Abstract
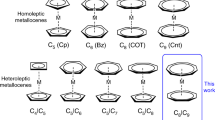
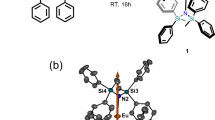
The best-performing single-molecule magnets (SMMs) have historically relied on pseudoaxial ligands delocalized across several coordinated atoms. This coordination environment has been found to elicit strong magnetic anisotropy, but lanthanide-based SMMs with low coordination numbers have remained synthetically elusive species. Here we report a cationic 4f complex bearing only two bis-silylamide ligands, Yb(III)[{N(SiMePh2)2}2][Al{OC(CF3)3}4], which exhibits slow relaxation of its magnetization. The combination of the bulky silylamide ligands and weakly coordinating [Al{OC(CF3)3}4]− anion provides a sterically hindered environment that suitably stabilizes the pseudotrigonal geometry necessary to elicit strong ground-state magnetic anisotropy. The resolution of the mJ states by luminescence spectroscopy is supported by ab initio calculations, which show a large ground-state splitting of approximately 1,850 cm−1. These results provide a facile route to access a bis-silylamido Yb(III) complex, and further underline the desirability of axially coordinated ligands with well-localized charges for high-performing SMMs.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2222783 (1 at 200 K), 2222786 (2 at 200 K) and 2222787 (2 at 90 K). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All other data generated or analysed are made available as Source Data or in the Supplementary Information. Source data are provided with this paper.
References
Leuenberger, M. N. & Loss, D. Quantum computing in molecular magnets. Nature 410, 789–793 (2001).
Ardavan, A. et al. Will spin-relaxation times in molecular magnets permit quantum information processing? Phys. Rev. Lett. 98, 057201 (2007).
Ungur, L. & Chibotaru, L. F. Magnetic anisotropy in the excited states of low symmetry lanthanide complexes. Phys. Chem. Chem. Phys. 13, 20086 (2011).
Ungur, L. & Chibotaru, L. F. Strategies toward high-temperature lanthanide-based single-molecule magnets. Inorg. Chem. 55, 10043–10056 (2016).
Chilton, N. F. Design criteria for high-temperature single-molecule magnets. Inorg. Chem. 54, 2097–2099 (2015).
Donati, F. et al. Magnetic remanence in single atoms. Science 352, 318–321 (2016).
Liu, F. et al. Single molecule magnet with an unpaired electron trapped between two lanthanide ions inside a fullerene. Nat. Commun. 8, 16098 (2017).
Goodwin, C. A. P., Ortu, F., Reta, D., Chilton, N. F. & Mills, D. P. Molecular magnetic hysteresis at 60 Kelvin in dysprosocenium. Nature 548, 439–442 (2017).
Guo, F.-S. et al. A dysprosium metallocene single-molecule magnet functioning at the axial limit. Angew. Chem. Int. Ed. 56, 11445–11449 (2017).
Gould, C. A. et al. Synthesis and magnetism of neutral, linear metallocene complexes of terbium(II) and dysprosium(II). J. Am. Chem. Soc. 141, 12967–12973 (2019).
Guo, F.-S. et al. Magnetic hysteresis up to 80 Kelvin in a dysprosium metallocene single-molecule magnet. Science 362, 1400–1403 (2018).
Willson, S. P. & Andrews, L. Characterization of the reaction products of laser-ablated late lanthanide metal atoms with molecular oxygen: infrared spectra of LnO, LnO+, LnO−, LnO2, LnO2−, LnO3−, and (LnO)2 in solid argon. J. Phys. Chem. A 103, 6972–6983 (1999).
Goodwin, C. A. P. et al. Heteroleptic samarium(III) halide complexes probed by fluorescence-detected L3-edge X-ray absorption spectroscopy. Dalton Trans. 47, 10613–10625 (2018).
Goodwin, C. A. P. et al. Physicochemical properties of near-linear lanthanide(II) bis(silylamide) complexes (Ln = Sm, Eu, Tm, Yb). Inorg. Chem. 55, 10057–10067 (2016).
