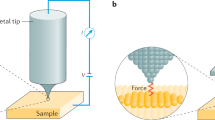
Abstract
Using atomic manipulation, one can dissociate, form and rearrange bonds, as well as alter the conformation or charge state of molecules. The molecular structures of reactants, intermediates and products are revealed at unprecedented resolution by using atomic force microscopy (AFM) and a suitably functionalized tip. Our present capabilities of manipulation and imaging of molecules by AFM approach the level of control predicted by Richard P. Feynman in his famous lecture ‘There's plenty of room at the bottom’, in which he described how molecules and materials might be formed by attaching and detaching individual atoms at will. In this Review, we discuss recent progress and the future prospects of molecule generation by atom manipulation and molecular characterization by AFM.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Binnig, G., Rohrer, H., Gerber, C. & Weibel, E. Tunneling through a controllable vacuum gap. Appl. Phys. Lett. 40, 178–180 (1982).
Binnig, G., Quate, C. F. & Gerber, C. Atomic force microscope. Phys. Rev. Lett. 56, 930–933 (1986).
Eigler, D. M. & Schweizer, E. K. Positioning single atoms with a scanning tunnelling microscope. Nature 344, 524–526 (1990).
Sugimoto, Y. et al. Atom inlays performed at room temperature using atomic force microscopy. Nat. Mater. 4, 156–159 (2005).
Sugimoto, Y., Miki, K., Abe, M. & Morita, S. Statistics of lateral atom manipulation by atomic force microscopy at room temperature. Phys. Rev. B 78, 205305 (2008).
Stipe, B. et al. Single-molecule dissociation by tunneling electrons. Phys. Rev. Lett. 78, 4410–4413 (1997).
Hla, S.-W., Bartels, L., Meyer, G. & Rieder, K.-H. Inducing all steps of a chemical reaction with the scanning tunneling microscope tip: towards single molecule engineering. Phys. Rev. Lett. 85, 2777–2780 (2000).
Okawa, Y. & Aono, M. Nanoscale control of chain polymerization. Nature 409, 683–684 (2001).
Repp, J., Meyer, G., Stojkovic, S. M., Gourdon, A. & Joachim, C. Molecules on insulating films: scanning-tunneling microscopy imaging of individual molecular orbitals. Phys. Rev. Lett. 94, 026803 (2005).
Repp, J., Meyer, G., Paavilainen, S., Olsson, F. E. & Persson, M. Imaging bond formation between a gold atom and pentacene on an insulating surface. Science 312, 1196–1199 (2006).
Liljeroth, P., Repp, J. & Meyer, G. Current-induced hydrogen tautomerization and conductance switching of naphthalocyanine molecules. Science 317, 1203–1206 (2007).
Gross, L., Mohn, F., Moll, N., Liljeroth, P. & Meyer, G. The chemical structure of a molecule resolved by atomic force microscopy. Science 325, 1110–1114 (2009).
Gross, L. et al. Organic structure determination using atomic resolution scanning probe microscopy. Nat. Chem. 2, 821–825 (2010).
Grill, L. et al. Nano-architectures by covalent assembly of molecular building blocks. Nat. Nanotechnol. 2, 687–691 (2007).
Cai, J. et al. Atomically precise bottom-up fabrication of graphene nanoribbons. Nature 466, 470–473 (2010).
de Oteyza, D. G. et al. Direct imaging of covalent bond structure in single-molecule chemical reactions. Science 340, 1434–1437 (2013).
Albrecht, F., Pavlicˇek, N., Herranz-Lancho, C., Ruben, M. & Repp, J. Characterization of a surface reaction by means of atomic force microscopy. J. Am. Chem. Soc. 137, 7424–7428 (2015).
Rogers, C. et al. Closing the nanographene gap: surface-assisted synthesis of peripentacene from 6,6′-bipentacene precursors. Angew. Chem. Int. Ed. 54, 15143–15146 (2015).
Kawai, S. et al. Atomically controlled substitutional boron-doping of graphene nanoribbons. Nat. Commun. 6, 8098 (2015).
Riss, A. et al. Imaging single-molecule reaction intermediates stabilized by surface dissipation and entropy. Nat. Chem. 8, 678–683 (2016).
Ruffieux, P. et al. On-surface synthesis of graphene nanoribbons with zigzag edge topology. Nature 531, 489–492 (2016).
He, Y. et al. Fusing tetrapyrroles to graphene edges by surface-assisted covalent coupling. Nat. Chem. http://dx.doi.org/10.1038/nchem.2600 (2016).
Schuler, B. et al. Adsorption geometry determination of single molecules by atomic force microscopy. Phys. Rev. Lett. 111, 106103 (2013).
Mohn, F., Schuler, B., Gross, L. & Meyer, G. Different tips for high-resolution AFM and STM imaging of single molecules. Appl. Phys. Lett. 102, 073109 (2013).
