Abstract
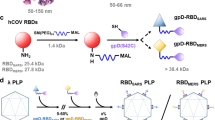
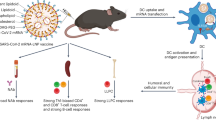
Messenger RNA vaccines lack specificity for dendritic cells (DCs)—the most effective cells at antigen presentation. Here we report the design and performance of a DC-targeting virus-like particle pseudotyped with an engineered Sindbis-virus glycoprotein that recognizes a surface protein on DCs, and packaging mRNA encoding for the Spike protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or for the glycoproteins B and D of herpes simplex virus 1. Injection of the DC-targeting SARS-CoV-2 mRNA vaccine in the footpad of mice led to substantially higher and durable antigen-specific immunoglobulin-G titres and cellular immune responses than untargeted virus-like particles and lipid–nanoparticle formulations. The vaccines also protected the mice from infection with SARS-CoV-2 or with herpes simplex virus 1. Virus-like particles with preferential uptake by DCs may facilitate the development of potent prophylactic and therapeutic vaccines.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Source data for the figures are available from figshare51 at https://doi.org/10.6084/m9.figshare.24516694. The raw and analysed datasets generated during the study are available for research purposes from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Roth, G. A. et al. Designing spatial and temporal control of vaccine responses. Nat. Rev. Mater. 7, 174–195 (2022).
Colby, D. J. et al. Safety and immunogenicity of Ad26 and MVA vaccines in acutely treated HIV and effect on viral rebound after antiretroviral therapy interruption. Nat. Med. 26, 498–501 (2020).
Ng’uni, T., Chasara, C. & Ndhlovu, Z. M. Major scientific hurdles in HIV vaccine development: historical perspective and future directions. Front. Immunol. 11, 590780 (2020).
Bernstein, D. I. et al. The R2 non-neuroinvasive HSV-1 vaccine affords protection from genital HSV-2 infections in a guinea pig model. npj Vaccines 5, 104 (2020).
Awasthi, S. et al. Nucleoside-modified mRNA encoding HSV-2 glycoproteins C, D, and E prevents clinical and subclinical genital herpes. Sci. Immunol. 4, eaaw7083 (2019).
Wang, W. et al. Dual-targeting nanoparticle vaccine elicits a therapeutic antibody response against chronic hepatitis B. Nat. Nanotechnol. 15, 406–416 (2020).
Roden, R. B. S. & Stern, P. L. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat. Rev. Cancer 18, 240–254 (2018).
Sahin, U. et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226 (2017).
Keskin, D. B. et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 565, 234–239 (2019).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021).
Widge, A. T. et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N. Engl. J. Med. 384, 80–82 (2021).
Thomas, S. J. et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N. Engl. J. Med. 385, 1761–1773 (2021).
Wang, Y. et al. mRNA vaccine: a potential therapeutic strategy. Mol. Cancer 20, 33 (2021).
Lindsay, K. E. et al. Visualization of early events in mRNA vaccine delivery in non-human primates via PET–CT and near-infrared imaging. Nat. Biomed. Eng. 3, 371–380 (2019).
Chen, J. et al. Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8+ T cell response. Proc. Natl Acad. Sci. USA 119, e2207841119 (2022).
Tenforde, M. W. et al. Effectiveness of severe acute respiratory syndrome coronavirus 2 messenger RNA vaccines for preventing coronavirus disease 2019 hospitalizations in the United States. Clin. Infect. Dis. 74, 1515–1524 (2022).
Eisenbarth, S. C. Dendritic cell subsets in T cell programming: location dictates function. Nat. Rev. Immunol. 19, 89–103 (2019).
Lanzavecchia, A. & Sallusto, F. Regulation of T cell immunity by dendritic cells. Cell 106, 263–266 (2001).
Heath, W. R., Kato, Y., Steiner, T. M. & Caminschi, I. Antigen presentation by dendritic cells for B cell activation. Curr. Opin. Immunol. 58, 44–52 (2019).
Wykes, M., Pombo, A., Jenkins, C. & MacPherson, G. G. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J. Immunol. 161, 1313–1319 (1998).
Igyarto, B. Z., Jacobsen, S. & Ndeupen, S. Future considerations for the mRNA-lipid nanoparticle vaccine platform. Curr. Opin. Virol. 48, 65–72 (2021).
Blumenthal, K. G. et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N. Engl. J. Med. 384, 1273–1277 (2021).
Yu, Y. et al. Antibody-dependent cellular cytotoxicity response to SARS-CoV-2 in COVID-19 patients. Signal Transduct. Target. Ther. 6, 346 (2021).
Svajger, U., Anderluh, M., Jeras, M. & Obermajer, N. C-type lectin DC-SIGN: an adhesion, signalling and antigen-uptake molecule that guides dendritic cells in immunity. Cell. Signal. 22, 1397–1405 (2010).
Yang, L. et al. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat. Biotechnol. 26, 326–334 (2008).
Wrapp, D. et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367, eabb2507 (2020).
Yin, D. et al. Targeting herpes simplex virus with CRISPR-Cas9 cures herpetic stromal keratitis in mice. Nat. Biotechnol. 39, 567–577 (2021).
Ling, S. et al. Lentiviral delivery of co-packaged Cas9 mRNA and a Vegfa-targeting guide RNA prevents wet age-related macular degeneration in mice. Nat. Biomed. Eng. 5, 144–156 (2021).
