Abstract
Increasing cannabinoid use with the incipient favorable public discourse raises concerns about their environmental release and potential impacts. Concentration of common cannabinoids and their metabolites (e.g., THC and THC-COOH) are already detected in source waters and engineered water systems across the globe with a rising trajectory. This study examines the adsorption of THC and THC-COOH onto activated carbons in surface water-relevant concentrations and investigates the effect of carbon’s physicochemical properties. At higher equilibrium concentration (i.e., Ce > 300 µg/L), adsorption of cannabinoids correlates with the sorbent-specific surface area. On the contrary, at lower concentrations (i.e., Ce = 0.1–100 µg/L), cannabinoid adsorption was predominantly controlled by pore size of the carbon. More specifically, when the surface area was not limited (i.e., dilution conditions), cannabinoid molecules appeared to have preferably attached within the mesopores. The adsorption mechanism deciphered in the study will facilitate in selection of commercial activated carbon to remove cannabinoids in drinking water treatment systems. This work will also provide a baseline for further research on removal of other cannabinoids (e.g., CBD), other structurally relevant drugs, their metabolites, and reaction byproduct from engineered treatment systems.
Similar content being viewed by others
Introduction
Cannabis is the most widely consumed Schedule I controlled substance with about 192 million users worldwide.1,2 The compassionate medical use of marijuana and continued nonfederal legalization efforts, e.g., Food and Drug Administration’s approval of the first cannabinoid-based epilepsy drug Epidiolex (cannabidiol), will further facilitate its use in the United States and across the globe. Its projected market share is expected to reach $22 billion by 2022.1,3 This raises questions on the environmental release and potential impacts of the compounds in the cannabinoid class.1 Due to a high rate of recreational consumption and its compassionate utilization as a medicine,4 THC and its metabolites have already been detected in natural and built aquatic environment across the globe5,6 and the release is expected to increase further.
The primary sources of cannabinoids in water are point sources, such as raw or treated wastewater from domestic households,7 as well as nonpoint sources, such as surface run-off from sewage sludge (biosolids) when applied to agricultural lands,8,9 seepage from landfills into aquifers,10 and infiltration from leaking sewer infrastructure.5 THC undergoes oxidative enzymatic metabolism by microsomal hydroxylation and catalytic oxidation that are induced by cytochrome P450 complex in the liver.11 The metabolic byproducts are excreted predominantly in the form of 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THC-COOH).12 THC-COOH, a nonpsychoactive metabolite and biomarker for THC, has been found as a stable compound over a 3-day period in wastewater samples at neutral pH and at 20 °C.13,14,15 In addition, only 1.3% of THC-COOH adsorption was estimated to be on sewer borne solids at pH 7.5.16 Since, wastewater treatment plants are only partially effective in removing this metabolite,6,15,17,18,19,20,21 THC-COOH is detected in concentrations up to 500 ng L−1 in raw fresh waters.22 THC-COOH is toxic for aquatic species at concentrations above 500 ng/L23,24 and may adversely affect human health.25 Furthermore, THC-COOH was detected in treated drinking waters;17 however, it is not currently being regulated or monitored for drinking water.26 Only a handful of studies have examined the destructive removal of THC-COOH from waste- and surface-waters with chlorination20,27,28 and photo-oxidation (zerovalent iron, UV, and UV/H2O2).20,22,29,30 These processes can transform THC-COOH.27,28 However, its transformation by-products can also be concerning for surface waters.27
The removal of cannabinoids from water thus continues to be the critical gap in the literature. This study examines adsorption mechanism of THC and THC-COOH onto activated carbons (ACs) in surface water-relevant concentrations. This work used three commercial ACs and various characterization techniques to investigate the effects of physicochemical properties on the adsorption of THC and THC-COOH. The adsorption mechanism deciphered in the study will facilitate in selection of commercial AC to remove cannabinoids in drinking water treatment systems. This work will also provide a baseline for further research on understanding the cannabinoid, its metabolites, and reaction byproduct removal from engineered treatment systems.
