Abstract
Trivalent oral poliovirus vaccine (tOPV) has been withdrawn and instead an inactivated poliovirus vaccine (IPV) and bivalent type 1 and type 3 OPV (bOPV) sequential immunization schedule has been implemented since 2016, but no immune persistence data are available for this polio vaccination strategy. This study aimed to assess immune persistence following different polio sequential immunization schedules. Venous blood was collected at 24, 36, and 48 months of age from participants who had completed sequential schedules of combined IPV and OPV in phase III clinical trials. The serum neutralizing antibody titers against poliovirus were determined, and the poliovirus-specific antibody-positive rates were evaluated. A total of 1104 participants were enrolled in this study. The positive rates of poliovirus type 1- and type 3-specific antibodies among the sequential immunization groups showed no significant difference at 24, 36, or 48 months of age. The positive rates of poliovirus type 2-specific antibody in the IPV-IPV-tOPV group at all time points were nearly 100%, which was significantly higher than the corresponding rates in other immunization groups (IPV-bOPV-bOPV and IPV-IPV-bOPV). Immunization schedules involving one or two doses of IPV followed by bOPV failed to maintain a high positive rate for poliovirus type 2-specific antibody.
Similar content being viewed by others
Introduction
As we approach the end of poliomyelitis (polio) eradication, cases caused by wild-type polio are decreasing, whereas vaccine-derived poliovirus (VDPV) cases caused by oral poliovirus vaccine (OPV) are predominant1 To reduce the cases of VDPV, it is imperative that OPV is phased out, and countries fully convert to an inactivated poliovirus vaccine (IPV) immunization2. Starting from May 1, 2016, developing countries, including China, implemented a new polio vaccine immunization strategy, discontinuing trivalent live attenuated polio vaccine (tOPV) and updating the polio immunization regimen to include one dose of inactivated polio vaccine (IPV) plus two doses of bivalent live attenuated polio vaccine (bOPV)2,3,4.
The persistence of poliovirus-specific antibody levels in individuals who receive combined sequential immunization with IPV and bOPV after switching immunization strategies is relevant to the prevention and control of wild-type polio cases and VDPV. Prior to the switching of immunization strategies, some studies focused on the immune persistence of combined sequential immunization with IPV and trivalent OPV5 and the immune persistence of combined vaccines containing IPV components6,7. To date, many clinical trials have investigated the safety and immunogenicity of IPV and bOPV combined sequential immunization8,9,10, but few studies have investigated its immune persistence. At present, the immunization policy of most developing countries, including China, is to use combined sequential immunization with IPV and bOPV for fundamental immunity and bOPV for booster immunization3,11. Whether such an immunization strategy and the timing of booster immunization are appropriate requires further investigation.
We completed a phase III clinical trial before tOPV was discontinued in 2015–2016 to evaluate the immunogenicity and safety of bOPV and Sabin strain-based IPV (sIPV) combined sequential immunization in 2-month-old infants9. To further observe the long-term immunogenicity of different sequential immunization schedules combining IPV and bOPV, this follow-up extension study was conducted on participants of the phase III clinical trial. Venous blood was collected at the ages of 24, 36, and 48 months from participants who had completed basic polio immunization, the serum was separated to determine the titers of neutralizing antibodies against poliovirus types 1, 2, and 3, and the antibody-positive rates and antibody levels were evaluated.
Through this clinical investigation, we were able to observe sustained levels of polio-neutralizing antibodies, particularly type 2 polio-neutralizing antibodies, in individuals who received a sequential immunization program with one dose of IPV and two doses of IPV. At present, China and some developing countries use sIPV for immunization12,13, and the difference in immune persistence between sIPV and Salk strain-based IPV (known as conventional IPV, cIPV) can also be reflected in this study. The investigation of immune persistence after fundamental immunization in infants and young children can help us find the appropriate timing of booster immunization and explain whether a single dose of bOPV at 48 months of age is justified in the current polio immunization strategy.
