfite. Inorg. Chem. 49, 4744–4746 (2010).
Shiramizu, M. & Toste, F. D. Deoxygenation of biomass-derived feedstocks: oxorhenium-catalyzed deoxydehydration of sugars and sugar alcohols. Angew. Chem. Int. Ed. 51, 8082–8086 (2012).
Boucher-Jacobs, C. & Nicholas, K. M. in Selective Catalysis for Renewable Feedstocks and Chemicals (ed. Nicholas, K. M.) 163–184 (Springer International Publishing, 2014).
Furimsky, E. Catalytic hydrodeoxygenation. Appl. Catal. A Gen. 199, 147–190 (2000).
Mascal, M. & Nikitin, E. B. High-yield conversion of plant biomass into the key value-added feedstocks 5-(hydroxymethyl)furfural, levulinic acid, and levulinic esters via 5-(chloromethyl)furfural. Green Chem. 12, 370–373 (2010).
Mascal, M. & Nikitin, E. B. Direct, high-yield conversion of cellulose into biofuel. Angew. Chem. Int. Ed. 47, 7924–7926 (2008).
Drosos, N. & Morandi, B. Boron-catalyzed regioselective deoxygenation of terminal 1,2-diols to 2-alkanols enabled by the strategic formation of a cyclic siloxane intermediate. Angew. Chem. Int. Ed. 54, 8814–8818 (2015).
Drosos, N., Ozkal, E. & Morandi, B. Catalytic selective deoxygenation of polyols using the B(C6F5)3/silane system. Synlett 27, 1760–1764 (2016).
Chatterjee, I., Porwal, D. & Oestreich, M. B(C6F5)3-catalyzed chemoselective defunctionalization of ether-containing primary alkyl tosylates with hydrosilanes. Angew. Chem. Int. Ed. 56, 3389–3391 (2017).
Yasuda, M., Onishi, Y., Ueba, M., Miyai, T. & Baba, A. Direct reduction of alcohols: highly chemoselective reducing system for secondary or tertiary alcohols using chlorodiphenylsilane with a catalytic amount of indium trichloride. J. Org. Chem. 66, 7741 (2001).
Adduci, L. L., Bender, T. A., Dabrowski, J. A. & Gagné, M. R. Chemoselective conversion of biologically sourced polyols into chiral synthons. Nat. Chem. 7, 576–581 (2015).
Kottke, R. H. in Kirk-Othmer Encyclopedia of Chemical Technology (ed. Seidel, A.) (John Wiley and Sons, 2000).
Ahmed Foskey, T. J., Heinekey, D. M. & Goldberg, K. I. Partial deoxygenation of 1,2-propanediol catalyzed by iridium pincer complexes. ACS Catal. 2, 1285–1289 (2012).
Brentzel, Z. J. et al. Chemicals from biomass: combining ring-opening tautomerization and hydrogenation reactions to produce 1,5-pentanediol from furfural. ChemSusChem 10, 1351–1355 (2017).
Wu, L., Moteki, T., Gokhale, A. A., Flaherty, D. W. & Toste, F. D. Production of fuels and chemicals from biomass: condensation reactions and beyond. Chem 1, 32–58 (2017).
Ghosh, A. K. & Brindisi, M. Achmatowicz reaction and its application in the syntheses of bioactive molecules. RSC Adv. 6, 111564–111598 (2016).
Li, Z. & Tong, R. Catalytic environmentally friendly protocol for Achmatowic rearrangement. J. Org. Chem. 81, 4847–4855 (2016).
Takeuchi, M., Taniguchi, T. & Ogasawara, K. Back to the Sugars: a new enantio and diastereocontrolled route to hexoses from furfural. Synthesis 1999, 341–354 (1999).
Fürstner, A. & Nagano, T. Total syntheses of ipomoeassin B and E. J. Am. Chem. Soc. 129, 1906–1907 (2007).
Jackson, K. L., Henderson, J. A., Morris, J. C., Motoyoshi, H. & Phillips, A. J. A synthesis of the C1–C15 domain of the halichondrins. Tetrahedron Lett. 49, 2939–2941 (2008).
Nagano, T. et al. Total synthesis and biological evaluation of the cytotoxic resin glycosides ipomoeassin A–F and analogues. Chem. Eur. J. 15, 9697–9706 (2009).
Prasad, K. R. & Pawar, A. B. Enantioselective formal synthesis of palmerolide A. Org. Lett. 13, 4252–4255 (2011).