Results
Physicochemical properties of adsorbents
Selected physicochemical properties of the adsorbents are presented in Table 1. The specific surface area (SSA) and pore volume distribution of AC represent the structural differences. The SSA of three carbons has the following trend; F400-PAC > Nano-PAC > HDB-PAC. All three carbons possess a wide heterogeneity in pore volume distribution; while HDB-PAC dominantly occupied mesoporous volume and F400-PAC is rich in micropores. As estimated from SEM micrographs (Fig. S2), the average particle sizes of F400-PAC, HDB-PAC, and Nano-PAC are found to be 59 ± 13, 10 ± 7, and 0.9 ± 0.4 µm, respectively. The particle size distributions are determined by SEM image analysis and are presented in Fig. S2. DLS determined mean particle size of Nano-PAC is 0.70 ± 0.12 µm (Fig. S3), which is comparable to the value calculated by SEM. The particle size of other two carbons are too large to be characterized with the DLS technique. Oxygen content of F400-PAC, HDB-PAC, and Nano-PAC are 5%, 9%, and 11%, respectively. FTIR spectra (Fig. S5) demonstrate various absorption peaks; e.g., the broad band at 1000–1300 cm−1 is usually assigned to functional groups containing single-bonded oxygen in compounds, such as phenols, lactones, ethers, and esters.31 The sharp band at 1500–1600 cm−1 can be attributed to overlapping aromatic ring vibrations of sp2 hybridized carbon atoms and carboxylate groups.32 The inverse proportionality of particle size (F400 > HDB > Nano) and total oxygen content (F400 < HDB < Nano) is attributed to the larger exterior surface area of smaller carbons that has more contact with ambient oxygen in the air. Similar results were reported regarding higher oxygen content of superfine pulverized AC that was attributed to rapid carbon shell oxidation in contrast to oxidation of interior pore network.33
Effect of adsorbent properties on adsorption of THC-COOH
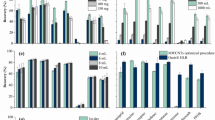
Adsorption isotherms of THC-COOH for three ACs are presented in Fig. 1a. All adsorbents demonstrated adsorption capacities (qe) ranging between 20 and 200 mg/g within the tested concentrations at equilibrium. The adsorbents capacities show notably different dependencies to aqueous concentration. Linear regression is performed to find the best-fit model parameters for Langmuir and Freundlich isotherms and to quantify the differences in equilibrium capacities. As shown in Table S2, Freundlich Model fits the experimental data better than Langmuir Model according to direct comparison of coefficient of determinations and is further analyzed to find the adsorption affinity at saturated (KFm) and dilute (KFu) conditions of THC-COOH solution presented in Fig. 1b.
a THC-COOH adsorption isotherms for AC. The lines show the Freundlich model fits to the data, b Freundlich adsorption affinity (\(K_F=q_{\rm{e}} C_{\rm{e}}^n\)) of AC for THC-COOH. KFm, as Ce and qe expressed in units of mg L−1 and mg g−1 and KFµ, as Ce and qe expressed in units of µg L−1 and mg g−1. Error bars indicate 95% level of confidence of the Freundlich K parameters. c Equilibrium adsorption capacity of AC for THC-COOH (C0 = 1000 ng/L). Error bars indicate ±1 std. dev. of analytical triplicates of experimental duplicates. d Comparison of THC and THC-COOH adsorption capacity (qe) at Ce = 100 µg L−1.