Results
Study population
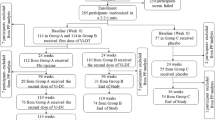
Of the 1200 participants in the previous phase III clinical trial, 35 did not receive all three doses of polio vaccine or lacked valid serum neutralizing antibody titer data; another 61 individuals voluntarily withdrew from the clinical study owing to a variety of reasons, such as moving away. The remaining 1104 participants enrolled in the present clinical study on the immunopersistence of sequential schedules of combined IPV and bOPV. In the previous trial, these 1104 participants had been assigned to six different immunization schedules: sIPV-bOPV-bOPV, sIPV-sIPV-bOPV, sIPV-sIPV-tOPV, cIPV-bOPV-bOPV, cIPV-cIPV-bOPV, or cIPV-cIPV-tOPV. The participant characteristics were compared among polio immunization schedule groups; there were no significant differences in participant age, sex, or race composition among groups (Table 1). At 24 months of age, 104 of the 1104 participants withdrew from blood collection; the other 1000 participants completed the 24-month-old immunopersistence observation. To compensate for the deficiency of poliovirus type 2-specific antibody in some participants, a single dose of IPV was administered to participants whose previous serum test revealed a poliovirus type 2-specific neutralizing antibody titer of <8. In total, a single dose of IPV was administered at 24 months of age to 85 participants who had a poliovirus type 2-specific antibody titer of <8 after primary immunization. Importantly, these individuals who were revaccinated with IPV were no longer included in the subsequent immunopersistence study. Thus, 1019 of the original 1104 participants were eligible for the 36-month-old immunopersistence observation. Of these 1019 participants, 113 participants withdrew; 906 participants completed the blood collection and antibody titer detection at 36 months of age. On the basis of those results, 122 participants received an additional dose of IPV after their 36-month blood collection, leaving 897 participants enrolled in the 48-month immunopersistence study. Finally, 284 participants withdrew over this final period; 613 participants completed the blood collection and antibody titer detection at 48 months of age (Fig. 1). The number of participants who received a supplementary dose of IPV in each group at the ages of 24, 36, and 48 months is shown in Supplementary Table 1-1, and the number of participants who completed blood collection for the observation of immune persistence is shown in Supplementary Table 1-2.
Comparison of poliovirus-specific antibody levels between different sequential immunization programs at the same time point
The serum poliovirus-specific neutralizing antibody titers were measured at 24, 36, and 48 months of age. A statistical analysis was conducted to compare the levels of poliovirus-specific neutralizing antibodies at 24, 36, or 48 months among participants who received different sequential polio immunizations. The positive rates for poliovirus type 1-specific antibody did not differ significantly among the sequential immunization groups (Table 2). The positive rates for poliovirus type 3-specific antibody did not differ significantly among the groups at 24 or 48 months of age, but a slight difference was observed at 36 months of age (Table 2, Supplemental Table 2).
By contrast, the positive rates for poliovirus type 2-specific antibody in serum collected at 24, 36, and 48 months of age differed significantly among the sequential immunization groups. The positive rates for poliovirus type 2-specific antibody were nearly 100% in the sIPV-sIPV-tOPV and cIPV-cIPV-tOPV groups at all time points; these positive rates were significantly higher than those in the other immunization groups. The positive rates for poliovirus type 2-specific antibody were significantly lower in the groups with an immunization schedule that contained only a single dose of IPV (sIPV-bOPV-bOPV, cIPV-bOPV-bOPV) than in those with an immunization schedule that contained two doses of IPV (cIPV-cIPV-bOPV, sIPV-sIPV-bOPV) (Table 2, Supplementary Table 2). In addition, for the immunization schedules with only one dose of IPV, the positive rates of poliovirus type 2-specific antibody in the group that injected cIPV were higher than those in the group that rejected sIPV at 24, 36, and 48 months of age separately (cIPV-bOPV-bOPV > sIPV-bOPV-bOPV, Table 2, Supplementary Table 2).
