Abstract
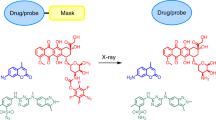
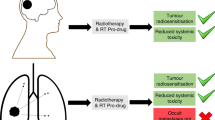
Radiotherapy-induced prodrug activation provides an ideal solution to reduce the systemic toxicity of chemotherapy in cancer therapy, but the scope of the radiation-activated protecting groups is limited. Here we present that the well-established photoinduced electron transfer chemistry may pave the way for developing versatile radiation-removable protecting groups. Using a functional reporter assay, N-alkyl-4-picolinium (NAP) was identified as a caging group that efficiently responds to radiation by releasing a client molecule. When evaluated in a competition experiment, the NAP moiety is more efficient than other radiation-removable protecting groups discovered so far. Leveraging this property, we developed a NAP-derived carbamate linker that releases fluorophores and toxins on radiation, which we incorporated into antibody–drug conjugates (ADCs). These designed ADCs were active in living cells and tumour-bearing mice, highlighting the potential to use such a radiation-removable protecting group for the development of next-generation ADCs with improved stability and therapeutic effects.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this study are included in the article and its Supplementary Information. Source data are provided with this paper.
References
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73, 17–48 (2023).
Delaney, G., Jacob, S., Featherstone, C. & Barton, M. The role of radiotherapy in cancer treatment. Cancer 104, 1129–1137 (2005).
Lan, G. et al. Nanoscale metal–organic layers for deeply penetrating X-ray-induced photodynamic therapy. Angew. Chem. Int. Ed. 56, 12102–12106 (2017).
Chen, X., Song, J., Chen, X. & Yang, H. X-ray-activated nanosystems for theranostic applications. Chem. Soc. Rev. 48, 3073–3101 (2019).
Dearnaley, D. P. et al. Comparison of radiation side-effects of conformal and conventional radiotherapy in prostate cancer: a randomised trial. Lancet 353, 267–272 (1999).
De Ruysscher, D. et al. Radiotherapy toxicity. Nat. Rev. Dis. Primers 5, 13 (2019).
Singleton, D. C., Macann, A. & Wilson, W. R. Therapeutic targeting of the hypoxic tumour microenvironment. Nat. Rev. Clin. Oncol. 18, 751–772 (2021).
Raza, A. et al. Endogenous and exogenous stimuli-responsive drug delivery systems for programmed site-specific release. Molecules 24, 1117 (2019).
Nishimoto, S., Hatta, H., Ueshima, H. & Kagiya, T. 1-(5′-fluoro-6′-hydroxy-5′,6′-dihydrouracil-5′-yl)-5-fluorouracil, a novel N(1)-C(5)-linked dimer that releases 5-fluorouracil by radiation activation under hypoxic conditions. J. Med. Chem. 35, 2711–2712 (1992).
Shibamoto, Y., Zhou, L., Hatta, H., Mori, M. & Nishimoto, S.-I. A novel class of antitumor prodrug, 1-(2′-oxopropyl)-5-fluorouracil (OFU001), that releases 5-fluorouracil upon hypoxic irradiation. Jpn J. Cancer Res. 91, 433–438 (2000).
Tanabe, K., Ishizaki, J., Ando, Y., Ito, T. & Nishimoto, S.-I. Reductive activation of 5-fluorodeoxyuridine prodrug possessing azide methyl group by hypoxic X-irradiation. Bioorg. Med. Chem. Lett. 22, 1682–1685 (2012).
Ahn, G. O. et al. Radiolytic and cellular reduction of a novel hypoxia-activated cobalt(III) prodrug of a chloromethylbenzindoline DNA minor groove alkylator. Biochem. Pharmacol. 71, 1683–1694 (2006).
Wu, L. et al. Generation of hydroxyl radical-activatable ratiometric near-infrared bimodal probes for early monitoring of tumor response to therapy. Nat. Commun. 12, 6145 (2021).
Hall, E. J. & Giaccia, A. J. Radiobiology for the Radiologist, Vol. 6 (Lippincott Wilkins & Williams, 2006).
Le Caer, S. Water radiolysis: influence of oxide surfaces on H2 production under ionizing radiation. Water 3, 235–253 (2011).
Buxton, G. V., Greenstock, C. L., Helman, W. P. & Ross, A. B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O– in aqueous solution. J. Phys. Chem. 17, 513–886 (1988).
Fu, Q. et al. External-radiation-induced local hydroxylation enables remote release of functional molecules in tumors. Angew. Chem. Int. Ed. 59, 21546–21552 (2020).
Geng, J. et al. Switching on prodrugs using radiotherapy. Nat. Chem. 13, 805–810 (2021).
