Abstract
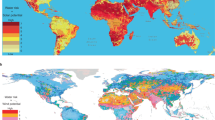
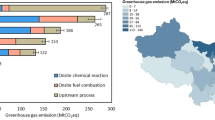
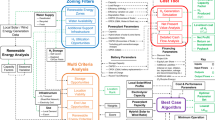
Maintaining global warming well below 2 °C, as stipulated in the Paris Agreement, will require a complete overhaul of the world energy system. Hydrogen is considered to be a key component of the decarbonization strategy for large parts of the transport system, as well as some heavy industries. Today, about 96% of current hydrogen production comes from the steam reforming of coal or natural gas (labelled black and grey hydrogen, respectively). If hydrogen is to become a solution, then black and grey hydrogen need to be replaced by a low-carbon option. One method that has received much attention is to produce so-called green hydrogen by coupling water electrolysis with renewable energies. However, green hydrogen is expensive and energy-intensive to produce. Here, we explore an alternative option and highlight the benefits of rock-based hydrogen (white and orange) compared with classic electrolysis-based technologies. We show that the exploitation of native hydrogen and its combination with carbon sequestration has the potential to fuel a large part of the energy transition without the substantial energy and raw material cost of green hydrogen.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
World Energy Outlook 2021 (IEA, 2021); www.iea.org/weo
Blanc, P. et al. Thermoddem: a geochemical database focused on low temperature water/rock interactions and waste materials. Appl. Geochem. 27, 2107–2116 (2012).
The Future of Hydrogen (IEA, 2019); https://www.iea.org/reports/the-future-of-hydrogen
Howarth, R. W. & Jacobson, M. Z. How green is blue hydrogen? Energy Sci. Eng. 9, 1676–1687 (2021).
Zgonnik, V. The occurrence and geoscience of natural hydrogen: a comprehensive review. Earth-Sci. Rev. 203, 103140 (2020).
Klein, F. et al. Iron partitioning and hydrogen generation during serpentinization of abyssal peridotites from 15°N on the Mid-Atlantic Ridge. Geochim. Cosmochim. Acta 73, 6868–6893 (2009).
Kelley, D. S. et al. A serpentinite-hosted ecosystem: the lost city hydrothermal field. Science 307, 1428–1434 (2005).
Cannat, M., Fontaine, F. & Escartín, J. in Serpentinization and Associated Hydrogen and Methane Fluxes at Slow Spreading Ridges (eds Rona, P. A. et al.) 241–264 (American Geophysical Union SERIES, 2010).
Neal, C. & Stanger, G. Hydrogen generation from mantle source rocks in Oman. Earth Planet. Sci. Lett. 66, 315–320 (1983).
Worman, S. L., Pratson, L. F., Karson, J. A. & Klein, E. M. Global rate and distribution of H2 gas produced by serpentinization within oceanic lithosphere. Geophys. Res. Lett. 43, 6435–6443 (2016).
Geymond, U., Ramanaidou, E., Lévy, D., Ouaya, A. & Moretti, I. Can weathering of banded iron formations generate natural hydrogen? Evidence from Australia, Brazil and South Africa. Minerals 12, 163 (2022).
Truche, L. et al. Hydrogen generation during hydrothermal alteration of peralkaline granite. Geochim. Cosmochim. Acta https://doi.org/10.1016/j.gca.2021.05.048 (2021).
Murray, J. et al. Abiotic hydrogen generation from biotite-rich granite: a case study of the Soultz-sous-Forêts geothermal site, France. Appl. Geochem. https://doi.org/10.1016/j.apgeochem.2020.104631 (2020).
Etiope, G., Schoell, M. & Hosgörmez, H. Abiotic methane flux from the Chimaera seep and Tekirova ophiolites (Turkey): understanding gas exhalation from low temperature serpentinization and implications for Mars. Earth Planet. Sci. Lett. 310, 96–104 (2011).