Pavlicˇek, N. et al. On-surface generation and imaging of arynes by atomic force microscopy. Nat. Chem. 7, 623–628 (2015).
Schuler, B. et al. Reversible Bergman cyclization by atomic manipulation. Nat. Chem. 8, 220–224 (2016).
Majzik, Z. et al. Synthesis of a naphthodiazaborinine and its verification by planarization with atomic force microscopy. ACS Nano 10, 5340–5345 (2016).
Mohn, F. et al. Reversible bond formation in a gold-atom–organic-molecule complex as a molecular switch. Phys. Rev. Lett. 105, 266102 (2010).
Albrecht, F., Neu, M., Quest, C., Swart, I. & Repp, J. Formation and characterization of a molecule–metal–molecule bridge in real space. J. Am. Chem. Soc. 135, 9200–9203 (2013).
Albrecht, T. R., Grütter, P., Horne, D. & Rugar, D. Frequency modulation detection using highQ cantilevers for enhanced force microscope sensitivity. J. Appl. Phys. 69, 668–673 (1991).
Morita, S., Giessibl, F. J., Meyer, E. & Wiesendanger, R. (eds) Noncontact Atomic Force Microscopy Vol. 3 (Springer, 2015).
Hölscher, H., Langkat, S. M., Schwarz, A. & Wiesendanger, R. Measurement of three-dimensional force fields with atomic resolution using dynamic force spectroscopy. Appl. Phys. Lett. 81, 4428–4430 (2002).
Sader, J. E. & Jarvis, S. P. Accurate formulas for interaction force and energy in frequency modulation force spectroscopy. Appl. Phys. Lett. 84, 1801–1803 (2004).
Giessibl, F. J. High-speed force sensor for force microscopy and profilometry utilizing a quartz tuning fork. Appl. Phys. Lett. 73, 3956–3958 (1999).
Giessibl, F. J. Advances in atomic force microscopy. Rev. Mod. Phys. 75, 949–983 (2003).
Giessibl, F. J., Bielefeldt, H., Hembacher, S. & Mannhart, J. Calculation of the optimal imaging parameters for frequency modulation atomic force microscopy. Appl. Surf. Sci. 140, 352–357 (1999).
Moreno, C., Stetsovych, O., Shimizu, T. K. & Custance, O. Imaging three-dimensional surface objects with submolecular resolution by atomic force microscopy. Nano Lett. 15, 2257–2262 (2015).
Iwata, K. et al. Chemical structure imaging of a single molecule by atomic force microscopy at room temperature. Nat. Commun. 6, 7766 (2015).
Moll, N., Gross, L., Mohn, F., Curioni, A. & Meyer, G. The mechanisms underlying the enhanced resolution of atomic force microscopy with functionalized tips. New J. Phys. 12, 125020 (2010).
Mohn, F., Gross, L., Moll, N. & Meyer, G. Imaging the charge distribution within a single molecule. Nat. Nanotechnol. 7, 227–231 (2012).
Bartels, L., Meyer, G. & Rieder, K.-H. Controlled vertical manipulation of single CO molecules with the scanning tunneling microscope: a route to chemical contrast. Appl. Phys. Lett. 71, 213–215 (1997).
Gross, L. et al. Bond-order discrimination by atomic force microscopy. Science 337, 1326–1329 (2012).
Hapala, P. et al. The mechanism of high-resolution STM/AFM imaging with functionalized tips. Phys. Rev. B 90, 085421 (2014).
Pavlicˇek, N. et al. Atomic force microscopy reveals bistable configurations of dibenzo[a,h]thianthrene and their interconversion pathway. Phys. Rev. Lett. 108, 086101 (2012).
Hämäläinen, S. K. et al. Intermolecular contrast in atomic force microscopy images without intermolecular bonds. Phys. Rev. Lett. 113, 186102 (2014).
Weymouth, A. J., Hofmann, T. & Giessibl, F. J. Quantifying molecular stiffness and interaction with lateral force microscopy. Science 343, 1120–1122 (2014).
Ellner, M. et al. The electric field of CO tips and its relevance for atomic force microscopy. Nano Lett. 16, 1974–1980 (2016).
Neu, M. et al. Image correction for atomic force microscopy images with functionalized tips. Phys. Rev. B 89, 205407 (2014).
Mönig, H. et al. Submolecular imaging by noncontact atomic force microscopy with an oxygen atom rigidly connected to a metallic probe. ACS Nano 10, 1201–1209 (2015).
Pavlicˇek, N., Swart, I., Niedenführ, J., Meyer, G. & Repp, J. Symmetry dependence of vibration-assisted tunneling. Phys. Rev. Lett. 110, 136101 (2013).