Guo, Z. et al. Radiotherapy-induced cleavage of quaternary ammonium groups activates prodrugs in tumors. Angew. Chem. Int. Ed. 61, e202205014 (2022).
Ding, Z. et al. Radiotherapy reduces N-oxides for prodrug activation in tumors. J. Am. Chem. Soc. 144, 9458–9464 (2022).
Yang, C., Yang, Y., Li, Y., Ni, Q. & Li, J. Radiotherapy-triggered proteolysis targeting chimera prodrug activation in tumors. J. Am. Chem. Soc. 145, 385–391 (2023).
Quintana, J. M. et al. Radiation cleaved drug-conjugate linkers enable local payload release. Bioconjugate Chem. 33, 1474–1484 (2022).
Petit, M. et al. X-ray photolysis to release ligands from caged reagents by an intramolecular antenna sensitive to magnetic resonance imaging. Angew. Chem. Int. Ed. 50, 9708–9711 (2011).
Barosi, A. et al. Synthesis and activation of an iron oxide immobilized drug-mimicking reporter under conventional and pulsed X-ray irradiation conditions. RSC Adv. 10, 3366–3370 (2020).
Guesdon-Vennerie, A. et al. Breaking photoswitch activation depth limit using ionising radiation stimuli adapted to clinical application. Nat. Commun. 13, 4102 (2022).
Fu, Q. et al. Bioorthogonal chemistry for prodrug activation in vivo. Chem. Soc. Rev. 52, 7737–7772 (2023).
Romero, N. A. & Nicewicz, D. A. Organic photoredox catalysis. Chem. Rev. 116, 10075–10166 (2016).
Weinstain, R., Slanina, T., Kand, D. & Klán, P. Visible-to-NIR-light activated release: from small molecules to nanomaterials. Chem. Rev. 120, 13135–13272 (2020).
Jonas, M., Blechert, S. & Steckhan, E. Photochemically induced electron transfer (PET) catalyzed radical cyclization: a practical method for inducing structural changes in peptides by formation of cyclic amino acid derivatives. J. Org. Chem. 66, 6896–6904 (2001).
Talla, A. et al. Metal-free photocatalytic aerobic oxidation of thiols to disulfides in batch and continuous-flow. Adv. Synth. Catal. 357, 2180–2186 (2015).
Holbrook, R. J., Weinberg, D. J., Peterson, M. D., Weiss, E. A. & Meade, T. J. Light-activated protein inhibition through photoinduced electron transfer of a ruthenium(II)–cobalt(III) bimetallic complex. J. Am. Chem. Soc. 137, 3379–3385 (2015).
Bogdanov, A. M. et al. Turning on and off photoinduced electron transfer in fluorescent proteins by π-stacking, halide binding, and tyr145 mutations. J. Am. Chem. Soc. 138, 4807–4817 (2016).
Niu, H. et al. Photoinduced electron transfer (PeT) based fluorescent probes for cellular imaging and disease therapy. Chem. Soc. Rev. 52, 2322–2357 (2023).
Sundararajan, C. & Falvey, D. E. C−O bond fragmentation of 4-picolyl- and N-methyl-4-picolinium esters triggered by photochemical electron transfer. J. Org. Chem. 69, 5547–5554 (2004).
Camble, R., Garner, R. & Young, G. T. Novel facilitation of peptide synthesis. Nature 217, 247–248 (1968).
Xu, M. et al. An antibody-radionuclide conjugate targets fibroblast activation protein for cancer therapy. Eur. J. Nucl. Med. Mol. Imaging 50, 3214–3224 (2023).
Wang, Q. et al. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature 579, 421–426 (2020).
Acknowledgements
We thank J. Li and M. Zhai at Peking University for 60Co Source. We thank the facility support from the Analytical Instrumentation Center of Peking University. This study was funded by the National Nature Science Foundation of China (grant no. 22225603), the Ministry of Science and Technology of the People’s Republic of China (grant no. 2021YFA1601400), the Beijing Municipal Natural Science Foundation (grant no. Z200018) and Changping Laboratory to Z.L.
Author information
Authors and Affiliations
Contributions
Z.L. conceived the study. Q.F., assisted by Z.G., S.S., Y.B., X.W. and P.S., performed chemical analysis, material synthesis and characterization. Q.F., assisted by S.S., J.C., M.X., Y.B., X.W. and D.L., performed radiosynthesis, positron emission tomography–computed tomography imaging, biodistribution and data analysis. Q.F., assisted by Z.G. and S.S., performed the cell viability assay. Z.G. analysed the NMR spectra and theoretical calculations. Q.F., assisted by Z.G. and S.S., performed all other experiments. Q.F., Z.G., S.S. and Z.L. analysed the data. Z.L. wrote the paper with inputs from all authors. All authors discussed the results and commented on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Benoit Paquette, Jeremy Quintana and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Mechanism study of radiation-induced functional molecule release from picolinium compound.
