Abstract
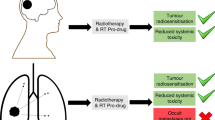
Chemotherapy is a powerful tool in the armoury against cancer, but it is fraught with problems due to its global systemic toxicity. Here we report the proof of concept of a chemistry-based strategy, whereby gamma/X-ray irradiation mediates the activation of a cancer prodrug, thereby enabling simultaneous chemo-radiotherapy with radiotherapy locally activating a prodrug. In an initial demonstration, we show the activation of a fluorescent probe using this approach. Expanding on this, we show how sulfonyl azide- and phenyl azide-caged prodrugs of pazopanib and doxorubicin can be liberated using clinically relevant doses of ionizing radiation. This strategy is different to conventional chemo-radiotherapy radiation, where chemo-sensitization of the cancer takes place so that subsequent radiotherapy is more effective. This approach could enable site-directed chemotherapy, rather than systemic chemotherapy, with ‘real time’ drug decaging at the tumour site. As such, it opens up a new era in targeted and directed chemotherapy.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All relevant data supporting the findings of this study are available within the paper and its Supplementary Information files.
References
Rautio, J. et al. Prodrugs: design and clinical applications. Nat. Rev. Drug Discov. 7, 255–270 (2008).
Miwa, M. et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur. J. Cancer 34, 1274–1281 (1998).
Krasnovskaya, O. O. et al. Thiourea modified doxorubicin: a perspective pH-sensitive prodrug. Bioconjug. Chem. 30, 741–750 (2019).
Ohwada, J. et al. Synthesis and biological activities of a pH-dependently activated water-soluble prodrug of a novel hexacyclic camptothecin analog. Bioorg. Med. Chem. Lett. 19, 2772–2776 (2009).
Swift, L. P., Cutts, S. M., Rephaeli, A., Nudelman, A. & Phillips, D. R. Activation of adriamycin by the pH-dependent formaldehyde-releasing prodrug hexamethylenetetramine. Mol. Cancer Ther. 2, 189–198 (2003).
Bentebibel, S.-E. et al. A first-in-human study and biomarker analysis of NKTR-214, a novel IL2Rβγ-biased cytokine, in patients with advanced or metastatic solid tumors. Cancer Discov. 9, 711–721 (2019).
Mustafa, A. A., Rajan, R., Suarez, J. D. & Alzghari, S. K. A review of the opioid analgesic benzhydrocodone-acetaminophen. Cureus 10, e2844 (2018).
Charych, D. et al. Modeling the receptor pharmacology, pharmacokinetics and pharmacodynamics of NKTR-214, a kinetically-controlled interleukin-2 (IL2) receptor agonist for cancer immunotherapy. PLoS ONE 12, e0179431 (2017).
Norman, D. J. et al. Electrodrugs: an electrochemical prodrug activation strategy. Chem. Commun. 54, 9242–9245 (2018).
Bezagu, M. et al. In situ targeted activation of an anticancer agent using ultrasound-triggered release of composite droplets. Eur. J. Med. Chem. 142, 2–7 (2017).
Hossion, A. M. L., Bio, M., Nkepang, G., Awuah, S. G. & You, Y. Visible light controlled release of anticancer drug through double activation of prodrug. ACS Med. Chem. Lett. 4, 124–127 (2012).
Sauer, R. et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. New Engl. J. Med. 351, 1731–1740 (2004).
Rosenzweig, K. E. & Gomez, J. E. Concurrent chemotherapy and radiation therapy for inoperable locally advanced non-small-cell lung cancer. J. Clin. Oncol. 35, 6–10 (2017).
Alvarado-Miranda, A. et al. Concurrent chemo-radiotherapy following neoadjuvant chemotherapy in locally advanced breast cancer. Radiat. Oncol. 4, 24–28 (2009).
Mao, X. et al. An agent-based model for drug-radiation interactions in the tumour microenvironment: hypoxia-activated prodrug SN30000 in multicellular tumour spheroids. PLoS Comput. Biol. 14, e1006469 (2018).
Takakusagi, Y. et al. Radiotherapy synergizes with the hypoxia-activated prodrug evofosfamide: in vitro and in vivo studies. Antioxid. Redox Signal. 28, 131–140 (2018).
Nytko, K. J. et al. The hypoxia-activated prodrug evofosfamide in combination with multiple regimens of radiotherapy. Oncotarget 8, 23702–23712 (2017).
Karzmark, C. J. Advances in linear accelerator design for radiotherapy, advances in linear accelerator design for radiotherapy. Med. Phys. 11, 105–128 (1984).
Wroe, L. M. et al. Comparative analysis of radiotherapy linear accelerator downtime and failure modes in the UK, Nigeria and Botswana. Clin. Oncol. 32, e111–e118 (2020).
Shtarkman, I. N., Gudkov, S. V., Chernikov, A. V. & Bruskov, V. I. X-ray- and heat-induced generation of hydrogen peroxide and hydroxyl radicals in aqueous solutions of l-amino acids. Biophysics 53, 1–7 (2008).
Riley, P. A. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int. J. Radiat. Biol. 65, 27–33 (2009).
Sutherland, B. M., Bennett, P. V., Sutherland, J. C. & Laval, J. Clustered DNA damages induced by X-rays in human cells. Radiat. Res. 157, 611–616 (2002).
Sutherland, B. M., Bennett, P. V., Sidorkina, O., Laval, J. & Clustered, D. N. A. Damages induced in isolated DNA and in human cells by low doses of ionizing radiation. Proc. Natl. Acad. Sci. USA 97, 103–108 (2000).
