Abstract
Bone is the most common site of metastasis, and although low proliferation and immunoediting at the early stage make existing treatment modalities less effective, the microenvironment-inducing behaviour could be a target for early intervention. Here we report on a spatiotemporal coupling interaction between tumour cells and osteoclasts, and named the tumour-associated osteoclast ‘tumasteoclast’—a subtype of osteoclasts in bone metastases induced by tumour-migrasome-mediated cytoplasmic transfer. We subsequently propose an in situ decoupling–killing strategy in which tetracycline-modified nanoliposomes encapsulating sodium bicarbonate and sodium hydrogen phosphate are designed to specifically release high concentrations of hydrogen phosphate ions triggered by tumasteoclasts, which depletes calcium ions and forms calcium-phosphorus crystals. This can inhibit the formation of migrasomes for decoupling and disrupt cell membrane for killing, thereby achieving early prevention of bone metastasis. This study provides a research model for exploring tumour cell behaviour in detail and a proof-of-concept for behaviour-targeting strategy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data that support the findings of this study are available within the paper and its Supplementary Information. The transcriptomic data of the OCPs, RA-OCPs, OCs and TAOCs can be found under accession no. PRJNA1045297. Further materials from this study are available from the corresponding author on reasonable request. Source Data are provided with this paper.
References
Coleman, R. E. et al. Bone metastases. Nat. Rev. Dis. Prim. 6, 83 (2020).
Chaffer, C. L. & Weinberg, R. A. A perspective on cancer cell metastasis. Science 331, 1559–1564 (2011).
von Moos, R. et al. Management of bone health in solid tumours: from bisphosphonates to a monoclonal antibody. Cancer Treat. Rev. 76, 57–67 (2019).
Massagué, J. & Obenauf, A. C. Metastatic colonization by circulating tumour cells. Nature 529, 298–306 (2016).
McAllister, S. S. & Weinberg, R. A. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat. Cell Biol. 16, 717–727 (2014).
Croucher, P. I., McDonald, M. M. & Martin, T. J. Bone metastasis: the importance of the neighbourhood. Nat. Rev. Cancer 16, 373–386 (2016).
Hosseini, H. et al. Early dissemination seeds metastasis in breast cancer. Nature 540, 552–558 (2016).
Dagogo-Jack, I. & Shaw, A. T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 15, 81–94 (2018).
Marusyk, A., Janiszewska, M. & Polyak, K. Intratumor heterogeneity: the Rosetta Stone of therapy resistance. Cancer Cell 37, 471–484 (2020).
Boumahdi, S. & de Sauvage, F. J. The great escape: tumour cell plasticity in resistance to targeted therapy. Nat. Rev. Drug Discov. 19, 39–56 (2020).
Ganesh, K. & Massagué, J. Targeting metastatic cancer. Nat. Med. 27, 34–44 (2021).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 70, 7–30 (2020).
Lin, Y., Xu, J. & Lan, H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J. Hematol. Oncol. 12, 76 (2019).
Zhang, J. et al. Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J. Clin. Invest. 107, 1235–1244 (2001).
Chen, Q., Zhang, X. H. & Massagué, J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell 20, 538–549 (2011).
Tsilimigras, D. I. et al. Liver metastases. Nat. Rev. Dis. Prim. 7, 27 (2021).
Sevenich, L. et al. Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat. Cell Biol. 16, 876–888 (2014).
Hofbauer, L. C. et al. Novel approaches to target the microenvironment of bone metastasis. Nat. Rev. Clin. Oncol. 18, 488–505 (2021).
Zhang, W. et al. The bone microenvironment invigorates metastatic seeds for further dissemination. Cell 184, 2471–2486 (2021).
Satcher, R. L. & Zhang, X. H. F. Evolving cancer–niche interactions and therapeutic targets during bone metastasis. Nat. Rev. Cancer 22, 85–101 (2022).
Weilbaecher, K. N., Guise, T. A. & McCauley, L. K. Cancer to bone: a fatal attraction. Nat. Rev. Cancer 11, 411–425 (2011).
Boyle, W. J., Simonet, W. S. & Lacey, D. L. Osteoclast differentiation and activation. Nature 423, 337–342 (2003).
Ell, B. & Kang, Y. SnapShot: bone metastasis. Cell 151, 690 (2012).