Chilton, N. F., Goodwin, C. A. P., Mills, D. P. & Winpenny, R. E. P. The first near-linear bis(amide) f-block complex: a blueprint for a high temperature single molecule magnet. Chem. Commun. 51, 101–103 (2015).
Eaborn, C., Hitchcock, P. B., Izod, K., Lu, Z.-R. & Smith, J. D. Alkyl Derivatives of europium(+2) and ytterbium(+2). Crystal structures of Eu[C(SiMe3)3]2, Yb[C(SiMe3)2(SiMe2CH=CH2)]I·OEt2 and Yb[C(SiMe3)2(SiMe2OMe)]I·OEt2. Organometallics 15, 4783–4790 (1996).
Eaborn, C., Hitchcock, P. B., Izod, K. & Smith, J. D. A monomeric solvent-free bent lanthanide dialkyl and a lanthanide analog of a Grignard reagent. Crystal structures of Yb{C(SiMe3)3}2 and [Yb{C(SiMe3)3}I·OEt2]2. J. Am. Chem. Soc. 116, 12071–12072 (1994).
Cotton, S. in Lanthanides and Actinides (ed. Cotton, S.) 10–84 (Macmillan, 1991).
Day, B. M. et al. Rare-earth cyclobutadienyl sandwich complexes: synthesis, structure and dynamic magnetic properties. Chem. Eur. J. 24, 16779–16782 (2018).
Evans, W. J., Davis, B. L. & Ziller, J. W. Synthesis and structure of tris(alkyl- and silyl-tetramethylcyclopentadienyl) complexes of lanthanum. Inorg. Chem. 40, 6341–6348 (2001).
Meng, Y.-S., Zhang, Y.-Q., Wang, Z.-M., Wang, B.-W. & Gao, S. Weak ligand-field effect from ancillary ligands on enhancing single-ion magnet performance. Chem. Eur. J. 22, 12724–12731 (2016).
Nicholas, H. M. et al. Electronic structures of bent lanthanide(III) complexes with two N-donor ligands. Chem. Sci. 10, 10493–10502 (2019).
Liu, J.-L. et al. A six-coordinate ytterbium complex exhibiting easy-plane anisotropy and field-induced single-ion magnet behavior. Inorg. Chem. 51, 8538–8544 (2012).
Pointillart, F., Cador, O., Le Guennic, B. & Ouahab, L. Uncommon lanthanide ions in purely 4f single molecule magnets. Coord. Chem. Rev. 346, 150–175 (2017).
Bartlett, R. A. & Power, P. P. Two-coordinate, nonlinear, crystalline d6 and d7 complexes: syntheses and structures of M{N(SiMePh2)2}2, M = Fe or Co. J. Am. Chem. Soc. 109, 7563–7564 (1987).
Chen, H., Bartlett, R. A., Dias, H. V. R., Olmstead, M. M. & Power, P. P. The use of very crowded silylamide ligands –N(SiMenPh3−n)2 (n = 0, 1, or 2) to synthesize crystalline, two-coordinate, derivatives to manganese(II), iron(II), and cobalt(II) and the free ion [Ph3SiNSiPh3]−. J. Am. Chem. Soc. 111, 4338–4345 (1989).
Power, P. P., Ruhlandt-Senge, K. & Shoner, S. C. Synthesis and characterization of the isoelectronic d10 species bis[bis(methyldiphenylsilyl)amido]cuprate(1−) and -zinc. Inorg. Chem. 30, 5013–5015 (1991).
Bartlett, R. A., Olmstead, M. M. & Power, P. P. Structural characterization of the ‘magnesylamine’ [(Et2O)Mg(Cl){N(SiMe3)2}]2 and the two-coordinate magnesium amide Mg{N(SiMePh2)2}2. Inorg. Chem. 33, 4800–4803 (1994).
Leng, J.-D., Goodwin, C. A. P., Vitorica-Yrezabal, I. J. & Mills, D. P. Salt metathesis routes to homoleptic near-linear Mg(II) and Ca(II) bulky bis(silyl)amide complexes. Dalton Trans. 47, 12526–12533 (2018).