At higher equilibrium concentration (i.e., close to saturation; Ce > 300 µg/L), adsorption of THC-COOH has the following trend; F400-PAC > Nano-PAC > HDB-PAC, which correlates to their SSAs. The collapse of THC isotherms at higher saturation concentrations when the adsorption capacity is normalized with respect to SSA aligns with this observation (Fig. 2). This is attributed to competition of cannabinoid molecules for SSA at higher concentrations due to limited availability of adsorption sites. On the contrary, at lower concentrations (i.e., Ce < 100 µg/L), the THC-COOH adsorption trend changes to HDB-PAC > F400-PAC > Nano-PAC. This indicates that when the surface area is not limiting, HDB-PAC has a notable advantage. The available pore size of mainly mesoporous HDB-PAC approaches the dimension of THC/THC-COOH molecules (~2 nm), enabling multiple contact points between cannabinoid molecules and the AC surface; which can be identified as the pore filling mechanism.34 In other words, when there is no site competition, cannabinoid molecules thermodynamically prefer to pack themselves in mesopores to minimize the overall energy of the system.
In order to investigate the adsorption mechanism at environmentally relevant levels; adsorption of THC-COOH, i.e., the primary urinary metabolite of THC, was tested via single-point sorption experiments at C0 = 1000 ng/L, as shown in Fig. 1c. Adsorption of the THC-COOH molecule is the highest for HDB-PAC among all ACs confirming that HDB-PAC has more affinity to accommodate THC-COOH molecules; despite its notably low SSA (483 m2/g) when compared to F400-PAC (948 m2/g) and Nano-PAC (857 m2/g). Nano-PAC and F400-PAC adsorbed 7–24% less THC-COOH molecules on a mass basis and 47 and 60% less molecules on a surface area basis. Results indicate that adsorption of cannabinoids at environmentally relevant, i.e., ng L−1 levels, may not necessarily be dictated by the SSA of AC. The packing of molecules may thermodynamically favor mesoporous ACs regardless of the total surface area.
Comparison of THC and THC-COOH adsorption by AC
THC isotherms are generated (Fig. S6) for side by side comparison with THC-COOH isotherms and gain insights into the adsorption mechanisms. It is to be noted that THC is not detected often in natural or engineered aquatic systems as much as its biomarker THC-COOH.33 Figure 1d shows the adsorption capacities of AC at 100 µg/L equilibrium concentration of THC and THC-COOH solutions. The values of adsorption capacities are estimated from Freundlich isotherm fits. For all ACs, THC adsorbs more than THC-COOH (Fig. S7). This is attributed to the lower solubility and higher Kow of THC compared to that of THC-COOH (Table S1); the carboxylic acid in THC-COOH possibly contributed in lowering the KOW. Also deprotonated carboxylic group on THC-COOH structure (pKa = 4.4) increases its participation within the polar water molecules. THC adsorption capacity (qe at 100 µg L−1) is 15–300% higher than THC-COOH depending the adsorbent type. The adsorption capacity differences between THC vs. THC-COOH follows the same order of AC oxygen content; i.e., Nano-PAC > HDB-PAC > F400-PAC, which indicates electrostatic repulsion between carboxylic functional group of THC-COOH and the oxygen containing functional groups on carbon surface. This was attributed to formation of water clusters,35 i.e., water molecules forming H-bonding with the oxygen containing functional groups on the carbon surfaces that can suppress the attachment of hydrophobic THC molecules when compared to THC-COOH. The THC/THC-COOH molecules are more likely to attach to hydrophobic sites of carbon surfaces via nonspecific van der Waals interactions (mainly caused by induced polarization or permanent uneven electron distribution on the molecular structure) or π–π interactions rather than by H-bonding of the functional groups. More research is required to understand the effect of functional group types on carbon surfaces under different background water conditions; although differences in solubility successfully captures the differences in adsorption of THC vs. THC-COOH by AC.36
It should be noted that the loss of THC to glass vials is considerably higher than THC-COOH; 26–49% for THC vs. 5–7% for THC-COOH, as shown in Fig. S8, which is accounted for in adsorption capacity estimations presented in Fig. 1d. Loss of THC on glassware especially at high concentrations has been reported in earlier literature and salinization of glass surface is recommended by researchers to minimize losses.11,37 Untreated glass surface consists of silicates groups, i.e, [SiO4−x(4−2×)−]n, which possibly promoted electrostatic interactions between its sites with hydroxyl groups (OH) of the THC molecules. Therefore, a greater loss of THC was observed in blank samples (no carbons) at high initial concentrations. However, the carboxylate anions (COO−) in the structure of THC-COOH suppresses the glass adsorption, probably due to the electrostatic repulsion between carboxylate (COO−) and silicate anions, despite the presence of -OH in THC-COOH molecules.