Segel, M. et al. Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. Science 373, 882–889 (2021).
Banskota, S. et al. Engineered virus-like particles for efficient in vivo delivery of therapeutic proteins. Cell 185, 250–265.e16 (2022).
Hu, B., Tai, A. & Wang, P. Immunization delivered by lentiviral vectors for cancer and infectious diseases. Immunol. Rev. 239, 45–61 (2011).
Chang, D. & Zaia, J. Why glycosylation matters in building a better flu vaccine. Mol. Cell. Proteomics 18, 2348–2358 (2019).
Watanabe, Y., Allen, J. D., Wrapp, D., McLellan, J. S. & Crispin, M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science 369, eabb9983 (2020).
Neerukonda, S. N. et al. Establishment of a well-characterized SARS-CoV-2 lentiviral pseudovirus neutralization assay using 293T cells with stable expression of ACE2 and TMPRSS2. PLoS ONE 16, e0248348 (2021).
Wienert, B., Shin, J., Zelin, E., Pestal, K. & Corn, J. E. In vitro-transcribed guide RNAs trigger an innate immune response via the RIG-I pathway. PLoS Biol. 16, e2005840 (2018).
Mu, X., Greenwald, E., Ahmad, S. & Hur, S. An origin of the immunogenicity of in vitro transcribed RNA. Nucleic Acids Res. 46, 5239–5249 (2018).
Ying, T. et al. Exceptionally potent neutralization of Middle East respiratory syndrome coronavirus by human monoclonal antibodies. J. Virol. 88, 7796–7805 (2014).
Ma, M. L. et al. Systematic profiling of SARS-CoV-2-specific IgG responses elicited by an inactivated virus vaccine identifies peptides and proteins for predicting vaccination efficacy. Cell Discov. 7, 67 (2021).
Jiang, H. W. et al. SARS-CoV-2 proteome microarray for global profiling of COVID-19 specific IgG and IgM responses. Nat. Commun. 11, 3581 (2020).
Li, Y. et al. Linear epitope landscape of the SARS-CoV-2 Spike protein constructed from 1,051 COVID-19 patients. Cell Rep. 34, 108915 (2021).
Lu, J. et al. A COVID-19 mRNA vaccine encoding SARS-CoV-2 virus-like particles induces a strong antiviral-like immune response in mice. Cell Res. 30, 936–939 (2020).
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020).
Bao, L. et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature 583, 830–833 (2020).
Kastenmuller, W., Kastenmuller, K., Kurts, C. & Seder, R. A. Dendritic cell-targeted vaccines—hope or hype? Nat. Rev. Immunol. 14, 705–711 (2014).
Ku, M.-W., Charneau, P. & Majlessi, L. Use of lentiviral vectors in vaccination. Expert Rev. Vaccines 20, 1571–1586 (2021).
Pollack, S. M. et al. First-in-human treatment with a dendritic cell-targeting lentiviral vector-expressing NY-ESO-1, LV305, induces deep, durable response in refractory metastatic synovial sarcoma patient. J. Immunother. 40, 302–306 (2017).
Ku, M. W. et al. Lentiviral vector induces high-quality memory T cells via dendritic cells transduction. Commun. Biol. 4, 713 (2021).
Ku, M. W. et al. Intranasal vaccination with a lentiviral vector protects against SARS-CoV-2 in preclinical animal models. Cell Host Microbe 29, 236–249.e6 (2021).
Norton, T. D. & Miller, E. A. Recent advances in lentiviral vaccines for HIV-1 infection. Front. Immunol. 7, 243 (2016).
Zhong, Y. SD_FIG1-7.xlsx.xlsx. figshare https://doi.org/10.6084/m9.figshare.24516694 (2023).
Acknowledgements
Y.C. is supported by the National Key R&D Program of China (2022YFC3400205), the National Natural Science Foundation of China (31971364) and Shanghai Jiao Tong University Scientific and Technological Innovation Funds (AF4150049). J.H. is supported by the National Natural Science Foundation of China (81970766 and 82171102) and the Shanghai Innovation Development Program (2020-RGZN-02033). T.Y. is supported by the National Key R&D Program of China (2019YFA0904400) and the National Natural Science Foundation of China (81822027 and 81630090). S.T. is supported by the National Natural Science Foundation of China (31970130) and the National Key R&D Program of China (2020YFE0202200).
Author information
Authors and Affiliations
Contributions
Y.C. conceived the study and designed the experiments; D.Y., Y. Zhong, S. Ling, S. Lu, Xiaoyuan Wang, Z.J., J.W., Y.D., X.T., Q.H., Xingbo Wang, J.C., Z.L., Y.L., Z.X., H.J., Y.W., Y.S., Q.W., J.X., W.H., H.X., H.Y., Y. Zhang, L.D., Z.H., S.T., R.D., T.Y. and J.H. performed the experiments or provided essential experimental resources; all the authors analysed the data; Y. Zhong, D.Y., S. Ling, and Y.C. wrote the manuscript with help from all the authors.
Corresponding authors
Ethics declarations
Competing interests
Y.C. is a co-founder and advisor of BDGENE Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Ke Cheng and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures, table and unprocessed blots.
Source data
Source Data Figs. 1–7
Source data and unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yin, D., Zhong, Y., Ling, S. et al. Dendritic-cell-targeting virus-like particles as potent mRNA vaccine carriers. Nat. Biomed. Eng (2024). https://doi.org/10.1038/s41551-024-01208-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-024-01208-4