(A) Proposed mechanism of the radiation-induced release reaction. The reaction yields radical intermediates and releases protected acetic acid by a one-electron reduction after 4-[(Acetyloxy)methyl]-1-methylpyridinium (compound 7’) irradiated with high-energy radiation. (B) 1H NMR spectrum of 7’ (up) and the radiation-irradiated product (bottom). A new single peak arose with a chemical shift of 2.10 ppm in D2O indicating the release of acetic acid. (C) UPLC chromatogram of a deoxygen solution containing 2 mM TEMPO and 1 mM 7’ with/without receiving a dose of 5,000 Gy γ-ray. The detector wavelength is 254 nm. (D) The mass spectral signal corresponding to the peak with a retention time of 2.26 minutes matches the molecular weight of the captured intermediate adduct. (E) UPLC chromatogram of 1 mM 7’ with/without received a dose of 5,000 Gy γ-ray. The detector wavelength is 254 nm. (F) The mass spectral signal corresponds to the peak with a retention time of 1.07 minutes, which matches the molecular weight of 7’. (G) The mass spectral signal corresponds to the peak with a retention time of 0.64 minutes, which matches the molecular weight of methylpicolinium.
Extended Data Fig. 2 Colony formation assay of 4T1-FAP cells treated with radiotherapy-induced NAPC-ADC activation.
4T1-FAP cells cultured in 6-well cell culture plates were treated as shown. (A) Photograph of colony formation in which colonies are stained with crystal violet. (B, C) Quantitative analysis of the number of colonies consisting of at least 50 cells under different treatments (n = 6, mean ± s.d.). The number of colonies in the group without ADC treatment and without X-ray irradiation in a normoxic environment was defined as 100%. Three independent experiments were performed and representative results are shown.
Extended Data Fig. 3 Pharmacokinetic studies of NAPC-ADC through positron emission tomography imaging and biodistribution.
(A) Representative positron emission tomography–computed tomography imaging of [89Zr]NAPC-ADC in BALB/c mice. Three independent experiments were performed and representative results are shown. (B) Time activity curves of [89Zr]NAPC-ADC in the blood (n = 3 independent experiments; 3 mice, mean ± s.d.). Mice received 14.8 MBq of [89Zr]NAPC-ADC (0.15 MBq/μg). Biodistribution of [89Zr]NAPC-ADC in both (C) BALB/c mice and (D) 4T1-FAP tumour-bearing BALB/c mice were performed at the indicated time points (n = 5 independent biological samples from 5 mice per group, mean ± s.d.). Mice received 0.74 MBq of [89Zr]NAPC-ADC (7.4 kBq/μg).
Extended Data Fig. 4 Blood biochemistry and complete blood panel analysis to evaluate the toxicology of NAPC-ADC in blood.
Healthy BALB/c mice were intravenously injected with PBS or NAPC-ADC (5 mg/kg) through the tail vein. Blood samples were collected at a series of time points: 1-, 3-, 7-, 14-, and 30-days post-injection (n = 6 independent biological samples, mean ± s.d.). The analysis reveals almost negligible difference in blood biochemical parameters and complete blood count, indicating the absence of any toxicological effects caused by NAPC-ADC on the blood system. (RBC, red blood cell; HGB, hemoglobin; HCT, hematocrit; MCHC, mean corpuscular hemoglobin concentration; WBC, white blood cells; NEUT, neutrophils; Lym, lymphocyte; Mon, monocyte; PLT, platelet; ALT, alanine transaminase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; TBIL, total bilirubin; TP, total protein; ALB, albumin; GLO, globulin; UREA, urea; CRE, creatinine; Ca, calcium; P, phosphate).
Extended Data Fig. 5 Synthetic routes of related compounds.
(A) Synthetic route of compounds 1 and 7. Compounds 2–6 and 8–9 shared the same synthesis route. (B) Synthetic route of NAPC-AMC. (C) Synthetic route of NAPC-MeRho. (D) Synthetic route of the payload of NAPC-ADC.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–17, NMR spectra and high-resolution mass spectra (Figs. 18–65).
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, Q., Gu, Z., Shen, S. et al. Radiotherapy activates picolinium prodrugs in tumours. Nat. Chem. (2024). https://doi.org/10.1038/s41557-024-01501-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41557-024-01501-4