Prasad, K. R. & Revu, O. Total synthesis of (+)-seimatopolide A. J. Org. Chem. 79, 1461–1466 (2014).
Ashmus, R. A., Jayasuriya, A. B., Lim, Y.-J., O’Doherty, G. A. & Lowary, T. L. De novo asymmetric synthesis of a 6-O-Methyl-d-glycero-l-gluco-heptopyranose-derived thioglycoside for the preparation of Campylobacter jejuni NCTC11168 capsular polysaccharide fragments. J. Org. Chem. 81, 3058–3063 (2016).
Monrad, R. N. & Madsen, R. Rhodium-catalyzed decarbonylation of aldoses. J. Org. Chem. 72, 9782–9785 (2007).
Fagan, P. J., Voges, M. H. & Bullock, R. M. Catalytic ionic hydrogenation of ketones by {[Cp*Ru(CO)2]2(μ-H)}+. Organometallics 29, 1045 (2010).
Liguori, F., Moreno-Marrodan, C. & Barbaro, P. Environmentally friendly synthesis of β-valerolactone by direct catalytic conversion of renewable sources. ACS Catal. 5, 1882–1894 (2015).
Goulas, K. A. & Toste, F. D. Combining microbial production with chemical upgrading. Curr. Opin. Biotechnol. 38, 47–53 (2016).
Cabrele, C. & Reiser, O. The modern face of synthetic heterocyclic chemistry. J. Org. Chem. 81, 10109–10125 (2016).
Marson, C. M. New and unusual scaffolds in medicinal chemistry. Chem. Soc. Rev. 40, 5514–5533 (2011).
Llevot, A. et al. Renewability is not enough: recent advances in the sustainable synthesis of biomass-derived monomers and polymers. Chem. Eur. J. 22, 11510–11521 (2016).
Foster, R. W., Tame, C. J., Bucar, D.-K., Hailes, H. C. & Sheppard, T. D. Sustainable synthesis of chiral tetrahydrofurans through the selective dehydration of pentoses. Chem. Eur. J. 21, 15947–15950 (2015).
Koh, P.-F., Wang, P., Huang, J.-M. & Loh, T.-P. Biomass derived furfural-based facile synthesis of protected (2S)-phenyl-3-piperidone, a common intermediate for many drugs. Chem. Commun. 50, 8324–8327 (2014).
Veits, G. K., Wenz, D. R. & Read de Alaniz, J. Versatile method for the synthesis of 4-aminocyclopentenones: dysprosium(III) triflate catalyzed aza-piancatelli rearrangement. Angew. Chem. Int. Ed. 49, 9484–9487 (2010).
Bloom, P. D. & Venkitasubramanian, P. Monomers and polymers from bioderived carbon. US Patent US20090018300A1 (2008).
Hammond, W., East, A., Jaffe, M. & Feng, X. Isosorbide-derived epoxy resins and methods of making same. US Patent US20150307650A1 (2015).
Tachdjian, C., Karanewsky, D. S., Adamski-Werner, S. L., Yamamoto, J. M. & Servant, G. Isosorbide derivatives and their use as flavor modifiers, tastants, and taste enhancers. US Patent US20130295261A1 (2013).
Bähn, S. et al. The catalytic amination of alcohols. ChemCatChem 3, 1853–1864 (2011).
Yang, Q., Wang, Q. & Yu, Z. Substitution of alcohols by N-nucleophiles via transition metal-catalyzed dehydrogenation. Chem. Soc. Rev. 44, 2305–2329 (2015).
Pera-Titus, M. & Shi, F. Catalytic amination of biomass-based alcohols. ChemSusChem 7, 720–722 (2014).
Fink, J. K. in High Performance Polymers (ed. Fink, J. K.) 449–474 (William Andrew Publishing, 2008).
Young, I. S. & Baran, P. S. Protecting-group-free synthesis as an opportunity for invention. Nat. Chem. 1, 193–205 (2009).
Baran, P. S., Maimone, T. J. & Richter, J. M. Total synthesis of marine natural products without using protecting groups. Nature 446, 404–408 (2007).
Wei, X.-F., Shimizu, Y. & Kanai, M. An expeditious synthesis of sialic acid derivatives by copper(I)-catalyzed stereodivergent propargylation of unprotected aldoses. ACS Cent. Sci. 2, 21–26 (2016).
Richter, C., Krumrey, M., Bahri, M., Trunschke, S. & Mahrwald, R. Amine-catalyzed cascade reactions of unprotected aldoses — an operationally simple access to defined configured stereotetrads or stereopentads. ACS Catal. 6, 5549–5552 (2016).