Gross, L. et al. High-resolution molecular orbital imaging using a p-wave STM tip. Phys. Rev. Lett. 107, 086101 (2011).
Temirov, R., Soubatch, S., Neucheva, O., Lassise, A. C. & Tautz, F. S. A novel method achieving ultra-high geometrical resolution in scanning tunnelling microscopy. New J. Phys. 10, 053012 (2008).
Kichin, G., Weiss, C., Wagner, C., Tautz, F. S. & Temirov, R. Single molecule and single atom sensors for atomic resolution imaging of chemically complex surfaces. J. Am. Chem. Soc. 133, 16847–16851 (2011).
Stipe, B., Rezaei, M. & Ho, W. Single-molecule vibrational spectroscopy and microscopy. Science 280, 1732–1735 (1998).
Lee, H. J. & Ho, W. Single-bond formation and characterization with a scanning tunneling microscope. Science 286, 1719–1722 (1999).
Lauhon, L. & Ho, W. Single-molecule chemistry and vibrational spectroscopy: pyridine and benzene on Cu(001). J. Phys. Chem. A 104, 2463–2467 (2000).
Chiang, C.-l., Xu, C., Han, Z. & Ho, W. Real-space imaging of molecular structure and chemical bonding by single-molecule inelastic tunneling probe. Science 344, 885–888 (2014).
Nonnenmacher, M., O'Boyle, M. P. & Wickramasinghe, H. K. Kelvin probe force microscopy. Appl. Phys. Lett. 58, 2921–2923 (1991).
Barth, C., Foster, A. S., Henry, C. R. & Shluger, A. L. Recent trends in surface characterization and chemistry with high-resolution scanning force methods. Adv. Mater. 23, 477–501 (2011).
Sadewasser, S. & Glatzel, T. (eds) Kelvin Probe Force Microscopy (Springer, 2011).
Gross, L. et al. Measuring the charge state of an adatom with noncontact atomic force microscopy. Science 324, 1428–1431 (2009).
Leoni, T. et al. Controlling the charge state of a single redox molecular switch. Phys. Rev. Lett. 106, 216103 (2011).
Steurer, W. et al. Toggling the local electric field with an embedded adatom switch. Nano Lett. 15, 5564–5568 (2015).
Schuler, B. et al. Contrast formation in kelvin probe force microscopy of single π-conjugated molecules. Nano Lett. 14, 3342–3346 (2014).
Gross, L. et al. Investigating atomic contrast in atomic force microscopy and Kelvin probe force microscopy on ionic systems using functionalized tips. Phys. Rev. B 90, 155455 (2014).
Bartels, L., Meyer, G. & Rieder, K.-H. Basic steps of lateral manipulation of single atoms and diatomic clusters with a scanning tunneling microscope tip. Phys. Rev. Lett. 79, 697–700 (1997).
Moresco, F. et al. Recording intramolecular mechanics during the manipulation of a large molecule. Phys. Rev. Lett. 87, 088302 (2001).
Ternes, M., Lutz, C. P., Hirjibehedin, C. F., Giessibl, F. J. & Heinrich, A. J. The force needed to move an atom on a surface. Science 319, 1066–1069 (2008).
Jung, T. A., Schlittler, R. R., Gimzewski, J. K., Tang, H. & Joachim, C. Controlled room-temperature positioning of individual molecules: molecular flexure and motion. Science 271, 181–184 (1996).
Moresco, F. et al. Conformational changes of single molecules induced by scanning tunneling microscopy manipulation: a route to molecular switching. Phys. Rev. Lett. 86, 672–675 (2001).
Loppacher, C. et al. Direct determination of the energy required to operate a single molecule switch. Phys. Rev. Lett. 90, 066107 (2003).
Grill, L. et al. Exploring the interatomic forces between tip and single molecules during STM manipulation. Nano Lett. 6, 2685–2689 (2006).
Pawlak, R. et al. Directed rotations of single porphyrin molecules controlled by localized force spectroscopy. ACS Nano 6, 6318–6324 (2012).
Ladenthin, J. N. et al. Force-induced tautomerization in a single molecule. Nat. Chem. 8, 935–940 (2016).
Kim, Y., Komeda, T. & Kawai, M. Single-molecule reaction and characterization by vibrational excitation. Phys. Rev. Lett. 89, 126104 (2002).
Qiu, X., Nazin, G. & Ho, W. Vibronic states in single molecule electron transport. Phys. Rev. Lett. 92, 206102 (2004).
Huang, K., Leung, L., Lim, T., Ning, Z. & Polanyi, J. C. Single-electron induces double-reaction by charge delocalization. J. Am. Chem. Soc. 135, 6220–6225 (2013).
Huang, K., Leung, L., Lim, T., Ning, Z. & Polanyi, J. C. Vibrational excitation induces double reaction. ACS Nano 8, 12468–12475 (2014).