Wu, K. et al. Exosomal miR-19a and IBSP cooperate to induce osteolytic bone metastasis of estrogen receptor-positive breast cancer. Nat. Commun. 12, 5196 (2021).
Lu, X. et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging α4β1-positive osteoclast progenitors. Cancer Cell 20, 701–714 (2011).
Lin, X. et al. Smart nanosacrificial layer on the bone surface prevents osteoporosis through acid-base neutralization regulated biocascade effects. J. Am. Chem. Soc. 142, 17543–17556 (2020).
Perrin, D. D. Binding of tetracyclines to bone. Nature 208, 787–788 (1965).
Krysko, D. V. et al. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 12, 860–875 (2012).
McDonald, M. M. et al. Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell 184, 1330–1347 (2021).
Ell, B. et al. Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer Cell 24, 542–5556 (2013).
Jiao, H. et al. Mitocytosis, a migrasome-mediated mitochondrial quality-control process. Cell 184, 2896–2910 (2021).
Hasegawa, T. et al. Identification of a novel arthritis-associated osteoclast precursor macrophage regulated by FoxM1. Nat. Immunol. 20, 1631–1643 (2019).
Chaffer, C. L. et al. Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell 154, 61–74 (2013).
Morel, A. P. et al. A stemness-related ZEB1-MSRB3 axis governs cellular pliancy and breast cancer genome stability. Nat. Med. 23, 568–578 (2017).
Matsuo, K. et al. Fosl1 is a transcriptional target of c-Fos during osteoclast differentiation. Nat. Genet. 24, 184–187 (2000).
Jardine, L. et al. Blood and immune development in human fetal bone marrow and Down syndrome. Nature 598, 327–331 (2021).
Li, Z. et al. ESR1 mutant breast cancers show elevated basal cytokeratins and immune activation. Nat. Commun. 13, 2011 (2022).
Miyazaki, T., Miyauchi, S., Anada, T., Imaizumi, H. & Suzuki, O. Evaluation of osteoclastic resorption activity using calcium phosphate coating combined with labeled polyanion. Anal. Biochem. 410, 7–12 (2011).
Wang, H. et al. Bone-in-culture array as a platform to model early-stage bone metastases and discover anti-metastasis therapies. Nat. Commun. 8, 15045 (2017).
Wang, H. et al. The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell 27, 193–210 (2015).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Ma, L. et al. Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration. Cell Res. 25, 24–38 (2015).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci USA 102, 15545–15550 (2005).
Mootha, V. K. et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273 (2003).
Keenan, A. B. et al. ChEA3: transcription factor enrichment analysis by orthogonal omics integration. Nucleic Acids Res. 47, W212–W224 (2019).
Tang, R. et al. Micro-computed tomography (Micro-CT): a novel approach for intraoperative breast cancer specimen imaging. Breast Cancer Res. Treat. 139, 311–316 (2013).
Acknowledgements
This work was supported by grants from the National Nature Science Fund of China (grant nos. 82322043, 92268113 and 82072414 to XF.L., 82372454 to PF.C. and 82330077 to S.F.), the Natural Science Fund of Zhejiang Province (grant no. LGF21H060005 to Y.H.), and the Zhejiang Provincial Department of Science and Technology “Leading Geese” research and development project (grant no. 2023C03091 to S.F.). We thank H. Jiao at the School of Life Sciences of Tsinghua University for his theoretical support on migrasome formation. We thank Q. Bian and Y. Zhou at the Zhejiang Province Key Laboratory of Anti-Cancer Drug Research for their technical support on animal experiments. We thank K. Kong at the Department of Chemistry of Zhejiang University for his theoretical support on CaP crystals. We thank L. Wu, P. Yang, D. Song and G. Zhu at the Center of Cryo-Electron Microscopy (CCEM), Zhejiang University for their technical assistance on cryo-TEM, TEM and SEM.
Author information
Authors and Affiliations
Contributions
C.G., PF.C. and XF.L. designed the project. C.G., PF.C., H.T., Y.Y., Z.H., H.Y., K.P. and PY.C. performed the experiments. C.T., J.X. and L.S. provided technical help. C.G. and H.Y. created the visualizations. C.G., PF.C., S.F. and XF.L. wrote the manuscript. XF.L. and S.F. supervised the project. All of the authors analysed and interpreted the data, and reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
XF.L., C.G. and S.F. are on a patent application (CN 2024100072569) filed by Zhejiang University related to this work. The other authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Hiroki Yokota, Xiang (H.-F.) Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Schematic diagram of migrasome-mediated tumour-TAOC coupling interaction and behaviour-targeted strategy with decoupling–killing therapy by HC&HP@TNL.