The GMTs of poliovirus-specific neutralizing antibody differed significantly among different immunization schedules (Table 2). For poliovirus type 1-specific neutralizing antibody levels: (1) at the same time point, the level of poliovirus type 1-specific neutralizing antibody was higher in the bOPV-immunized group than in the tOPV-immunized group (relative poliovirus type 1-specific neutralizing antibody levels: sIPV-bOPV-bOPV and sIPV-sIPV-bOPV > sIPV-sIPV-tOPV; cIPV-bOPV-bOPV and cIPV-cIPV-bOPV > cIPV-cIPV-tOPV); (2) within the same sequential immunization schedule, the poliovirus type 1-specific neutralizing antibody levels were higher at the same time point in the sIPV-immunized group than in the cIPV-immunized group (relative poliovirus type 1-specific neutralizing antibody levels: sIPV-bOPV-bOPV > cIPV-bOPV-bOPV; sIPV-sIPV-bOPV > cIPV-cIPV-bOPV; sIPV-sIPV-tOPV > cIPV-cIPV-tOPV) (Table 2, Supplementary Tables 3-1, 3-4, 3-7), It may be that the Sabin strain is used as a challenge strain for testing serum neutralizing antibody, causing the titer of poliovirus-specific antibody to be higher for sIPV than cIPV under the same immunization program. For poliovirus type 2-specific neutralizing antibody levels: in the case of inoculation with the same IPV (all sIPV or all cIPV), the levels were higher in the tOPV-immunized group than in the bOPV-immunized group at the same time point (relative poliovirus type 2-specific neutralizing antibody levels: sIPV-sIPV-tOPV > sIPV-sIPV-bOPV > sIPV-bOPV-bOPV; cIPV-cIPV-tOPV > cIPV-cIPV-bOPV > cIPV-bOPV-bOPV) (Table 2, Supplementary Tables 3-2, 3-5, 3-8). There was little difference in poliovirus type 3-specific neutralizing antibody levels among the groups, but the level of poliovirus type 3-specific neutralizing antibody was slightly lower in the tOPV-immunized group than in the bOPV-immunized group (sIPV-sIPV-tOPV < sIPV-sIPV-bOPV; cIPV-cIPV-tOPV < cIPV-cIPV-bOPV) (Table 2, Supplementary Tables 3-3, 3-6, 3-9).
Changes in poliovirus-specific antibody levels along the sampling time series axis
The changes in poliovirus-specific neutralizing antibody titers were observed longitudinally, by comparing the levels at 5 (28 days after the completion of basic immunization with three doses of polio vaccine), 24, 36, and 48 months of age. In order to more effectively analyze the overall changes in antibody level over time, these analyses were conducted on the set of data from the 496 participants who had antibody titers determined for blood samples collected at 24, 36, and 48 months of age and had not been additionally vaccinated with a supplementary dose of IPV. Participants who had received booster immunization or did not have an effective serum antibody titer at 24–48 months of age were excluded. The seropositive rates and GMTs in this set of data from 496 participants are shown in Supplementary Table 4, and the results of differential analysis among the sequential immunization groups at the same time point (24, 36, and 48 months of age separately) were similar to those of the full data set above.
The changes in poliovirus-specific antibody titers over time in each immunization schedule group are displayed in Fig. 2. For poliovirus type 1- and type 3-specific neutralizing antibodies, the patterns of change in poliovirus-specific neutralizing antibody titers over time were similar among the different sequential immunization schedule groups (Fig. 2a, c). At 28 days after the completion of three polio vaccine doses (5 months), almost all participants had a high titer of poliovirus type 1- and type 3-specific neutralizing antibody. During the period from 5 to 24 months, the reverse distribution curves of poliovirus type 1- and type 3-specific neutralizing antibody changed significantly, with a significant decrease in the population with a high titer of poliovirus-specific neutralizing antibody. During the period from 24 to 48 months, there was no significant change in the reverse distribution curve of poliovirus type 1- and type 3-specific neutralizing antibody. During the period from 5 to 48 months, the positive rates of poliovirus type 1- and type 3-specific neutralizing antibody (>23) did not change significantly; all immunization schedule groups maintained a positive rate of almost 100%.
The patterns of change in poliovirus type 2-specific neutralizing antibody titer over time were different among the sequential immunization schedule groups (Fig. 2b). Owing to the absence of poliovirus type 2 components in bOPV, the level of poliovirus type 2-specific neutralizing antibody was low in the sIPV-bOPV-bOPV and cIPV-bOPV-bOPV groups; correspondingly, the inverse antibody distribution curve decreased rapidly. The inverse antibody distribution curves of the groups that received two doses of IPV (sIPV-sIPV-bOPV and cIPV-cIPV-bOPV) declined more slowly than those of the groups that received only one dose of IPV (sIPV-bOPV-bOPV and cIPV-bOPV-bOPV); however, the distribution curve of poliovirus type 2-specific neutralizing antibody did not plateau, and there were no significant changes in the titers during the 5–48-month period. In the groups that received two doses of IPV and one dose of tOPV (sIPV-sIPV-tOPV and cIPV-cIPV-tOPV), the reverse distribution curves of poliovirus type 2-specific neutralizing antibody tended to be high and flat, and then decrease when the titer reached a relatively large value; in addition, the curves revealed a significant difference between the 5-month time point and the other time points, with a significant decrease in the proportion of participants with a high poliovirus type 2-specific neutralizing antibody titer, while there was no significant change during the 24–48-month period.