Discussion
Cannabinoids are in natural and engineered aquatic systems and more will likely be introduced in the near future. Although, impacts of chronic involuntary consumption of cannabinoids in water are not well understood, it is evident that these molecules are classified as Schedule I drugs and their presence in source waters require attention. Though fate and chemical transformation of these molecules are critical scientific questions, the engineering relevance centers around their removal from our water systems. Understanding removal of cannabinoids and their metabolites with a conventional contaminant removal process (i.e., adsorption) is a step toward engineering solution of this problem. Further refining for molecular selectivity by AC surface tailoring and even molecular recognition mimicking the THC binding to the cannabinoid receptors in nervous system (i.e., CB1–CB2) are research venues to improve their targeted removal from complex solutions. To advance in this field, the impact of background solution must be explored with respect to ambient temperature and pH, ionic strength, as well as natural organic matter to simulate more complex and controlled aquatic environments. The mechanisms deciphered in this study will pave a path for designing and selecting adsorption process parameters to effectively remove these compounds. The main implication of this study is that THC and THC-COOH can be effectively removed with AC adsorbents and that in waters similar to those studied herein, cannabinoid removal will not be governed by AC physicochemical properties. Further research is warranted to gain a better understanding of the role of water chemistry, presence of natural organic matter, and interaction of transformation products of THC and THC-COOH in the removal of these compounds from water. Research in this field is scarce; therefore, exploring cannabinoid removal in simulated complex waters (e.g., higher ionic strength, natural organic matter) and eventually in actual surface waters should be the next logical steps to unlock fate and transformation of cannabinoids in water.
Methods
ACs and reagents
Three types of well-characterized AC with a range of particle sizes and physicochemical properties were used as representative adsorbents in this study; these include F400-GAC (Granular AC, Calgon Corp.), HDB-PAC (Powdered AC, HydroDarco B, Cabot Corp., Boston, MA), and Nano-PAC (Superfine AC, US Research Nanomaterials Inc., Houston, TX). HDB-PAC and Nano-PAC were used as received from the suppliers, while F400-GAC was ground and sieved to 200–325 mesh size prior to use for creating a homogenous batch of PAC. Federally exempt THC and THC-COOH standards (1 mg/mL in methanol solution) were procured from Cerilliant Corp. (Sigma Aldrich, St. Louis, MO). Molecular structures and properties of these compounds are summarized in Supplementary Information (SI) in Fig. S1 and Table S1, respectively. Deionized (DI) water (18.2 MΩ cm at 25 °C) was obtained from Synergy UV Purification System (MilliporeSigma, Burlington, MA).
Characterization of adsorbents
SSA and pore size distribution was conducted by nitrogen gas adsorption at 77 °K with an ASAP 2460 analyzer (Micromeritics Instrument Corp. U.S., Norcross, GA). Elemental analysis of carbon (C), hydrogen (H), nitrogen (N), and oxygen (O) was conducted on a FlashEA™ 1112 Elemental Analyzer (Thermo Electron Corporation, Waltham, MA). Scanning electron microscopy (SEM) of all samples was carried out with a JEOL JSM-7401F FE-SEM at an operating voltage of 15 kV. The samples were coated with 5 nm thick gold layer using Denton Vacuum Desk IV Standard Sputter Coater. Dynamic light scattering (DLS) was conducted on a Malvern Instrument, Nano-sizer S90, using 1 mg carbon suspension in 10 mL aqueous solution. Fourier Transform Infrared (FTIR) spectroscopy of ACs was conducted with a Bruker IFS 66 V FTIR (Bruker Optics, Billerica, MA) using potassium bromide (KBr) pellets. Pellets were formed by mixing carbons with finely divided KBr at a ratio of 1:100.
