Abstract
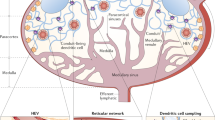
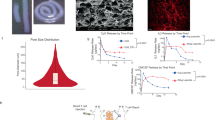
Lymph nodes are crucial organs of the adaptive immune system, orchestrating T cell priming, activation and tolerance. T cell activity and function are highly regulated by lymph nodes, which have a unique structure harbouring distinct cells that work together to detect and respond to pathogen-derived antigens. Here we show that implanted patient-derived freeze-dried lymph nodes loaded with chimeric antigen receptor T cells improve delivery to solid tumours and inhibit tumour recurrence after surgery. Chimeric antigen receptor T cells can be effectively loaded into lyophilized lymph nodes, whose unaltered meshwork and cytokine and chemokine contents promote chimeric antigen receptor T cell viability and activation. In mouse models of cell-line-derived human cervical cancer and patient-derived pancreatic cancer, delivery of chimeric antigen receptor T cells targeting mesothelin via the freeze-dried lymph nodes is more effective in preventing tumour recurrence when compared to hydrogels containing T-cell-supporting cytokines. This tissue-mediated cell delivery strategy holds promise for controlled release of various cells and therapeutics with long-term activity and augmented function.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All the data supporting the findings of this study are available within the article and its Supplementary Information and from the corresponding authors upon reasonable request. Source data are provided with this paper. The GO analysis was based on the Panther database (https://www.pantherdb.org/).
References
Brentjens, R. J. et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 5, 177ra138 (2013).
Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517 (2014).
Hou, A. J., Chen, L. C. & Chen, Y. Y. Navigating CAR T cells through the solid-tumour microenvironment. Nat. Rev. Drug Discov. 20, 531–550 (2021).
Milone, M. C. et al. Engineering-enhanced CAR T cells for improved cancer therapy. Nat. Cancer 2, 780–793 (2021).
Hong, M., Clubb, J. D. & Chen, Y. Y. Engineering CAR T cells for next-generation cancer therapy. Cancer Cell 38, 473–488 (2020).
Rafiq, S., Hackett, C. S. & Brentjens, R. J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 17, 147–167 (2020).
Labanieh, L., Majzner, R. G. & Mackall, C. L. Programming CAR T cells to kill cancer. Nat. Biomed. Eng. 2, 377–391 (2018).
Fu, R. et al. Delivery techniques for enhancing CAR T cell therapy against solid tumors. Adv. Funct. Mater. 31, 2009489 (2021).
Wang, H. & Mooney, D. J. Biomaterial-assisted targeted modulation of immune cells in cancer treatment. Nat. Mater. 17, 761–772 (2018).
Hu, Q. et al. Inhibition of post-surgery tumour recurrence via a hydrogel releasing CAR T cells and anti-PDL1-conjugated platelets. Nat. Biomed. Eng. 5, 1038–1047 (2021).
Stephan, S. B. et al. Biopolymer implants enhance the efficacy of adoptive T cell therapy. Nat. Biotechnol. 33, 97–101 (2015).
Pérez del Río, E. et al. CCL21-loaded 3D hydrogels for T cell expansion and differentiation. Biomaterials 259, 120313 (2020).
Suematsu, S. & Watanabe, T. Generation of a synthetic lymphoid tissue-like organoid in mice. Nat. Biotechnol. 22, 1539–1545 (2004).
Cheung, A. S., Zhang, D. K. Y., Koshy, S. T. & Mooney, D. J. Scaffolds that mimic antigen-presenting cells enable ex vivo expansion of primary T cells. Nat. Biotechnol. 36, 160–169 (2018).
Coon, M. E., Stephan, S. B., Gupta, V., Kealey, C. P. & Stephan, M. T. Nitinol thin films functionalized with CAR T cells for the treatment of solid tumours. Nat. Biomed. Eng. 4, 195–206 (2020).
Grosskopf, A. K. et al. Delivery of CAR T cells in a transient injectable stimulatory hydrogel niche improves treatment of solid tumors. Sci. Adv. 8, eabn8264 (2022).
Wang, K. et al. GD2-specific CAR T cells encapsulated in an injectable hydrogel control retinoblastoma and preserve vision. Nat. Cancer 1, 990–997 (2020).
Ma, L. et al. Enhanced CAR T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science 365, 162–168 (2019).
Krishnamurty, A. T. & Turley, S. J. Lymph node stromal cells: cartographers of the immune system. Nat. Immunol. 21, 369–380 (2020).
Bousso, P. T cell activation by dendritic cells in the lymph node: lessons from the movies. Nat. Rev. Immunol. 8, 675–684 (2008).
Saxena, V. et al. Role of lymph node stroma and microenvironment in T cell tolerance. Immunol. Rev. 292, 9–23 (2019).
Cyster, J. G. Chemokines and cell migration in secondary lymphoid organs. Science 286, 2098–2102 (1999).
von Andrian, U. H. & Mempel, T. R. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 3, 867–878 (2003).
Morton, D. L. et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N. Engl. J. Med. 370, 599–609 (2014).
Leone, A., Diorio, G. J., Pettaway, C., Master, V. & Spiess, P. E. Contemporary management of patients with penile cancer and lymph node metastasis. Nat. Rev. Urol. 14, 335–347 (2017).
Cochran, A. J. et al. Tumour-induced immune modulation of sentinel lymph nodes. Nat. Rev. Immunol. 6, 659–670 (2006).
Zhang, C. et al. Mapping the spreading routes of lymphatic metastases in human colorectal cancer. Nat. Commun. 11, 1993 (2020).