Bartoli, G. et al. SiO2-supported CeCl3·7H2O−NaI Lewis acid promoter: investigation into the Garcia Gonzalez reaction in solvent-free conditions. J. Org. Chem. 72, 6029–6036 (2007).
Calter, M. A., Zhu, C. & Lachicotte, R. J. Rapid synthesis of the 7-deoxy zaragozic acid core. Org. Lett. 4, 209–212 (2002).
Paterson, I., Feßner, K., Finlay, M. R. V. & Jacobs, M. F. Studies towards the synthesis of the zaragozic acids: a novel epoxide cyclisation approach to the formation of the bicyclic acetal core. Tetrahedron Lett. 37, 8803–8806 (1996).
Liu, J.-H. & Long, Y.-Q. Studies toward the total synthesis of cyclodidemniserinol trisulfate. Part II: 3,5,7-Trisubstituted 6,8-dioxabicyclo [3.2.1] octane core structure construction via I2-mediated deprotection and ring closure tandem reaction. Tetrahedron Lett. 50, 4592–4594 (2009).
Shing, T. K. M. & Cheng, H. M. Intramolecular direct aldol reactions of sugar 2,7-diketones: syntheses of hydroxylated cycloalka(e)nones. Org. Biomol. Chem. 13, 4795–4802 (2015).
Bauer, J. & Osborn, H. M. I. Sialic acids in biological and therapeutic processes: opportunities and challenges. Future Med. Chem. 7, 2285–2299 (2015).
Bender, T. A., Dabrowski, J. A., Zhong, H. & Gagné, M. R. Diastereoselective B(C6F5)3-catalyzed reductive carbocyclization of unsaturated carbohydrates. Org. Lett. 18, 4120–4123 (2016).
Hazra, C. K., Gandhamsetty, N., Park, S. & Chang, S. Borane catalysed ring opening and closing cascades of furans leading to silicon functionalized synthetic intermediates. Nat. Commun. 7, 13431 (2016).
Wong, H. N. C. et al. Use of cyclopropanes and their derivatives in organic synthesis. Chem. Rev. 89, 165–198 (1989).
Böhm, C. et al. A new strategy for the stereoselective synthesis of 1,2,3-trisubstituted cyclopropanes. Eur. J. Org. Chem. 2000, 2955–2965 (2000).
Kalidindi, S. et al. Enantioselective synthesis of arglabin. Angew. Chem. Int. Ed. 46, 6361–6363 (2007).
Mix, K. A., Aronoff, M. R. & Raines, R. T. Diazo compounds: versatile tools for chemical biology. ACS Chem. Biol. 11, 3233–3244 (2016).
Li, W., Niu, Y., Xiong, D.-C., Cao, X. & Ye, X.-S. Highly substituted cyclopentane–CMP conjugates as potent sialyltransferase inhibitors. J. Med. Chem. 58, 7972–7990 (2015).
Quintard, A., Lefranc, A. & Alexakis, A. Highly enantioselective direct vinylogous michael addition of γ-butenolide to enals. Org. Lett. 13, 1540–1543 (2011).
Wu, B., Yu, Z., Gao, X., Lan, Y. & Zhou, Y.-G. Regioselective α-addition of deconjugated butenolides: enantioselective synthesis of dihydrocoumarins. Angew. Chem. Int. Ed. 56, 4006–4010 (2017).
Mäki-Arvela, P., Simakova, I. L., Salmi, T. & Murzin, D. Y. Production of lactic acid/lactates from biomass and their catalytic transformations to commodities. Chem. Rev. 114, 1909–1971 (2014).
Chen, K., Li, X., Zhang, S.-Q. & Shi, B.-F. Synthesis of chiral α-hydroxy acids via palladium-catalyzed C(sp3)-H alkylation of lactic acid. Chem. Commun. 52, 1915–1918 (2016).
Ansari, A. A., Lahiri, R. & Vankar, Y. D. The carbon-Ferrier rearrangement: an approach towards the synthesis of C -glycosides. Arkivoc, 316–362 (2013).
Dechert-Schmitt, A.-M. R., Schmitt, D. C. & Krische, M. J. Protecting-group-free diastereoselective C–C coupling of 1,3-glycols and allyl acetate through site-selective primary alcohol dehydrogenation. Angew. Chem. Int. Ed. 52, 3195–3198 (2013).