Gaucher, E. C. New perspectives in the industrial exploration for native hydrogen. Elements 16, 8–9 (2020).
Lefeuvre, N. et al. Native H2 exploration in the western Pyrenean foothills. Geochem. Geophys. Geosyst. 22, e2021GC009917 (2021).
Moretti, I., Brouilly, E., Loiseau, K., Prinzhofer, A. & Deville, E. Hydrogen emanations in intracratonic areas: new guide lines for early exploration basin screening. Geosciences 11, 145 (2021).
Guélard, J. et al. Natural H2 in Kansas: deep or shallow origin? Geochem. Geophys. Geosyst. 18, 1841–1865 (2017).
Prinzhofer, A., Tahara Cissé, C. S. & Diallo, A. B. Discovery of a large accumulation of natural hydrogen in Bourakebougou (Mali). Int. J. Hydrog. Energy 43, 19315–19326 (2018).
Kularatne, K. et al. Simultaneous ex-situ CO2 mineral sequestration and hydrogen production from olivine-bearing mine tailings. Appl. Geochem. 95, 195–205 (2018).
Kelemen, P. B. et al. Rates and mechanisms of mineral carbonation in peridotite: natural processes and recipes for enhanced, in situ CO2 capture and storage. Annu. Rev. Earth Planet. Sci. 39, 545–576 (2011).
Kelemen, P. et al. In situ carbon mineralization in ultramafic rocks: natural processes and possible engineered methods. Energy Procedia 146, 92–102 (2018).
Gaillardet, J., Dupré, B., Louvat, P. & Allègre, C. J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 159, 3–30 (1999).
Matter, J. M. & Kelemen, P. B. Permanent storage of carbon dioxide in geological reservoirs by mineral carbonation. Nat. Geosci. 2, 837–841 (2009).
Gíslason, S. R., Sigurdardóttir, H., Aradóttir, E. S. & Oelkers, E. H. A brief history of Carbfix: challenges and victories of the project’s pilot phase. Energy Procedia 146, 103–114 (2018).
Snæbjörnsdóttir, S. et al. Carbon dioxide storage through mineral carbonation. Nat. Rev. Earth Environ. 1, 90–102 (2020).
McGrail, B. P. et al. Wallula Basalt Pilot Demonstration Project: post-injection results and conclusions. Energy Procedia 114, 5783–5790 (2017).
Combaudon, V. & Moretti, I. Generation of hydrogen along the Mid-Atlantic Ridge: onshore and offshore. Geol. Earth Mar. Sci. 3, 1–14 (2021).
Voigt, M. et al. An experimental study of basalt-seawater-CO2 interaction at 130 °C. Geochim. Cosmochim. Acta 308, 21–41 (2021).
Lawley, C. J. et al. Precious metal mobility during serpentinization and breakdown of base metal sulphide. Lithos 105278, 354–355 (2020).
Lagneau, V., Regnault, O. & Descostes, M. Industrial deployment of reactive transport simulation: an application to uranium in situ recovery. Rev. Mineral. Geochem. 85, 499–528 (2019).
Stringfellow, W. T. & Dobson, P. F. Technology for the recovery of lithium from geothermal brines. Energies 14, 6805 (2021).
O’Connor, W. et al. Aqueous Mineral Carbonation: Mineral Availability, Pretreatment, Reaction Parametrics, and Process Studies Technical Report DOE/ARC-TR-04-002 (US DOE, 2005).
Oelkers, E. H., Declercq, J., Saldi, G. D., Gislason, S. R. & Schott, J. Olivine dissolution rates: a critical review. Chem. Geol. 500, 1–19 (2018).
Wang, J., Watanabe, N., Okamoto, A., Nakamura, K. & Komai, T. Pyroxene control of H2 production and carbon storage during water-peridotite-CO2 hydrothermal reactions. Int. J. Hydrog. Energy 44, 26835–26847 (2019).