Discussion
Many studies14,15 have shown that seroconversion rates against polioviruses types 1 and 3 were non-inferior in sequential schedules containing IPV and bOPV, compared with all-IPV or all-tOPV schedule, and the levels of poliovirus type 2-specific neutralizing antibody exhibited a significant difference among different immunization schedules (IPV-bOPV-bOPV < IPV-IPV-bOPV < IPV-IPV-tOPV or tOPV-tOPV-tOPV), the results were in concordance with the observation of immune persistence. In our clinical trial, the immune persistence of poliovirus types 1 and 3-specific neutralizing antibody performed well in both of the sequential vaccination schedules. For the type 2 polio-specific neutralizing antibody positive rate observed in the persistence study, the type 2 polio-specific neutralizing antibody positive rate decreased more strongly between 24 and 48 months of age as the number of doses of type 2 polio-related vaccine received decreased. The seroprevalence of type 2 polio-specific neutralizing antibody decreased to 35.8% and 73.5% at 24 months of age in the sIPV-bOPV-bOPV and cIPV-bOPV-bOPV groups who received only one dose of type 2 polio-related vaccine. In the sIPV-sIPV-bOPV and cIPV-cIV-bOPV groups who received two doses of type 2 polio-related vaccine, the antibody positive rates decreased to 84.6% and 89.9% at 24 months of age. In the sIPV-sIPV-tOPV and cIPV-cIPV-tOPV groups vaccinated with three doses of type 2 polio-related vaccine, antibody positive rates were 100% and 99.4% at 24 months of age, respectively, and remained high at 24–48 months of age. In this clinical trial, a booster dose of IPV was administered to individuals who tested negative for polio neutralizing antibodies at 24, 36, and 48 months of age. The number of participants receiving a booster IPV dose in each group, as shown in Supplement Table 1, was in the order of IPV–bOPV–bOPV > IPV–IPV–bOPV > IPV–IPV–tOPV, which also reflected the immune persistence of each immunization schedule.
The clinical trial data suggest that vaccination with one dose of IPV does not provide long-term persistent immunity specific to type 2 poliovirus, and it is recommended that infants who have previously received one dose of IPV plus two doses of bOPV need to be vaccinated early with a vaccine containing type 2 poliovirus before 48 months of age. The clinical trial data also suggest that vaccination with two doses of IPV is more effective than a single dose of IPV for long-term persistence. Three doses of vaccine containing the type 2 polio component is optimal and will be progressively achieved as IPV production increases to address the lack of persistence of type 2 polio antibody in the population.
The clinical trial data revealed that the positive rate of type 2 polio antibody in the group vaccinated with one dose of cIPV (cIPV-bOPV-bOPV) was higher than that in the group vaccinated with one dose of sIPV (sIPV -bOPV-bOPV) during the period of 24–48 months, which may be caused by the difference in immunogenicity between the Salk and Sabin strains16,17. When two doses of IPV were administered, the difference in positive rates due to different types of IPV was eliminated.
After the switch of immunization strategy in 2016, the number of circulating vaccine-derived poliovirus type 2 (cVDPV2) cases has increased, triggering a public health emergency of international concern18,19,20,21. There are several reasons for this, including factors such as insufficient IPV production capacity, backward economic development, imperfect medical systems, religious beliefs, an IPV shortage, and low immunization coverage in some parts of the world22,23. Sequential immunization schedules, including IPV, cannot be established in many countries after the cessation of tOPV, resulting in a decline in population immunity against poliovirus24,25. Clinical studies have shown that a sequential immunization schedule of one dose of IPV plus two doses of bOPV is not sufficient to induce high level and long-lasting immune persistence of polio type 2-specific antibody.
In response to the high cost and shortage of IPV, some developing countries administer a fractional dose of IPV (fIPV)26,27. Studies have shown that two doses of fIPV induce the same antibody positive rate as two doses of full-dose IPV 1 month after vaccination, and that the slope of neutralizing antibody decay appears to be similar between the study groups28. Therefore, vaccination with fIPV is an effective method in the absence of IPV.