Adsorption experiments
Constant carbon dose single-solute isotherm experiments were conducted using completely mixed batch reactors (i.e., 255 mL glass bottles with Teflon-lined screw caps). Bottles containing 1.0 ± 0.1 mg of AC were first filled with DI water leaving no headspace to minimize volatilization of the compounds and spiked with predetermined volumes of adsorbates from their concentrated stock solutions (1 mg/mL of methanol). The volume percentage of the methanol-spiked solution per bottle was kept below 0.1% (v/v) to minimize the co-solvent effect. Later, the bottles with no headspace were secured horizontally in a sealed box to prevent photodegradation and shaken at constant speed and at room temperature (25 ± 1 °C) for 7 days to reach pseudo-equilibrium according to preliminary kinetic tests (Fig. S4). The solution was not buffered and the pH was determined to 6.5 prior to commencing the experiments. The pH remained constant during THC (pKa = 9.81) adsorption tests and decreased slightly, i.e., between 0.1 and 1.0 unit for THC-COOH (pKa = 4.66) tests, likely due to the dissociation of carboxylic acid group. Addition of 4 mg L−1 carbon adsorbent did not influence the solution pH.
After the equilibrium period, the sealed boxes were placed on a bench to allow settling of the adsorbents, and remaining adsorbate concentration in the supernatant were analyzed by either UV–vis spectrometer (Hach DR6000, Loveland, CO) at wavelength of 209 nm or liquid chromatography mass spectrometry (LC–MS) (Thermo Scientific Dionex Ultimate 3000 HPLC), as discussed in Text S1 in the SI. All LC–MS sample including ‘no carbon controls’ were filtered with 0.45 μm PTFE syringe filter. Samples prior to UV–vis spectroscopy analysis were taken from the supernatant of settled samples and full UV spectrum (200–800 nm) was scanned to ensure no interferences are present in the suspension. Bottles without any adsorbents served as blanks to monitor any loss of adsorbates during the experiments. Losses observed (only for THC) in blank samples were not accounted during computation of carbon adsorption capacity. Freundlich and Langmuir isotherm models were employed to fit the adsorption data as shown in Eqs. (1) and (2).
where qe and Ce represent the solid-phase equilibrium concentration (mg/g) and the liquid-phase equilibrium concentration (μg/L or mg/L), respectively, KF is the unit-capacity parameter ((mg/g)/Cen), equal to the amount adsorbed at a value of Ce equal to unity, and n is a dimensionless parameter related to the surface heterogeneity. Two KF parameters (KFμ and KFm) were examined for THC and THC-COOH adsorption capacities to simulate the dilution and saturation conditions at equilibrium concentrations of 1 μg/L and 1 mg/L, respectively.
where qm is the maximum adsorption capacity of adsorbent (mg/g) and KL is the Langmuir constant (L/mg). The goodness-of-fit was indicated by coefficient of determination (r2). Parameters for Langmuir and Freundlich isotherm models are presented in Table S2.
Data availability
Supplementary Information contains details of analytical techniques, molecular structures and physicochemical properties of adsorbates, details of adsorbent characterization, raw data for adsorption kinetics and isotherms, and linear regression parameters for Langmuir and Freundlich isotherm models.
References
Saleh, N. B., Apul, O. & Karanfil, T. The genesis of a critical environmental concern: cannabinoids in our water systems. Environ. Sci. Technol. 53, 1746 (2019).
UNDOC. World Drug Report 2018. Global Overview of Drug Demand and Supply. (2018).
Business Marijuana Daily. Exclusive: US marijuana sales may reach $10 billion this year, $22 billion by 2022. Available at: https://mjbizdaily.com/exclusive-marijuana-sales-may-reach-10-billion-this-year-22-billion-by-2022. (2018). Accessed 24 Sept 2018.
Mehmedic, Z. et al. Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J. Forensic Sci. 55, 1209 (2010).