Ulbrich, K., Kreitmeier, P. & Reiser, O. Microwave- or microreactor-assisted conversion of furfuryl alcohols into 4-hydroxy-2-cyclopentenones. Synlett 2010, 2037–2040 (2010).
Dobler, D. & Reiser, O. Synthesis of 6-substituted 2-pyrones starting from renewable resources: total synthesis of sibirinone, (e)-6-(pent-1-en-1-yl)-2h-pyran-2-one, and (e)-6-(hept-1-en-1-yl)-2h-pyran-2-one. J. Org. Chem. 81, 10357–10365 (2016).
Zheng, X.-Y. et al. Selective formation of chromogen I from N-acetyl-D-glucosamine upon lanthanide coordination. Inorg. Chem. 56, 110–113 (2017).
Brovetto, M., Gamenara, D., Saenz Méndez, P. & Seoane, G. A. C−C bond-forming lyases in organic synthesis. Chem. Rev. 111, 4346–4403 (2011).
Nestl, B. M., Hammer, S. C., Nebel, B. A. & Hauer, B. New generation of biocatalysts for organic synthesis. Angew. Chem. Int. Ed. 53, 3070–3095 (2014).
Matsubara, K. et al. One-step synthesis of 2-keto-3-deoxy-d-gluconate by biocatalytic dehydration of d-gluconate. J. Biotechnol. 191, 69–77 (2014).
Van de Vyver, S., Odermatt, C., Romero, K., Prasomsri, T. & Román-Leshkov, Y. Solid Lewis acids catalyze the carbon–carbon coupling between carbohydrates and formaldehyde. ACS Catal. 5, 972–977 (2015).
Wheeldon, I., Christopher, P. & Blanch, H. Integration of heterogeneous and biochemical catalysis for production of fuels and chemicals from biomass. Curr. Opin. Biotechnol. 45, 127–135 (2017).
Luo, H. Y., Consoli, D. F., Gunther, W. R. & Román-Leshkov, Y. Investigation of the reaction kinetics of isolated Lewis acid sites in Beta zeolites for the Meerwein–Ponndorf–Verley reduction of methyl levulinate to γ-valerolactone. J. Catal. 320, 198–207 (2014).
Acknowledgements
The authors thank the US Department of Energy Office of Basic Energy Sciences for their support of this work (DE-FG02-05ER15630). J.A.D. thanks Elon University for teaching relief during manuscript preparation.
Author information
Authors and Affiliations
Contributions
All authors contributed to researching data for the article, discussion of content and writing and reviewing of the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Biorefinery
-
Infrastructure that converts biomass or biomaterials into value-added products.
- Cellulosics
-
Materials that are derived from cellulose, typically C6 sugars.
- Stereoablative
-
A process that removes the stereochemistry within a molecule, often by transforming a stereogenic centre to an achiral centre.
- Redox neutral
-
A chemical reaction in which the overall oxidation state of the molecule does not change. Although these transformations are powerful, their utilization in the field of cellulosic derivatization is underexplored.
- Catalyst control
-
A reaction in which the product outcome is dictated by the nature of the catalyst, which supersedes any biases imposed by the substrate. This may be in the form of differences in reactivity or selectivity. The pre-existing stereochemical complexity of cellulosics makes imposing control of both site-selectivity and stereoselectivity in a transformation challenging.
- Substrate control
-
A paradigm wherein the inherent nature of the reactant dictates the product outcome. In cellulosics, the existing stereochemistry of the alcohols can often control how reactions proceed.
Rights and permissions
About this article
Cite this article
Bender, T.A., Dabrowski, J.A. & Gagné, M.R. Homogeneous catalysis for the production of low-volume, high-value chemicals from biomass. Nat Rev Chem 2, 35–46 (2018). https://doi.org/10.1038/s41570-018-0005-y
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-018-0005-y
This article is cited by
-
Kinetic and hydrogen production analysis in the sequential valorization of a Populus clone by cold alkaline extraction and pyrolysis
Scientific Reports (2024)
-
Functional characterization of fungal lytic polysaccharide monooxygenases for cellulose surface oxidation
Biotechnology for Biofuels and Bioproducts (2023)
-
Analysis of the Scale of Global Human Needs and Opportunities for Sustainable Catalytic Technologies
Topics in Catalysis (2023)
-
Two Fusarium copper radical oxidases with high activity on aryl alcohols
Biotechnology for Biofuels (2021)
-
Transforming biorefinery designs with ‘Plug-In Processes of Lignin’ to enable economic waste valorization
Nature Communications (2021)