Lomax, M. E., Folkes, L. K. & O’Neill, P. Biological consequences of radiation-induced DNA damage: relevance to radiotherapy. Clin. Oncol. 25, 578–585 (2013).
Valerie, K. et al. Radiation-induced cell signaling: inside-out and outside-in. Mol. Cancer Ther. 6, 789–801 (2007).
Sahin, E. et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 470, 359–365 (2011).
O’Neill, P. & Wardman, P. Radiation chemistry comes before radiation biology. Int. J. Radiat. Biol. 85, 9–25 (2009).
Azzam, E. I., Jay-Gerin, J.-P. & Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 327, 48–60 (2012).
Nordsmark, M. et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother. Oncol. 77, 18–24 (2005).
Kuzmin, G. N., Knatko, M. V. & Kurganov, S. V. Light and X-ray-induced chemistry of methane on TiO2. React. Kinet. Catal. Lett. 23, 313–317 (1983).
Barner-Kowollik, C., Vana, P., Quinn, J. F. & Davis, T. P. Long-lived intermediates in reversible addition-fragmentation chain-transfer (RAFT) polymerization generated by γ radiation. J. Polym. Sci. Pol. Chem. 40, 1058–1063 (2002).
Yang, Y. et al. Photodecomposition of thienylsulfonyl azides: generation and spectroscopic characterization of triplet thienylsulfonyl nitrenes and 3-thienylnitrene. J. Phys. Chem. A 123, 9311–9320 (2019).
Reagan, M. T. & Nickon, A. The photolysis of sulfonyl azides in isopropyl alcohol. J. Am. Chem. Soc. 90, 4096–4105 (1968).
Dermer, O. C. & Edmison, M. T. Orientation in aromatic substitution by the benzenesulfonimido radical. J. Am. Chem. Soc. 77, 70–73 (1955).
Bukowski, R. M., Yasothan, U. & Kirkpatrick, P. Pazopanib. Nat. Rev. Drug Discov. 9, 17–18 (2010).
Sleijfer, S. et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European Organisation for Research and Treatment of Cancer–Soft Tissue and Bone Sarcoma Group (EORTC study 62043). J. Clin. Oncol. 27, 3126–3132 (2009).
Stevens, M. Y., Sawant, R. T. & Odell, L. R. Synthesis of sulfonyl azides via diazotransfer using an imidazole-1-sulfonyl azide salt: scope and 15N NMR labeling experiments. J. Org. Chem. 79, 4826–4831 (2014).
Zhu, X.-D. et al. Antiangiogenic effects of pazopanib in xenograft hepatocellular carcinoma models: evaluation by quantitative contrast-enhanced ultrasonography. BMC Cancer 11, 307 (2011).
Matikonda, S. S. et al. Mechanistic evaluation of bioorthogonal decaging with trans-cyclooctene: the effect of fluorine substituents on aryl azide reactivity and decaging from the 1,2,3-triazoline. Bioconj. Chem. 29, 324–334 (2018).
Doroshow, J. H. Effect of anthracycline antibiotics on oxygen radical formation in rat heart. Cancer Res. 43, 460–472 (1983).
Olson, R. D. & Mushlin, P. S. Doxorubicin cardiotoxicity: analysis of prevailing hypotheses. FASEB J. 4, 3076–3086 (1990).
Benati, L. et al. Radical reduction of aromatic azides to amines with triethylsilane. J. Org. Chem. 71, 5822–5825 (2006).
Acknowledgements
We thank the ERC (ERC-2013-ADG 340469 ADREEM) for funding M.B., J.G., K.N. and Y.Z. J.G. acknowledges support from the National Natural Science Foundation of China (22071263), the Natural Science Foundation of Guangdong Province, China (2020A1515010994), Guangdong Province Zhujiang Talents Program (2019QN01Y127), Shenzhen Fundamental Research Program (JCYJ20200109110215774) and ‘Hundred Talents Program’ of the Chinese Academy of Sciences. Y.Z. acknowledges support from the National Natural Science Foundation of China (22001261) and the China Postdoctoral Science Foundation (2020M672873). Q.G. acknowledges support from the China Postdoctoral Science Foundation (2020M682976).
Author information
Authors and Affiliations
Contributions
M.B. and J.G. conceived, designed and directed the project. Y.Z., K.N., H.D. and H.P. conducted the compound syntheses, X-ray activation and characterizations. Y.Z. and J.G. conducted the MTT, flow cytometry, transwell and wound healing assays. Y.Z. and Q.G. conducted the in vivo experiments. J.G., Y.Z., M.P., H.R., D.A. and M.B. co-wrote the manuscript. All authors analysed the data, contributed to the scientific discussion and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks Peng Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–44 and Discussion.
Rights and permissions
About this article
Cite this article
Geng, J., Zhang, Y., Gao, Q. et al. Switching on prodrugs using radiotherapy. Nat. Chem. 13, 805–810 (2021). https://doi.org/10.1038/s41557-021-00711-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00711-4
This article is cited by
-
Radiotherapy activates picolinium prodrugs in tumours
Nature Chemistry (2024)
-
Targeted activation in localized protein environments via deep red photoredox catalysis
Nature Chemistry (2023)
-
Hydrogen-bonded organic framework-based bioorthogonal catalysis prevents drug metabolic inactivation
Nature Catalysis (2023)
-
Radiation-sensitive genetic prognostic model identifies individuals at risk for radiation resistance in head and neck squamous cell carcinoma
Journal of Cancer Research and Clinical Oncology (2023)
-
DNA-based platform for efficient and precisely targeted bioorthogonal catalysis in living systems
Nature Communications (2022)