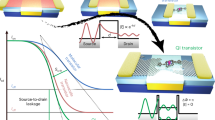
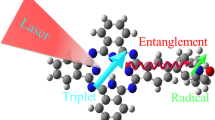
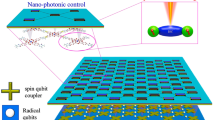
Abstract
Free radicals, generally formed through the cleavage of covalent electron-pair bonds, play an important role in diverse fields ranging from synthetic chemistry to spintronics and nonlinear optics. However, the characterization and regulation of the radical state at a single-molecule level face formidable challenges. Here we present the detection and sophisticated tuning of the open-shell character of individual diradicals with a donor–acceptor structure via a sensitive single-molecule electrical approach. The radical is sandwiched between nanogapped graphene electrodes via covalent amide bonds to construct stable graphene–molecule–graphene single-molecule junctions. We measure the electrical conductance as a function of temperature and track the evolution of the closed-shell and open-shell electronic structures in real time, the open-shell triplet state being stabilized with increasing temperature. Furthermore, we tune the spin states by external stimuli, such as electrical and magnetic fields, and extract thermodynamic and kinetic parameters of the transition between closed-shell and open-shell states. Our findings provide insights into the evolution of single-molecule radicals under external stimuli, which may proof instrumental for the development of functional quantum spin-based molecular devices.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and Supplementary Information. The datasets used in Supplementary Information are available online from the Zenodo repository at https://zenodo.org/records/10603012. Source data are provided with this paper.
References
Dery, H., Dalal, P., Cywinski, L. & Sham, L. J. Spin-based logic in semiconductors for reconfigurable large-scale circuits. Nature 447, 573–576 (2007).
Vincent, R., Klyatskaya, S., Ruben, M., Wernsdorfer, W. & Balestro, F. Electronic read-out of a single nuclear spin using a molecular spin transistor. Nature 488, 357–360 (2012).
Baek, S. H. C. et al. Complementary logic operation based on electric-field controlled spin-orbit torques. Nat. Electron. 1, 398–403 (2018).
Fert, A., Reyren, N. & Cros, V. Magnetic skyrmions: advances in physics and potential applications. Nat. Rev. Mater. 2, 17031 (2017).
Gehring, P., Thijssen, J. M. & van der Zant, H. S. J. Single-molecule quantum-transport phenomena in break junctions. Nat. Rev. Phys. 1, 381–396 (2019).
Berciu, M., Rappoport, T. G. & Janko, B. Manipulating spin and charge in magnetic semiconductors using superconducting vortices. Nature 435, 71–75 (2005).
Bracher, T. et al. Detection of short-waved spin waves in individual microscopic spin-wave waveguides using the inverse spin hall effect. Nano Lett. 17, 7234–7241 (2017).
Tong, M. et al. Light-driven spintronic heterostructures for coded terahertz emission. ACS Nano 16, 8294–8300 (2022).
Simao, C. et al. A robust molecular platform for non-volatile memory devices with optical and magnetic responses. Nat. Chem. 3, 359–364 (2011).
Warner, M. et al. Potential for spin-based information processing in a thin-film molecular semiconductor. Nature 503, 504–508 (2013).
Lombardi, F. et al. Quantum units from the topological engineering of molecular graphenoids. Science 366, 1107–1110 (2019).
Abe, M. Diradicals. Chem. Rev. 113, 7011–7088 (2013).
Zeng, Z. et al. Pro-aromatic and anti-aromatic pi-conjugated molecules: an irresistible wish to be diradicals. Chem. Soc. Rev. 44, 6578–6596 (2015).
Naghibi, S. et al. Redox-addressable single-molecule junctions incorporating a persistent organic radical. Angew. Chem. Int. Ed. 61, e202116985 (2022).
Bejarano, F. et al. Robust organic radical molecular junctions using acetylene terminated groups for C–Au bond formation. J. Am. Chem. Soc. 140, 1691–1696 (2018).
Zhang, X. et al. Electron spin resonance of single iron phthalocyanine molecules and role of their non-localized spins in magnetic interactions. Nat. Chem. 14, 59–65 (2021).
Shen, Y. et al. Normal & reversed spin mobility in a diradical by electron-vibration coupling. Nat. Commun. 12, 6262 (2021).
Patera, L. L. et al. Resolving the unpaired-electron orbital distribution in a stable organic radical by Kondo resonance mapping. Angew. Chem. Int. Ed. 58, 11063–11067 (2019).