Tumour cells induce spatially contacted RANKL-activated osteoclast precursor (RA-OCP) into “Tumasteoclast” (TAOC) to form tumour-TAOC spatiotemporal coupling interaction via migrasome-mediated cytoplasmic transfer. Based on the spatiotemporal characteristics of tumour-TAOC coupling in the initial metastasis, we constructed tetracycline-modified nanoliposomes encapsulating sodium bicarbonate and sodium hydrogen phosphate (HC&HP@TNL). When the bone metastasis reactivates and tumour-TAOC coupling is formed, HC&HP@TNL will be triggered by TAOC to release high concentrations of sodium hydrogen phosphate. Hydrogen phosphate combines with calcium ions in the microenvironment to form in situ calcium-phosphorus (CaP) crystals, which reduce the calcium concentration to inhibit migrasome formation, and disrupt the cell membrane integrity to induce immunogenic cell death (ICD) for immune response.
Extended Data Fig. 2 HC&HP@TNL activates the antitumour immune response.
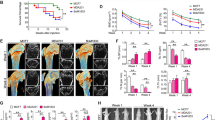
a-c, Schematic representation of an ectopic injection model after CaP-treated 4T1 cell injection (a), distant mammary tumour image (b) and tumour volume (c) (scale bar, 10 mm, n = 8 independent mice, mean ± SD). d-f, Schematic representation of an ectopic injection model after bone metastasis treatment (d), distant mammary tumour image (e) and tumour volume (f) (scale bar, 10 mm, n = 8 independent mice, mean ± SD). g, Flow cytometric analysis of CD80 + CD86+ DCs in draining lymph nodes. h, i, Flow cytometric analysis of CD4 + T cells (h) and CD8 + T cells (i) in the distant mammary tumours (n = 6 independent mice, mean ± SD). j, HE staining and immunofluorescence images of CD8 and CD4 (scale bar, 100μm). k, Distant tumour volume in an ectopic injection model after CaP-treated 4T1 cell injection in immunocompromised mice (scale bar, 10 mm, n = 8 independent mice, mean ± SD). l, Distant tumour volume in an ectopic injection model after bone metastasis treatment in immunocompromised mice (scale bar, 10 mm, n = 4 independent mice for Cl@TNL as control, n = 8 independent mice for HC&HP@TNL, mean ± SD). m, Tumour volume of bone metastasis in BALB/c normal mice (n = 8 independent mice, mean ± SD) and immunocompromised mice (n = 4 independent mice for Cl@TNL, n = 7 independent mice for HC&HP@TNL, mean ± SD). n, Tumour inhibition rate of bone metastasis by HC&HP@TNL in normal BALB/c, immuno-enhanced BALB/c, and immunocompromised BALB/c (Cl@TNL as control of 0%, n = 8 independent mice for normal BALB/c and BALB/c+anti-PD-1, n = 7 independent mice for BALB/c-nu/nu, mean ± SD). o, Inhibition effect percent of CaP effect, ICD effect, and ICB effect in bone metastasis by HC&HP@TNL+anti-PD-1. (n = 8 independent mice, mean ± SD). P values were determined using two-way ANOVA with Šidák’s multiple-comparison test (c, f, k, l), or two-tailed one-way ANOVA with a Tukey post-hoc test (g-i, m-o).
Supplementary information
Supplementary information
Supplementary Discussion 1–12, Figs. 1–16, notes and unprocessed blot images.
Supplementary Data 1
Source Data for the Supplementary Figures.
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 4
Statistical Source Data.
Source Data Fig. 5
Statistical Source Data.
Source Data Extended Data Fig./Table 1
Statistical Source Data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gu, C., Chen, P., Tian, H. et al. Targeting initial tumour–osteoclast spatiotemporal interaction to prevent bone metastasis. Nat. Nanotechnol. (2024). https://doi.org/10.1038/s41565-024-01613-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41565-024-01613-5