Pal, R., Megharaj, M., Kirkbride, K. P. & Naidu, R. Illicit drugs and the environment—a review. Sci. Total Environ. 463–464, 1079 (2013).
Park, Y. R., Mackie, A. L. & Gagnon, G. A. A critical review of the occurrence, detection, and treatment of Δ9-tetrahydrocannabinol in aquatic environments. Environ. Rev. 25, 255 (2017).
Boleda, M. R., Galceran, M. T. & Ventura, F. Monitoring of opiates, cannabinoids and their metabolites in wastewater, surface water and finished water in Catalonia, Spain. Water Res. 43, 1126 (2009).
Jones-Lepp, T. L. & Stevens, R. Pharmaceuticals and personal care products in biosolids/sewage sludge: the interface between analytical chemistry and regulation. Anal. Bioanal. Chem. 387, 1173 (2007).
Richardson, S. D. & Kimura, S. Y. Emerging environmental contaminants: challenges facing our next generation and potential engineering solutions. Environ. Technol. Innov. 8, 40 (2017).
Kaleta, A., Ferdig, M. & Buchberger, W. Semiquantitative determination of residues of amphetamine in sewage sludge samples. J. Sep. Sci. 29, 1662 (2006).
Sharma, P., Murthy, P. & Bharath, M. M. S. Chemistry, metabolism, and toxicology of cannabis: clinical implications. Iran. J. Psychiatry 7, 149 (2012).
Huestis, M. A. Pharmacokinetics and metabolism of the plant cannabinoids, delta9-tetrahydrocannabinol, cannabidiol and cannabinol. Handb. Exp. Pharmacol. 657 (2005). https://doi.org/10.1007/3-540-26573-2-23
Senta, I., Krizman, I., Ahel, M. & Terzic, S. Assessment of stability of drug biomarkers in municipal wastewater as a factor influencing the estimation of drug consumption using sewage epidemiology. Sci. Total Environ. 487, 659 (2014).
McCall, A. K. et al. Critical review on the stability of illicit drugs in sewers and wastewater samples. Water Res. 88, 933 (2016).
Devault, D. A., Néfau, T., Levi, Y. & Karolak, S. The removal of illicit drugs and morphine in two waste water treatment plants (WWTPs) under tropical conditions. Environ. Sci. Pollut. Res. 24, 25645 (2017).
Khan, U. & Nicell, J. A. Sewer epidemiology mass balances for assessing the illicit use of methamphetamine, amphetamine and tetrahydrocannabinol. Sci. Total Environ. 421–422, 144 (2012).
Carmona, E., Andreu, V. & Picó, Y. Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: From waste to drinking water. Sci. Total Environ. 484, 53 (2014).
Boleda, M. R., Galceran, M. T. & Ventura, F. Trace determination of cannabinoids and opiates in wastewater and surface waters by ultra-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 1175, 38 (2007).
Postigo, C., Lopez De Alda, M. J. & Barceló, D. Fully automated determination in the low nanogram per liter level of different classes of drugs of abuse in sewage water by on-line solid-phase extraction-liquid chromatography-electrospray-tandem mass spectrometry. Anal. Chem. 80, 3123 (2008).
Boix, C., Ibáñez, M., Bijlsma, L., Sancho, J. V. & Hernández, F. Investigation of cannabis biomarkers and transformation products in waters by liquid chromatography coupled to time of flight and triple quadrupole mass spectrometry. Chemosphere 99, 64 (2014).
Yadav, M. K. et al. Occurrence of illicit drugs in water and wastewater and their removal during wastewater treatment. Water Res. 124, 713 (2017).
Park, Y., Mackie, A. L., MacIsaac, S. A. & Gagnon, G. A. Photo-oxidation of 11-nor-9-carboxy-Δ9-tetrahydrocannabinol using medium-pressure UV and UV-H2O2—a kinetic study. Environ. Sci. Water Res. Technol. 4, 1262 (2018).