Demir, S., Zadrozny, J. M. & Long, J. R. Large spin-relaxation barriers for the low-symmetry organolanthanide complexes [Cp*2Ln(BPh4)] (Cp* = pentamethylcyclopentadienyl; Ln = Tb, Dy). Chem. Eur. J. 20, 9524–9529 (2014).
Day, B. M., Guo, F.-S. & Layfield, R. A. Cyclopentadienyl ligands in lanthanide single-molecule magnets: one ring to rule them all? Acc. Chem. Res. 51, 1880–1889 (2018).
McClain, K. R. et al. High-temperature magnetic blocking and magneto-structural correlations in a series of dysprosium(III) metallocenium single-molecule magnets. Chem. Sci. 9, 8492–8503 (2018).
Hitchcock, P. B., Lappert, M. F., Smith, R. G., Bartlett, R. A. & Power, P. P. Synthesis and structural characterisation of the first neutral homoleptic lanthanide metal(III) alkyls: [LnR3] [Ln = La or Sm, R = CH(SiMe3)2]. Chem. Commun. 1007–1009 (1988).
Clark, D. L., Gordon, J. C., Hay, P. J., Martin, R. L. & Poli, R. DFT study of tris(bis(trimethylsilyl)methyl)lanthanum and -samarium. Organometallics 21, 5000–5006 (2002).
Perrin, L., Maron, L., Eisenstein, O. & Lappert, M. F. γ Agostic C–H or β agostic Si–C bonds in La{CH(SiMe3)2}3? A DFT study of the role of the ligand. New J. Chem. 27, 121–127 (2003).
Boyde, N. C., Chmely, S. C., Hanusa, T. P., Rheingold, A. L. & Brennessel, W. W. Structural distortions in M[E(SiMe3)2]3 complexes (M = group 15, f-element; E = N, CH): is three a crowd? Inorg. Chem. 53, 9703–9714 (2014).
Goodwin, C. A. P. et al. Homoleptic trigonal planar lanthanide complexes stabilized by superbulky silylamide ligands. Organometallics 34, 2314–2325 (2015).
Bryan, A. M., Merrill, W. A., Reiff, W. M., Fettinger, J. C. & Power, P. P. Synthesis, structural, and magnetic characterization of linear and bent geometry cobalt(II) and nickel(II) amido complexes: evidence of very large spin–orbit coupling effects in rigorously linear coordinated Co2+. Inorg. Chem. 51, 3366–3373 (2012).
Weller, R., Ruppach, L., Shlyaykher, A., Tambornino, F. & Werncke, C. G. Homoleptic quasilinear metal(I/II) silylamides of Cr–Co with phenyl and allyl functions—impact of the oxidation state on secondary ligand interactions. Dalton Trans. 50, 10947–10963 (2021).
Weller, R. et al. Quasilinear 3d-metal(I) complexes [KM(N(Dipp)SiR3)2] (M = Cr–Co)—structural diversity, solution state behaviour and reactivity. Dalton Trans. 50, 4890–4903 (2021).
Occhipinti, G. et al. Synthesis and stability of homoleptic metal(III) tetramethylaluminates. J. Am. Chem. Soc. 133, 6323–6337 (2011).
Harder, S., Ruspic, C., Bhriain, N. N., Berkermann, F. & Schürmann, M. Benzyl complexes of lanthanide(II) and lanthanide(III) metals: trends and comparisons. Z. Naturforsch. B. 63, 267–274 (2008).
Goodwin, C. A. P., Reta, D., Ortu, F., Chilton, N. F. & Mills, D. P. Synthesis and electronic structures of heavy lanthanide metallocenium cations. J. Am. Chem. Soc. 139, 18714–18724 (2017).
Parker, D., Suturina, E. A., Kuprov, I. & Chilton, N. F. How the ligand field in lanthanide coordination complexes determines magnetic susceptibility anisotropy, paramagnetic NMR shift, and relaxation behavior. Acc. Chem. Res. 53, 1520–1534 (2020).
Van Vleck, J. H. Paramagnetic relaxation times for titanium and chrome alum. Phys. Rev. 57, 426–447 (1940).
Ding, M. et al. Magnetization slow dynamics in ferrocenium complexes. Chem. Eur. J. 25, 10625–10632 (2019).