Despite the ongoing decline in the number of cVDPV2, the risk of international spread of cVDPV2 remains high29. The long distance international spread of VDPV2 between Jerusalem, London, New York, and Montreal has revealed a new risk phenomenon, namely, the evolution of vaccine-derived polioviruses in under-immunized pockets of the population who lack intestinal mucosal immunity in IPV-using countries29,30,31. Andino et al. constructed a novel live attenuated polio vaccine (nOPV), which not only succeeded OPV in rapidly stimulating the intestinal mucosal immunity of the population against polio, but also improved the genetic stability of the attenuated Sabin strain and reduced the possibility of recovering neurotoxicity, thus minimizing the risk of producing cVDPVs32,33. nOPV was authorized for outbreak response use under a World Health Organization Emergency Use Listing34. However, nOPV2 surveillance should continue for the duration of the Emergency Use Listing35.
The global effort to eradicate polio has made significant progress over the years, but continued vigilance and investment in immunization programs are needed to achieve the ultimate goal of a polio-free world.
Methods
Phase III clinical trial
This open-label, descriptive, single-centered, uncontrolled, extension study was conducted between 2015 and 2016 on selected individuals who had participated in a previous randomized, parallel-controlled trial (ClinicalTrials.gov number: NCT03614702) in Guangxi Province, China. The design of the previous randomized, parallel-controlled study has already been described in detail. Briefly, the eligible infants were randomized to receive one of six different sequential polio immunization schedules (sIPV-bOPV-bOPV, sIPV-sIPV-bOPV, sIPV-sIPV-tOPV, cIPV-bOPV-bOPV, cIPV-cIPV-bOPV, or cIPV-cIPV-tOPV; 200 participants per group). The three vaccine doses were administered at the ages of 2, 3, and 4 months old, respectively, and the antibody seroconversion rate was determined at 28 days after the third dose.
Immune persistence study design
This extension study builds on the previous phase III study. At the ages of 24, 36, and 48 months, venous blood (2–3 ml) was collected from participants who had completed a basic three-dose polio immunization. The serum was separated from these blood samples and used for determining the neutralizing antibody titers against poliovirus types 1, 2, and 3. The Sabin strain are used as a challenge strain for testing of serum neutralizing antibody. The antibody-positive rate was determined; antibody positive was defined as having a neutralizing antibody titer of ≥1:8 against type 1, type 2, and type 3 poliovirus.
After each blood collection, a decision on whether to inoculate each participant with IPV to enhance their immunity depended on their previous neutralizing antibody results against poliovirus. The serum neutralizing antibody titer was measured 28 days after IPV inoculation.
All of the above neutralizing antibody titer tests were conducted by the National Institutes for Food and Drug Control, following the protocol recommended by the World Health Organization.
The present trial(ClinicalTrials.gov ID: NCT03821441) launched in 2018 and completed in 2020, was designed by the Institute of Medical Biology, Chinese Academy of Medical Sciences and the Guangxi Center for Disease Prevention and Control and were approved by the State Food and Drug Administration of China. The clinical trial protocol was verified and approved by the ethical committee of Guangxi Zhuang Autonomous Region for Disease Control and Prevention (approval number: GXIRB2017-0009-2) and was conducted in accordance with the Declaration of Helsinki (revised 2013). Informed consent was obtained in writing from the legal guardians of all study participants.
Participants
The participants involved in the previous randomized, parallel-controlled study in Guangxi were recruited again. Eligible participants had completed a full course of primary vaccination against polio (three-dose immunization) and had a complete set of serum neutralizing antibody titer results available from the previous study. In addition, participants were required to be at least 24 months of age at the time of participating in the present clinical study. The exclusion criteria were as follows: (1) history of enhanced immunization with polio vaccine after completion of the previous clinical study; (2) history of confirmed infection with poliovirus; (3) participation in another study; and (4) another serious acute chronic disease or abnormality that could significantly increase the participant’s risk or interfere with the interpretation of our study results.
The guardians and families of the participants voluntarily complied with the requirements of the clinical trial protocol. Participants were permitted to voluntarily withdraw at any time during the trial. Participants could be withdrawn from the study in cases of failure to complete the follow-up visits, violation of or deviation from the trial protocol, or the appearance of other abnormal symptoms that interfered with the trial.
Study objective
The primary objective of the present study was to evaluate the persistence of the poliovirus-specific antibody-positive rate in participants who received sequential immunization of IPV combined with bOPV.
Statistical analysis
The seroconversion rates for antibody against poliovirus types 1, 2, and 3 were calculated in terms of each immunization schedule at 24, 36, and 48 months. Chi-squared tests and Fisher’s exact probability tests were used to compare the differences in seroconversion rates between groups.