Schendel, V. et al. Remotely controlled isomer selective molecular switching. Nano Lett. 16, 93–97 (2015).
Ladenthin, J. N. et al. Hot carrier-induced tautomerization within a single porphycene molecule on Cu(111). ACS Nano 9, 7287–7295 (2015).
Auwärter, W. et al. A surface-anchored molecular four-level conductance switch based on single proton transfer. Nat. Nanotechnol. 7, 41–46 (2012).
Kumagai, T. et al. Controlling intramolecular hydrogen transfer in a porphycene molecule with single atoms or molecules located nearby. Nat. Chem. 6, 41–46 (2014).
Bennewitz, R. et al. Ultrathin films of NaCl on Cu(111): a LEED and dynamic force microscopy study. Surf. Sci. 438, 289–296 (1999).
Hanssen, K. O. et al. A combined atomic force microscopy and computational approach for structural elucidation of breitfussin A and B, highly modified halogenated dipeptides from the Arctic hydrozoan Thuiaria breitfussi. Angew. Chem. Int. Ed. 51, 12238–12241 (2012).
Schuler, B. et al. From perylene to a 22-ring aromatic hydrocarbon in one-pot. Angew. Chem. Int. Ed. 126, 9150–9152 (2014).
Schuler, B., Meyer, G., Peña, D., Mullins, O. C. & Gross, L. Unraveling the molecular structures of asphaltenes by atomic force microscopy. J. Am. Chem. Soc. 137, 9870–9876 (2015).
van der Lit, J. et al. Suppression of electron–vibron coupling in graphene nanoribbons contacted via a single atom. Nat. Commun. 4, 2023 (2013).
Dienel, T. et al. Resolving atomic connectivity in graphene nanostructure junctions. Nano Lett. 15, 5185–5190 (2015).
Zhao, A. et al. Controlling the Kondo effect of an adsorbed magnetic ion through its chemical bonding. Science 309, 1542–1544 (2005).
van der Lit, J., Jacobse, P. H., Vanmaekelbergh, D. & Swart, I. Bending and buckling of narrow armchair graphene nanoribbons via STM manipulation. New J. Phys. 17, 053013 (2015).
Blanksby, S. J. & Ellison, G. B. Bond dissociation energies of organic molecules. Acc. Chem. Res. 36, 255–263 (2003).
Leung, L., Lim, T., Ning, Z. & Polanyi, J. C. Localized reaction at a smooth metal surface: p-diiodobenzene at Cu(110). J. Am. Chem. Soc. 134, 9320–9326 (2012).
Rauschenbach, S. et al. Electrospray ion beam deposition: soft-landing and fragmentation of functional molecules at solid surfaces. ACS Nano 3, 2901–2910 (2009).
Hamann, C. et al. Ultrahigh vacuum deposition of organic molecules by electrospray ionization. Rev. Sci. Instrum. 82, 033903 (2011).
Deng, Z. et al. A close look at proteins: submolecular resolution of two-and three-dimensionally folded cytochrome C at surfaces. Nano Lett. 12, 2452–2458 (2012).
Mohn, F., Gross, L. & Meyer, G. Measuring the short-range force field above a single molecule with atomic resolution. Appl. Phys. Lett. 99, 053106 (2011).
Schuler, B., Mohn, F., Gross, L., Meyer, G. & Jaspars, M. in Modern NMR Approaches to the Structure Elucidation of Natural Products Vol. 1 (eds Williams, A., Martin, G. & Rovnyak, D. ) 306–318 (Royal Society of Chemistry, 2015).
Acknowledgements
The authors thank B. Schuler, N. Moll, Z. Majzik, S. Fatayer, G. Meyer, D. Peña and R. Allenspach for discussions. They acknowledge financial support from the European Research Council Consolidator Grant AMSEL (agreement no. 682144), the European Research Council Advanced Grant CEMAS (agreement no. 291194), the European Union project PAMS (agreement no. 610446) and the Initial Training Network QTea (agreement no. 317485).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Pavliček, N., Gross, L. Generation, manipulation and characterization of molecules by atomic force microscopy. Nat Rev Chem 1, 0005 (2017). https://doi.org/10.1038/s41570-016-0005
Published:
DOI: https://doi.org/10.1038/s41570-016-0005
This article is cited by
-
Single-molecule chemistry with a smart robot
Nature Synthesis (2024)
-
Molecular identification with atomic force microscopy and conditional generative adversarial networks
npj Computational Materials (2024)
-
The role of halogens in Au–S bond cleavage for energy-differentiated catalysis at the single-bond limit
Nature Communications (2023)
-
Reactions in single-molecule junctions
Nature Reviews Materials (2022)
-
Capillary grip-induced stick-slip motion
Nano Research (2022)