Chizmeshya, A. V. G., McKelvy, M. J., Squires, K., Carpenter, R. W. & Bearat, H. A Novel Approach to Mineral Carbonation: Enhancing Carbonation While Avoiding Mineral Pretreatment Process Cost (Arizona State University, 2007); http://www.osti.gov/servlets/purl/924162-e8RuYF/.arXiv:1011.1669v3
Lamadrid, H. M., Zajacz, Z., Klein, F. & Bodnar, R. J. Synthetic fluid inclusions XXIII. Effect of temperature and fluid composition on rates of serpentinization of olivine. Geochim. Cosmochim. Acta https://doi.org/10.1016/j.gca.2020.08.009 (2020).
Kelemen, P. B. & Matter, J. M. In situ carbonation of peridotite for CO2 storage. Proc. Natl Acad. Sci. USA 105, 17295–17300 (2008).
Gunnarsson, I. et al. The rapid and cost-effective capture and subsurface mineral storage of carbon and sulfur at the Carbfix2 site. Int. J. Greenh. Gas. Control 79, 117–126 (2018).
Klein, F. et al. Fluids in the crust. Experimental constraints on fluid-rock reactions during incipient serpentinization of harzburgite. Am. Mineral. https://doi.org/10.2138/am-2015-5112 (2015).
Grozeva, N. G., Klein, F., Seewald, J. S. & Sylva, S. P. Experimental study of carbonate formation in oceanic peridotite. Geochim. Cosmochim. Acta 199, 264–286 (2017).
Fauguerolles, C. Etude Expérimentale de la Production d’H2 Associée à la Serpentinisation des Péridotites au Niveau des Dorsales Océaniques Lentes. PhD thesis, Univ. d’Orléans (2016).
Osselin, F., Pichavant, M., Champallier, R., Ulrich, M. & Raimbourg, H. Reactive transport experiments of coupled carbonation and serpentinization in a natural serpentinite. Implication for hydrogen production and carbon geological storage. Geochim. Cosmochim. Acta 318, 165–189 (2022).
Menzel, M. D. et al. Carbonation of mantle peridotite by CO2-rich fluids: the formation of listvenites in the Advocate ophiolite complex (Newfoundland, Canada). Lithos 323, 238–261 (2018).
Jamtveit, B., Austrheim, H. & Malthe-Sorenssen, A. Accelerated hydration of the Earth’s deep crust induced by stress perturbations. Nature 408, 75–78 (2000).
Osselin, F. et al. Stress from NaCl crystallisation by carbon dioxide injection in aquifers. Environ. Geotech. 2, 280–291 (2015).
Zhu, W. et al. Experimental evidence of reaction-induced fracturing during olivine carbonation. Geophys. Res. Lett. 43, 9535–9543 (2016).
van Noort, R., Wolterbeek, T., Drury, M., Kandianis, M. & Spiers, C. The force of crystallization and fracture propagation during in-situ carbonation of peridotite. Minerals 7, 190 (2017).
Luhmann, A. J. et al. Chemical and physical changes during seawater flow through intact dunite cores: an experimental study at 150-200 °C. Geochim. Cosmochim. Acta 214, 86–114 (2017).
The Role of Critical Minerals in Clean Energy Transitions (IEA, 2021); https://www.iea.org/reports/the-role-of-critical-minerals-in-clean-energy-transitions
Author information
Authors and Affiliations
Contributions
F.O. was responsible for the design of the study, drafting the article and data acquisition. C.F. carried out data acquisition. C.S., E.C.G. and B.S. made revisions. M.P. was responsible for the design of the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Geoscience thanks Isabelle Moretti and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Osselin, F., Soulaine, C., Fauguerolles, C. et al. Orange hydrogen is the new green. Nat. Geosci. 15, 765–769 (2022). https://doi.org/10.1038/s41561-022-01043-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41561-022-01043-9
This article is cited by
-
Risk of the hydrogen economy for atmospheric methane
Nature Communications (2022)