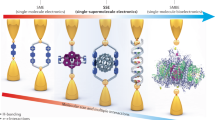
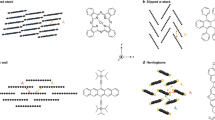
Abstract
Intermolecular charge transport plays an essential role in organic electronic materials and biological systems. To date, experimental investigations of intermolecular charge transport in molecular materials and electronic devices have been restricted to conjugated systems in which π–π stacking interactions are involved. Herein we demonstrate that the σ–σ stacking interactions between neighbouring non-conjugated molecules offer an efficient pathway for charge transport through supramolecular junctions. The conductance of σ–σ stacked molecular junctions formed between two non-conjugated cyclohexanethiol or single-anchored adamantane molecules is comparable to that of π–π stacked molecular junctions formed between π-conjugated benzene rings. The current–voltage characteristics and flicker noise analysis demonstrate the existence of stacked molecular junctions formed between the electrode pairs and exhibit the characteristics of through-space charge transport. Density functional theory calculations combined with the non-equilibrium Green’s function method reveal that efficient charge transport occurs between two molecules configured with σ–σ stacking interactions.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data that support the findings of this study are available within this article and its Supplementary Information. Source data are provided with this paper.
Code availability
The data analysis of conductance measurements in this work was performed using our open-source code XMe analysis, which is available at Github (https://github.com/Pilab-XMU/XMe_DataAnalysis) and Zenodo (https://doi.org/10.5281/zenodo.6578853).
References
Kim, T., Park, J. Y., Hwang, J., Seo, G. & Kim, Y. Supramolecular two-dimensional systems and their biological applications. Adv. Mater. 32, 2002405 (2020).
Kilchherr, F. et al. Single-molecule dissection of stacking forces in DNA. Science 353, aaf5508 (2016).
Neel, A. J., Hilton, M. J., Sigman, M. S. & Toste, F. D. Exploiting non-covalent π interactions for catalyst design. Nature 543, 637–646 (2017).
Olivo, G., Capocasa, G., Del Giudice, D., Lanzalunga, O. & Di Stefano, S. New horizons for catalysis disclosed by supramolecular chemistry. Chem. Soc. Rev. 50, 7681–7772 (2021).
Peng, W., Qu, X., Shaik, S. & Wang, B. Deciphering the oxygen activation mechanism at the CuC site of particulate methane monooxygenase. Nat. Catal. 4, 266–273 (2021).
de Greef, T. F. A. & Meijer, E. W. Supramolecular polymers. Nature 453, 171–173 (2008).
Amabilino, D. B., Smith, D. K. & Steed, J. W. Supramolecular materials. Chem. Soc. Rev. 46, 2404–2420 (2017).
Li, C. et al. Supramolecular-covalent hybrid polymers for light-activated mechanical actuation. Nat. Mater. 19, 900–909 (2020).
Xu, L. et al. Enantiomeric separation of semiconducting single-walled carbon nanotubes by acid cleavable chiral polyfluorene. ACS Nano 15, 4699–4709 (2021).
Tang, M. et al. Tailoring π-conjugated systems: from π-π stacking to high-rate-performance organic cathodes. Chem 4, 2600–2614 (2018).
Chen, H. & Fraser Stoddart, J. From molecular to supramolecular electronics. Nat. Rev. Mater. 6, 804–828 (2021).
Hunter, C. A., Lawson, K. R., Perkins, J. & Urch, C. J. Aromatic interactions. J. Chem. Soc. Perkin Trans. 2, 651–669 (2001).
Tsuzuki, S., Honda, K., Uchimaru, T., Mikami, M. & Tanabe, K. Origin of attraction and directionality of the π/π interaction: model chemistry calculations of benzene dimer interaction. J. Am. Chem. Soc. 124, 104–112 (2001).
Parrish, R. M. & Sherrill, C. D. Quantum-mechanical evaluation of π–π versus substituent–π interactions in π stacking: direct evidence for the Wheeler–Houk picture. J. Am. Chem. Soc. 136, 17386–17389 (2014).
Hunter, C. A. & Sanders, J. K. M. The nature of π–π interactions. J. Am. Chem. Soc. 112, 5525–5534 (1990).
Martinez, C. R. & Iverson, B. L. Rethinking the term “pi-stacking”. Chem. Sci. 3, 2191–2201 (2012).
Sinnokrot, M. O. & Sherrill, C. D. High-accuracy quantum mechanical studies of π–π interactions in benzene dimers. J. Phys. Chem. A 110, 10656–10668 (2006).
Alonso, M. et al. Understanding the fundamental role of π/π, σ/σ, and σ/π dispersion interactions in shaping carbon-based materials. Chem. Eur. J. 20, 4931–4941 (2014).
Grimme, S. Do special noncovalent π–π stacking interactions really exist? Angew. Chem. Int. Ed. 47, 3430–3434 (2008).
Bloom, J. W. G. & Wheeler, S. E. Taking the aromaticity out of aromatic interactions. Angew. Chem. Int. Ed. 50, 7847–7849 (2011).