Baum, T. Y., Andez, S. F., Pena, D. G. & van der Zant, H. S. J. Magnetic fingerprints in an all-organic radical molecular break junction. Nano Lett. 22, 8086–8092 (2022).
Hayakawa, R. et al. Large magnetoresistance in single-radical molecular junctions. Nano Lett. 16, 4960–4967 (2016).
Mitra, G. et al. Interplay between magnetoresistance and Kondo resonance in radical single-molecule junctions. Nano Lett. 22, 5773–5779 (2022).
Pyurbeeva, E. et al. Controlling the entropy of a single-molecule junction. Nano Lett. 21, 9715–9719 (2021).
Pyurbeeva, E. & Mol, J. A. A thermodynamic approach to measuring entropy in a few-electron nanodevice. Entropy 23, 640 (2021).
Bajaj, A., Khurana, R. & Ali, M. E. Quantum interference and spin filtering effects in photo-responsive single molecule devices. J. Mater. Chem. C 9, 11242–11251 (2021).
Han, Y. et al. Electric-field-driven dual-functional molecular switches in tunnel junctions. Nat. Mater. 19, 843–848 (2020).
Fock, J. et al. Manipulation of organic polyradicals in a single-molecule transistor. Phys. Rev. B 86, 235403 (2012).
Li, L. et al. Highly conducting single-molecule topological insulators based on mono- and di-radical cations. Nat. Chem. 14, 1061–1067 (2022).
Chen, Z. et al. Evolution of the electronic structure in open-shell donor–acceptor organic semiconductors. Nat. Commun. 12, 5889 (2021).
Li, Y., Li, L., Wu, Y. & Li, Y. A review on the origin of synthetic metal radical: singlet open-shell radical ground state? J. Phys. Chem. C 121, 8579–8588 (2017).
Chen, Z. et al. Aggregation-induced radical of donor–acceptor organic semiconductors. J. Phys. Chem. Lett. 12, 9783–9790 (2021).
Chen, Z., Li, Y. & Huang, F. Persistent and stable organic radicals: design, synthesis, and applications. Chem 7, 288–332 (2021).
Lörtscher, E. Wiring molecules into circuits. Nat. Nanotechnol. 8, 381–384 (2013).
Xin, N. et al. Concepts in the design and engineering of single-molecule electronic devices. Nat. Rev. Phys. 1, 211–230 (2019).
Jia, C. et al. Covalently bonded single-molecule junctions with stable and reversible photoswitched conductivity. Science 352, 1443–1445 (2016).
Meng, L. et al. Dual-gated single-molecule field-effect transistors beyond Moore’s law. Nat. Commun. 13, 1410 (2022).
Yang, C. et al. Complete deciphering of the dynamic stereostructures of a single aggregation-induced emission molecule. Matter 5, 1224–1234 (2022).
Xin, N. et al. Stereoelectronic effect-induced conductance switching in aromatic chain single-molecule junctions. Nano Lett. 17, 856–861 (2017).
Yang, C. et al. Unveiling the full reaction path of the Suzuki–Miyaura cross-coupling in a single-molecule junction. Nat. Nanotechnol. 16, 1214–1223 (2021).
Yang, C. et al. Electric field-catalyzed single-molecule Diels–Alder reaction dynamics. Sci. Adv. 7, eabf0689 (2021).
Cao, Y. et al. Building high-throughput molecular junctions using indented graphene point contacts. Angew. Chem. Int. Ed. 51, 12228–12232 (2012).
Yang, C., Yang, C., Guo, Y., Feng, J. & Guo, X. Graphene–molecule–graphene single-molecule junctions to detect electronic reactions at the molecular scale. Nat. Protoc. 18, 1958–1978 (2023).
Mol, J. A. et al. Graphene–porphyrin single-molecule transistors. Nanoscale 7, 13181–13185 (2015).
Gehring, P. et al. Field-effect control of graphene–fullerene thermoelectric nanodevices. Nano Lett. 17, 7055–7061 (2017).
Gehring, P. et al. Quantum interference in graphene nanoconstrictions. Nano Lett. 16, 4210–4216 (2016).
Hayashi, H. et al. Monoradicals and diradicals of dibenzofluoreno[3,2-b]fluorene isomers: mechanisms of electronic delocalization. J. Am. Chem. Soc. 142, 20444–20455 (2020).