Maniaki, D. et al. Asymmetric dinuclear lanthanide(III) complexes from the use of a ligand derived from 2-acetylpyridine and picolinoylhydrazide: synthetic, structural and magnetic studies. Molecules 25, 3153 (2020).
Amoza, M., Maxwell, L., Aliaga-Alcalde, N., Gómez-Coca, S. & Ruiz, E. Spin–phonon coupling and slow-magnetic relaxation in pristine ferrocenium. Chem. Eur. J. 27, 16440–16447 (2021).
Pointillart, F., Gal, Y. L., Golhen, S., Cador, O. & Ouahab, L. Single-molecule magnet behaviour in a tetrathiafulvalene-based electroactive antiferromagnetically coupled dinuclear dysprosium(III) complex. Chem. Eur. J. 17, 10397–10404 (2011).
Li, Q.-W. et al. ‘Half-sandwich’ YbIII single-ion magnets with metallacrowns. Chem. Commun. 51, 10291–10294 (2015).
Soussi, K. et al. Magnetic and photo-physical investigations into DyIII and YbIII complexes involving tetrathiafulvalene ligand. Inorg. Chem. Front. 2, 1105–1117 (2015).
Pedersen, K. S. et al. Design of single-molecule magnets: insufficiency of the anisotropy barrier as the sole criterion. Inorg. Chem. 54, 7600–7606 (2015).
Long, J., Guari, Y., Ferreira, R. A. S., Carlos, L. D. & Larionova, J. Recent advances in luminescent lanthanide based single-molecule magnets. Coord. Chem. Rev. 363, 57–70 (2018).
Ziessel, R. F. et al. NIR lanthanide luminescence by energy transfer from appended terpyridine–boradiazaindacene dyes. Chem. Eur. J. 12, 5060–5067 (2006).
Pointillart, F. et al. A redox-active luminescent ytterbium based single molecule magnet. Chem. Commun. 49, 615–617 (2012).
Gonçalves e Silva, F. R. et al. Visible and near-infrared luminescence of lanthanide-containing dimetallic triple-stranded helicates: energy transfer mechanisms in the SmIII and YbIII molecular edifices. J. Phys. Chem. A 106, 1670–1677 (2002).
Roos, B. O. in Advances in Chemical Physics: Ab Initio Methods in Quantum Chemistry II Vol. 69 (ed. Lawley, K. P.) 399–455 (Wiley, 1987).
Roos, B. O., Lindh, R., Malmqvist, P. Å., Veryazov, V. & Widmark, P.-O. Multiconfigurational Quantum Chemistry (Wiley, 2016).
Angeli, C., Cimiraglia, R., Evangelisti, S., Leininger, T. & Malrieu, J.-P. Introduction of n-electron valence states for multireference perturbation theory. J. Chem. Phys. 114, 10252 (2001).
Angeli, C., Cimiraglia, R. & Malrieu, J.-P. n-Electron valence state perturbation theory: a fast implementation of the strongly contracted variant. Chem. Phys. Lett. 350, 297–305 (2001).
Angeli, C., Cimiraglia, R. & Malrieu, J.-P. n-Electron valence state perturbation theory: a spinless formulation and an efficient implementation of the strongly contracted and of the partially contracted variants. J. Chem. Phys. 117, 9138–9153 (2002).
Lang, L., Sivalingam, K. & Neese, F. The combination of multipartitioning of the Hamiltonian with canonical Van Vleck perturbation theory leads to a Hermitian variant of quasidegenerate n-electron valence perturbation theory. J. Chem. Phys. 152, 014109 (2020).
Angeli, C., Borini, S., Cestari, M. & Cimiraglia, R. A quasidegenerate formulation of the second order n-electron valence state perturbation theory approach. J. Chem. Phys. 121, 4043–4049 (2004).
Nakano, H. Quasidegenerate perturbation theory with multiconfigurational self‐consistent‐field reference functions. J. Chem. Phys. 99, 7983–7992 (1993).
Neese, F. Software update: the ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 8, e1327 (2018).
Neese, F., Wennmohs, F., Becker, U. & Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 152, 224108 (2020).