Fokin, A. A., Gerbig, D. & Schreiner, P. R. σ/σ- and π/π-interactions are equally important: multilayered graphanes. J. Am. Chem. Soc. 133, 20036–20039 (2011).
Wagner, J. P. & Schreiner, P. R. London dispersion in molecular chemistry—reconsidering steric effects. Angew. Chem. Int. Ed. 54, 12274–12296 (2015).
Slowinski, K., Chamberlain, R. V., Miller, C. J. & Majda, M. Through-bond and chain-to-chain coupling. Two pathways in electron tunneling through liquid alkanethiol monolayers on mercury electrodes. J. Am. Chem. Soc. 119, 11910–11919 (1997).
Duati, M. et al. Electron transport across hexa-peri-hexabenzocoronene units in a metal–self-assembled monolayer–metal junction. Adv. Mater. 18, 329–333 (2006).
York, R. L., Nguyen, P. T. & Slowinski, K. Long-range electron transfer through monolayers and bilayers of alkanethiols in electrochemically controlled Hg–Hg tunneling junctions. J. Am. Chem. Soc. 125, 5948–5953 (2003).
Liu, Y., Qiu, X., Soni, S. & Chiechi, R. C. Charge transport through molecular ensembles: recent progress in molecular electronics. Chem. Phys. Rev. 2, 021303 (2021).
Wu, S. et al. Molecular junctions based on aromatic coupling. Nat. Nanotechnol. 3, 569–574 (2008).
Batra, A. et al. Quantifying through-space charge transfer dynamics in π-coupled molecular systems. Nat. Commun. 3, 1086 (2012).
Carini, M. et al. High conductance values in π-folded molecular junctions. Nat. Commun. 8, 15195 (2017).
Stefani, D. et al. Large conductance variations in a mechanosensitive single-molecule junction. Nano Lett. 18, 5981–5988 (2018).
Song, P. et al. Stable molecular diodes based on π–π interactions of the molecular frontier orbitals with graphene electrodes. Adv. Mater. 30, 1706322 (2018).
Li, X. et al. Structure-independent conductance of thiophene-based single-stacking junctions. Angew. Chem. Int. Ed. 59, 3280–3286 (2020).
Frisenda, R., Janssen, V. A. E. C., Grozema, F. C., van der Zant, H. S. J. & Renaud, N. Mechanically controlled quantum interference in individual π-stacked dimers. Nat. Chem. 8, 1099–1104 (2016).
Kiguchi, M. et al. Highly conductive [3×n] gold-ion clusters enclosed within self-assembled cages. Angew. Chem. Int. Ed. 52, 6202–6205 (2013).
Kiguchi, M. et al. Electron transport through single molecules comprising aromatic stacks enclosed in self-assembled cages. Angew. Chem. Int. Ed. 50, 5708–5711 (2011).
Schneebeli, S. T. et al. Single-molecule conductance through multiple π–π-stacked benzene rings determined with direct electrode-to-benzene ring connections. J. Am. Chem. Soc. 133, 2136–2139 (2011).
Gruber, C. M. et al. Resonant optical antennas with atomic-sized tips and tunable gaps achieved by mechanical actuation and electrical control. Nano Lett. 20, 4346–4353 (2020).
Bai, J. et al. Anti-resonance features of destructive quantum interference in single-molecule thiophene junctions achieved by electrochemical gating. Nat. Mater. 18, 364–369 (2019).
Zang, Y. et al. Voltage-induced single-molecule junction planarization. Nano Lett. 21, 673–679 (2021).
Magyarkuti, A., Adak, O., Halbritter, A. & Venkataraman, L. Electronic and mechanical characteristics of stacked dimer molecular junctions. Nanoscale 10, 3362–3368 (2018).
Hong, W. et al. An MCBJ case study: the influence of pi-conjugation on the single-molecule conductance at a solid/liquid interface. Beilstein J. Nanotechnol. 2, 699–713 (2011).
Liu, J. et al. Transition from tunneling leakage current to molecular tunneling in single-molecule junctions. Chem 5, 390–401 (2019).
Venkataraman, L. et al. Single-molecule circuits with well-defined molecular conductance. Nano Lett. 6, 458–462 (2006).
Leary, E. et al. Incorporating single molecules into electrical circuits. The role of the chemical anchoring group. Chem. Soc. Rev. 44, 920–942 (2015).
Hong, W. et al. Single molecular conductance of tolanes: experimental and theoretical study on the junction evolution dependent on the anchoring group. J. Am. Chem. Soc. 134, 2292–2304 (2012).
Adak, O. et al. Flicker noise as a probe of electronic interaction at metal–single molecule interfaces. Nano Lett. 15, 4143–4149 (2015).
Tang, C. et al. Identifying the conformational isomers of single-molecule cyclohexane at room temperature. Chem 6, 2770–2781 (2020).
Zhang, Q. et al. Graphene as a promising electrode for low-current attenuation in nonsymmetric molecular junctions. Nano Lett. 16, 6534–6540 (2016).