Tasnim, H. et al. Quantitative measurement of naïve T cell association with dendritic cells, FRCs, and blood vessels in lymph nodes. Front. Immunol. 9, 1571 (2018).
Liu, W. F. et al. Real-time in vivo detection of biomaterial-induced reactive oxygen species. Biomaterials 32, 1796–1801 (2011).
Vella, A. T., Dow, S., Potter, T. A., Kappler, J. & Marrack, P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc. Natl Acad. Sci. USA 95, 3810–3815 (1998).
Hermans, D. et al. Lactate dehydrogenase inhibition synergizes with IL-21 to promote CD8+ T cell stemness and antitumor immunity. Proc. Natl Acad. Sci. USA 117, 6047–6055 (2020).
Castro, F., Cardoso, A. P., Gonçalves, R. M., Serre, K. & Oliveira, M. J. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front. Immunol. 9, 847 (2018).
Assarsson, E. et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS ONE 9, e95192 (2014).
Onder, L. et al. IL-7–producing stromal cells are critical for lymph node remodeling. Blood 120, 4675–4683 (2012).
Mueller, S. N. & Germain, R. N. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat. Rev. Immunol. 9, 618–629 (2009).
Gattinoni, L., Speiser, D. E., Lichterfeld, M. & Bonini, C. T memory stem cells in health and disease. Nat. Med. 23, 18–27 (2017).
Mahnke, Y. D., Brodie, T. M., Sallusto, F., Roederer, M. & Lugli, E. The who’s who of T cell differentiation: human memory T cell subsets. Eur. J. Immunol. 43, 2797–2809 (2013).
Schluns, K. S., Kieper, W. C., Jameson, S. C. & Lefrançois, L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat. Immunol. 1, 426–432 (2000).
Geginat, J., Sallusto, F. & Lanzavecchia, A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4+ T cells. J. Exp. Med. 194, 1711–1720 (2001).
Grabstein, K. H. et al. Cloning of a T cell growth factor that interacts with the β chain of the interleukin-2 receptor. Science 264, 965–968 (1994).
Schluns, K. S. & Lefrançois, L. Cytokine control of memory T cell development and survival. Nat. Rev. Immunol. 3, 269–279 (2003).
Melenhorst, J. J. et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 602, 503–509 (2022).
Propper, D. J. & Balkwill, F. R. Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 19, 237–253 (2022).
Briukhovetska, D. et al. Interleukins in cancer: from biology to therapy. Nat. Rev. Cancer 21, 481–499 (2021).
Kagoya, Y. et al. A novel chimeric antigen receptor containing a JAK–STAT signaling domain mediates superior antitumor effects. Nat. Med. 24, 352–359 (2018).
Hurton, L. V. et al. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc. Natl Acad. Sci. USA 113, E7788–E7797 (2016).
Tang, L. et al. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat. Biotechnol. 36, 707–716 (2018).
Liu, Y. et al. Cytokine conjugation to enhance T cell therapy. Proc. Natl Acad. Sci. USA 120, e2213222120 (2023).
Li, A. M. et al. Checkpoint inhibitors augment CD19-directed chimeric antigen receptor (CAR) T cell therapy in relapsed B cell acute lymphoblastic leukemia. Blood 132, 556 (2018).
Zhang, J. et al. Non-viral, specifically targeted CAR T cells achieve high safety and efficacy in B-NHL. Nature 609, 369–374 (2022).
Link, A. et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat. Immunol. 8, 1255–1265 (2007).
Horsnell, H. L. et al. Lymph node homeostasis and adaptation to immune challenge resolved by fibroblast network mechanics. Nat. Immunol. 23, 1169–1182 (2022).
Nam, S., Seo, B. R., Najibi, A. J., McNamara, S. L. & Mooney, D. J. Active tissue adhesive activates mechanosensors and prevents muscle atrophy. Nat. Mater. 22, 249–259 (2023).
Gong, N. et al. In situ PEGylation of CAR T cells alleviates cytokine release syndrome and neurotoxicity. Nat. Mater. 22, 1571–1580 (2023).
Acknowledgements
We thank the National Key R&D Program of China (2021YFA0909900), the National Natural Science Foundation of China (52173142 and 52233013) and Zhejiang University for the grants from the Startup Package.
Author information
Authors and Affiliations
Contributions
Z.G., H.L. and P.Z. initiated the project concept. J. Shi, W. Wu, D.C., Z.L., T.S., Y.W., Y.Y., Q.W., F.L., R.Z., C.Z. and X.S. performed the experiments and collected the data. Z.M., Y.D. and W. Wang provided support for the PDX model. G.D., J. Sun, X.L., W.F. and D.C. helped with data analysis. J. Shi, W. Wu, H.L. and Z.G. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
Related patents have been applied for by Z.G., H.L., J. Shi and W. Wu (patent applicant: Zhejiang University; name of inventors: Zhen Gu, Hongjun Li, Jiaqi Shi, Wei Wu; application number: 2022113666477; status of application: substantive examination; specific aspect of paper covered in patent application: preparation of CAR T cell-loaded lyophilizing lymph nodes and its anti-tumour application). Z.G. is the cofounder of Zenomics Inc., ZCapsule Inc. and µZen Inc. The other authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Christopher Jewell and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–26 and Table 1.
Source data
Source Data Figs. 1–5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, J., Wu, W., Chen, D. et al. Lyophilized lymph nodes for improved delivery of chimeric antigen receptor T cells. Nat. Mater. (2024). https://doi.org/10.1038/s41563-024-01825-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41563-024-01825-z