Parolini, M. & Binelli, A. Oxidative and genetic responses induced by Δ-9-tetrahydrocannabinol (Δ-9-THC) to Dreissena polymorpha. Sci. Total Environ. 68, 468–469 (2014).
Parolini, M., Castiglioni, S., Magni, S., Della Torre, C. & Binelli, A. Increase in cannabis use may indirectly affect the health status of a freshwater species. Environ. Toxicol. Chem. 36, 472 (2017).
Stewart, A. M. & Kalueff, A. V. The behavioral effects of acute Delta(9)-tetrahydrocannabinol and heroin (diacetylmorphine) exposure in adult zebrafish. Brain Res. 1543, 109 (2014).
Wee, S. Y. & Aris, A. Z. Occurrence and public-perceived risk of endocrine disrupting compounds in drinking water. npj Clean. Water 2, 4 (2019).
González-Marino, I., Rodríguez, I., Quintana, J. B. & Cela, R. Investigation of the transformation of TCH-OOH during water chlorination by liquid-chromatography-quadrupole-time-of-flight-mass spectrometry. J. Hazard. Mater. 261, 636 (2013).
Mackie, A. L., Park, Y. R. & Gagnon, G. A. Chlorination kinetics of 11-nor-9-carboxy-Δ9-tetrahydrocannabinol—effects of pH and humic acid. Environ. Sci. Technol. 51, 10711 (2017).
Mackuľak, T. et al. Zerovalent iron and iron(VI): effective means for the removal of psychoactive pharmaceuticals and illicit drugs from wastewaters. Sci. Total Environ. 539, 420 (2016).
Catalá, M. et al. Elimination of drugs of abuse and their toxicity from natural waters by photo-Fenton treatment. Sci. Total Environ. 520, 198 (2015).
Khalid, A., Zubair, M. & Ihsanullah., A. Comparative study on the adsorption of eriochrome black T dye from aqueous solution on graphene and acid-modified. Arab. J. Sci. Eng. 43, 2167 (2018).
Chen, J. P. & Wu, S. Acid/base-treated activated carbons: characterization of functional groups and metal adsorptive properties. Langmuir 20, 2233 (2004).
Partlan, E. et al. Effect of bead milling on chemical and physical characteristics of activated carbons pulverized to superfine sizes. Water Res. 89, 161 (2016).
Zhang, S., Shao, T., Bekaroglu, S. S. K. & Karanfil, T. Adsorption of synthetic organic chemicals by carbon nanotubes: effects of background solution chemistry. Water Res. 44, 2067 (2010).
Apul, O. & Karanfil, T. Adsorption of synthetic organic contaminants by carbon nanotubes: a critical review. Water Res. 68, 34 (2015).
Schwarzenbach, R. P., Gschwend, P. M., & Imboden, D. M. Environmental Organic Chemistry (John Wiley & Sons, 2016).
Baker, D. R. & Kasprzyk-Hordern, B. Critical evaluation of methodology commonly used in sample collection, storage and preparation for the analysis of pharmaceuticals and illicit drugs in surface water and wastewater by solid phase extraction and liquid chromatography-mass spectrometry. J. Chromatogr. A 1218, 8036 (2011).
Acknowledgements
Authors thank Pagsuyoin Research Group for their support during preliminary efforts of cannabinoids’ detection. This work was supported by the faculty start-up grant from University of Massachusetts Lowell. However, the paper has not been subjected to the peer and policy review of the agency and therefore does not necessarily reflect their views.
Author information
Authors and Affiliations
Contributions
All authors have substantial contributions to (1) the conception or design of the work or the acquisition, analysis, or interpretation of the data, (2) drafting the work or revising it critically for important intellectual content, (3) final approval of the completed version, and (4) accountability for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khalid, A., Rowles, L.S., Ateia, M. et al. Mesoporous activated carbon shows superior adsorption affinity for 11-nor-9-carboxy-Δ9-tetrahydrocannabinol in water. npj Clean Water 3, 2 (2020). https://doi.org/10.1038/s41545-019-0049-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41545-019-0049-7