Cheng, Z.-L. et al. In situ formation of highly conducting covalent Au–C contacts for single-molecule junctions. Nat. Nanotechnol. 6, 353–357 (2011).
Chen, F., Li, X., Hihath, J., Huang, Z. & Tao, N. Effect of anchoring groups on single-molecule conductance: comparative study of thiol-, amine-, and carboxylic-acid-terminated molecules. J. Am. Chem. Soc. 128, 15874–15881 (2006).
Tsutsui, M., Shoji, K., Morimoto, K., Taniguchi, M. & Kawai, T. Thermodynamic stability of single molecule junctions. Appl. Phys. Lett. 92, 223110 (2008).
González, M. T. et al. Stability of single- and few-molecule junctions of conjugated diamines. J. Am. Chem. Soc. 135, 5420–5426 (2013).
Song, H. et al. Observation of molecular orbital gating. Nature 462, 1039–1043 (2009).
Xiang, A. et al. The origin of the transition voltage of gold–alkanedithiol–gold molecular junctions. Chem. Phys. 465–466, 40–45 (2016).
Guo, S., Hihath, J., Díez-Pérez, I. & Tao, N. Measurement and statistical analysis of single-molecule current–voltage characteristics, transition voltage spectroscopy, and tunneling barrier height. J. Am. Chem. Soc. 133, 19189–19197 (2011).
Beebe, J. M., Kim, B., Gadzuk, J. W., Frisbie, C. D. & Kushmerick, J. G. Transition from direct tunneling to field emission in metal-molecule-metal junctions. Phys. Rev. Lett. 97, 026801 (2006).
Mirjani, F., Thijssen, J. M. & van der Molen, S. J. Advantages and limitations of transition voltage spectroscopy: a theoretical analysis. Phys. Rev. B 84, 115402 (2011).
Bâldea, I. Ambipolar transition voltage spectroscopy: analytical results and experimental agreement. Phys. Rev. B 85, 035442 (2012).
Trouwborst, M. L. et al. Transition voltage spectroscopy and the nature of vacuum tunneling. Nano Lett. 11, 614–617 (2011).
Gryn’ova, G. & Corminboeuf, C. Topology-driven single-molecule conductance of carbon nanothreads. J. Phys. Chem. Lett. 10, 825–830 (2019).
Tang, C. et al. Multicenter-bond-based quantum interference in charge transport through single-molecule carborane junctions. Angew. Chem. Int. Ed. 58, 10601–10605 (2019).
Chen, Z. et al. Modularized tuning of charge transport through highly twisted and localized single-molecule junctions. J. Phys. Chem. Lett. 10, 3453–3458 (2019).
Capozzi, B. et al. Single-molecule diodes with high rectification ratios through environmental control. Nat. Nanotechnol. 10, 522–527 (2015).
Lovat, G. et al. Room-temperature current blockade in atomically defined single-cluster junctions. Nat. Nanotechnol. 12, 1050–1054 (2017).
Huisman, E. H., Guédon, C. M., van Wees, B. J. & van der Molen, S. J. Interpretation of transition voltage spectroscopy. Nano Lett. 9, 3909–3913 (2009).
Chai, J.-D. & Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 10, 6615–6620 (2008).
Frisch, M. et al. Gaussian 16, revision A.03 (Gaussian, Inc., 2016).
Boys, S. F. & Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 19, 553–566 (1970).
Smidstrup, S. et al. QuantumATK: an integrated platform of electronic and atomic-scale modelling tools. J. Phys. Condens. Matter 32, 015901 (2020).
Brandbyge, M., Mozos, J.-L., Ordejón, P., Taylor, J. & Stokbro, K. Density-functional method for nonequilibrium electron transport. Phys. Rev. B 65, 165401 (2002).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (nos 21905238, 21673195, 21722305, 21973079), National Key R&D Program of China (2017YFA0204902) and Natural Science Foundation of Fujian Province (2021J06008). We thank J. Liu, J. Zheng, J. Bai, X. Li, Z. Tan and Y. Zhang for discussion during the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
W.H. and Y.Y. supervised this project. A.F. conceived the idea of molecular design and wrote the first version of this manuscript. A.F. and M.A.Y.A.-S. carried out the break junction experiments. A.F. analysed the data with the help of Z.P., S.Z. and Z.X.; Y.Z. performed theoretical calculations with the help of B.Z. and L.C.; and W.X. synthesized the molecules for the control experiment. All authors conceived the work and contributed to the revision of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Mercedes Alonso, Ganna Gryn’ova and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–42, Table 1 and Methods.
Supplementary Data 1
Source data of Supplementary Figs.13, 18–22, 32 and 33.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Rights and permissions
About this article
Cite this article
Feng, A., Zhou, Y., Al-Shebami, M.A.Y. et al. σ–σ Stacked supramolecular junctions. Nat. Chem. 14, 1158–1164 (2022). https://doi.org/10.1038/s41557-022-01003-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-01003-1