Liu, Y. et al. Magnetization dynamics on isotope-isomorphic holmium single-molecule magnets. Angew. Chem. Int. Ed. 60, 27282–27287 (2021).
Bader, R. F. W. A quantum theory of molecular structure and its applications. Chem. Rev. 91, 893–928 (1991).
Bader, R. F. W. Atoms in Molecules: A Quantum Theory (Oxford University Press, 1994).
Reta, D. & Chilton, N. F. Uncertainty estimates for magnetic relaxation times and magnetic relaxation parameters. Phys. Chem. Chem. Phys. 21, 23567–23575 (2019).
Krossing, I. The facile preparation of weakly coordinating anions: structure and characterisation of silverpolyfluoroalkoxyaluminates AgAl(ORF)4, calculation of the alkoxide ion affinity. Chem. Eur. J. 7, 490–502 (2001).
Sheldrick, G. M. SHELXT—integrated space-group and crystal-structure determination. Acta Crystallogr. A 71, 3–8 (2015).
Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 71, 3–8 (2015).
Acknowledgements
The authors acknowledge the University of Ottawa, the Natural Sciences and Engineering Research Council of Canada and the Canadian Foundation for Innovation for funding and supporting this work. A.M. acknowledges funding provided by the Academy of Finland (grant number 332294) and the University of Oulu (Kvantum Institute). Computational resources were provided by CSC-IT Center for Science in Finland and the Finnish Grid and Cloud Infrastructure (persistent identifier urn:nbn:fi:research-infras-2016072533). The authors sincerely thank S. Hill and E. V. Salerno for their assistance with attempted EPR spectroscopy experiments. We also thank D. Chartrand and M. Thierry from the Université de Montréal for the single-crystal X-ray diffraction data collection of compound 2 performed at low temperature (90 K).
Author information
Authors and Affiliations
Contributions
D.E. and K.L.M.H. synthesized and characterized the compounds and collected and interpreted the magnetic data. D.E. and K.L.M.H. collected X-ray diffraction data on samples, and D.E. and A.A.K. performed structure determination and refinement. A.M. performed the ab initio calculations and analysis. D.A.G. collected and interpreted luminescence data. D.E. and K.L.M.H. wrote the manuscript with contributions from all authors. M.M. supervised all aspects of the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Floriana Tuna and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
X-ray crystallography data, Supplementary Tables 1–14, Figs. 1–11 and computational details.
Supplementary Data 1
Crystallographic data for compound 1 at 200 K; CCDC 2222783.
Supplementary Data 2
Crystallographic data for compound 2 at 200 K; CCDC 2222786.
Supplementary Data 3
Crystallographic data for compound 2 at 90 K, CCDC 2222787.
Supplementary Data 4
Atomic coordinates used in the computational analysis. The molecules are named by their numerical identifier, with labels ‘a’ and ‘b’ referring to the two structures in the asymmetric unit. The optimized atomic coordinates where the phenyl rings have been replaced by methyl groups are indicated with a prime suffix (2′).
Supplementary Data 5
Source data for Supplementary Fig. 2 (Photoluminescence spectroscopy source data for 1); Supplementary Fig. 3 (Molar magnetic susceptibility-temperature product source data for 2); Supplementary Fig. 4 (Magnetization field dependence source data for 2); Supplementary Fig. 5 (Unprocessed in-phase molar magnetic susceptibility source data for 2 under variable field); Supplementary Fig. 7 (Unprocessed in-phase molar magnetic susceptibility source data for 2 under variable temperature); and Supplementary Fig. 10 (Calculated molar magnetic susceptibility-temperature product source data for 2).
Source data
Source Data Fig. 2
Unprocessed out-of-phase molar magnetic susceptibility source data for 2 under variable field and temperature.
Source Data Fig. 3
Photoluminescence spectroscopy source data for 2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Errulat, D., Harriman, K.L.M., Gálico, D.A. et al. A trivalent 4f complex with two bis-silylamide ligands displaying slow magnetic relaxation. Nat. Chem. 15, 1100–1107 (2023). https://doi.org/10.1038/s41557-023-01208-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01208-y
This article is cited by
-
Slow magnetic relaxation in a europium(II) complex
Nature Communications (2024)