Dressler, J. J. et al. Thiophene and its sulfur inhibit indenoindenodibenzothiophene diradicals from low-energy lying thermal triplets. Nat. Chem. 10, 1134–1140 (2018).
Qin, F., Auerbach, A. & Sachs, F. Estimating single-channel kinetic parameters from idealized patch clamp data containing missed events. Biophys. J. 70, 264–280 (1996).
Huang, X. et al. Electric field–induced selective catalysis of single-molecule reaction. Sci. Adv. 5, eaaw3072 (2019).
Frisch, M. J. et al. Gaussian16 Revision C.01 (Gaussian, 2016).
Yamaguchi, K. The electronic structures of biradicals in the unrestricted Hartree–Fock approximation. Chem. Phys. Lett. 33, 330–335 (1975).
Schleyer, P. V. R. et al. Dissected nucleus-independent chemical shift analysis of π-aromaticity and antiaromaticity. Org. Lett. 3, 2465–2468 (2001).
Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2, 73–78 (2012).
Grimme, S. & Hansen, A. A practicable real‐space measure and visualization of static electron correlation effects. Angew. Chem. Int. Ed. 54, 12308–12313 (2005).
Wang, M. et al. Donor-acceptor conjugated polymer based on naphtho[1,2-c:5,6-c]bis[1,2,5]thiadiazole for high-performance polymer solar cells. J. Am. Chem. Soc. 133, 9638–9641 (2011).
Brandbyge, M., Mozos, J. L., Ordejón, P., Taylor, J. & Stokbro, K. Density-functional method for nonequilibrium electron transport. Phys. Rev. B 65, 165401 (2002).
Wang, B., Wang, J. & Guo, H. Current partition: a nonequilibrium Green’s function approach. Phys. Rev. Lett. 82, 398–401 (1999).
Taylor, J., Guo, H. & Wang, J. Ab initio modeling of quantum transport properties of molecular electronic devices. Phys. Rev. B 63, 245407 (2001).
Soler, J. M. et al. The SIESTA method for ab initio order-N materials simulations. J. Phys. Condens. Matter 14, 2745–2779 (2002).
Troullier, N. & Martins, J. A straightforward method for generating soft transferable pseudopotentials. Solid State Commun. 74, 613–616 (1990).
Heyd, J., Scuseria, G. E. & Ernzerhof, M. Hybrid functionals based on a screened coulomb potential. J. Chem. Phys. 118, 8207–8215 (2003).
Acknowledgements
The authors acknowledge primary financial support from the National Key R&D Program of China (2021YFA1200101 (to X.G.) and 2022YFA0128700 (to X.G.)), the National Natural Science Foundation of China (22150013 (to X.G.), 21727806 (to X.G.), 21933001(to X.G.), 22288201 (to X.L.), 22322304 (to X.L.), 22375065 (to Y.L.) and 51973063 (to Y.L.)), the Tencent Foundation through the XPLORER PRIZE (to X.G.), ‘Frontiers Science Center for New Organic Matter’ at Nankai University (63181206 (to X.G.)), the Natural Science Foundation of Beijing (2222009 (to X.G.)), the Tip-top Scientific and Technical Innovative Youth Talents of Guangdong Special Support Program (2019TQ05C890 (to Y.L.)) and the National Science Foundation (OIA-1757220 (to N.R.)).
Author information
Authors and Affiliations
Contributions
X.G., F.H. and Y.L. conceived the idea for the paper. C.Ya., J.C. and G.K. fabricated the devices and performed the device measurements. Z.C., W.Z., W.L., J.H. and W.C. carried out the molecular synthesis and characterization. C.S., M.A.S. and N.R. performed calculations for the analysis of the open-shell character and aromaticity. C.Yu., X.L. and J.Y. built and analysed the theoretical model and performed the quantum transport calculation. X.G., F.H., Y.L. and C.Ya. analysed the data and wrote the paper. All the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Pascal Gehring, Weida Hu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–51, Notes 1 and 2, and Supplementary Tables 1 and 2.
Source data
Source Data Fig. 1
Data for Figs. 1–4 in the main text.
Source Data Fig. 2
Chemical structures in Fig. 1b.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, C., Chen, Z., Yu, C. et al. Regulation of quantum spin conversions in a single molecular radical. Nat. Nanotechnol. (2024). https://doi.org/10.1038/s41565-024-01632-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41565-024-01632-2