The titers of antibodies against poliovirus types 1, 2, and 3 in all groups are presented as the geometric mean titers (GMTs) and their 95% confidence intervals. The differences in GMT between groups were compared by performing an analysis of variance after logarithmic transformation of the data. All statistical tests were considered significant at p < 0.05, two-sided.
Reverse cumulative distribution curves were plotted for the subsets of participants with effective antibody titers at 5 (i.e., 28 days after completion of basic immunization with three doses of vaccine), 24, 36, and 48 months of age.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ming, L. C., Hussain, Z., Yeoh, S. F., Koh, D. & Lee, K. S. Circulating vaccine-derived poliovirus: a menace to the end game of polio eradication. Glob. Health 16, 63 (2020).
Hampton, L.M. et al. Cessation of use of trivalent oral polio vaccine and introduction of inactivated poliovirus vaccine worldwide, 2016. Wkly Epidemiol. Rec. 91, 421–427 (2016).
Garon, J. et al. Polio endgame: the global switch from tOPV to bOPV. Expert Rev. Vaccines 15, 693–708 (2016).
Chinese Center for Disease Control and Prevention. Decision to Implement New Polio Vaccine Immunization Strategy, http://www.chinacdc.cn/jkzt/crb/zl/jshzy/ (2016).
Lu, L. et al. Immunogenicity and persistence from different 3-dose schedules of live and inactivated polio vaccines in Chinese infants. Vaccine 33, 4653–4658 (2015).
Mangarule, S. et al. Antibody persistence following administration of a hexavalent DTwP-IPV-HB-PRP approximately T vaccine versus separate DTwP-HB-PRP approximately T and IPV vaccines at 12-24 months of age and safety and immunogenicity of a booster dose of DTwP-IPV-HB-PRP approximately T in healthy infants in India. Vaccin. X 11, 100190 (2022).
Embree, J., Law, B., Voloshen, T. & Tomovici, A. Immunogenicity, safety, and antibody persistence at 3, 5, and 10 years postvaccination in adolescents randomized to booster immunization with a combined tetanus, diphtheria, 5-component acellular pertussis, and inactivated poliomyelitis vaccine administered with a hepatitis B virus vaccine concurrently or 1 month apart. Clin. Vaccin. Immunol. 22, 282–290 (2015).
Sutter, R. W. et al. Immunogenicity of a new routine vaccination schedule for global poliomyelitis prevention: an open-label, randomised controlled trial. Lancet 386, 2413–2421 (2015).
Zhao, T. et al. Post hoc analysis of two clinical trials to compare the immunogenicity and safety of different polio immunization schedules in Chinese infants. Ann. Transl. Med. 9, 253 (2021).
Brickley, E. B. et al. Intestinal Immunity to Poliovirus Following Sequential Trivalent Inactivated Polio Vaccine/Bivalent Oral Polio Vaccine and Trivalent Inactivated Polio Vaccine-only Immunization Schedules: Analysis of an Open-label, Randomized, Controlled Trial in Chilean Infants. Clin. Infect. Dis. 67, S42–S50 (2018).
Childhood Immunization Schedule for National Immunization Program Vaccines — China (Version 2021), https://www.chinacdc.cn/nip/kyjz/mycxbjsm/mycxb/.
Bakker, W. A. et al. Inactivated polio vaccine development for technology transfer using attenuated Sabin poliovirus strains to shift from Salk-IPV to Sabin-IPV. Vaccine 29, 7188–7196 (2011).
Okayasu, H., Sein, C., Hamidi, A., Bakker, W. A. & Sutter, R. W. Development of inactivated poliovirus vaccine from Sabin strains: A progress report. Biologicals 44, 581–587 (2016).
O’Ryan, M. et al. Inactivated poliovirus vaccine given alone or in a sequential schedule with bivalent oral poliovirus vaccine in Chilean infants: a randomised, controlled, open-label, phase 4, non-inferiority study. Lancet Infect. Dis. 15, 1273–1282 (2015).
Qiu, J. et al. Immunogenicity and safety evaluation of bivalent types 1 and 3 oral poliovirus vaccine by comparing different poliomyelitis vaccination schedules in China: A randomized controlled non-inferiority clinical trial. Hum. Vaccin Immunother. 13, 1–10 (2017).
Chen, H. et al. Immunogenicity and safety of sabin-strain based inactivated poliovirus vaccine replacing salk-strain based inactivated poliovirus vaccine: An innovative application of different strain-IPVs replacement. Vaccine 39, 2467–2474 (2021).
Kumar, P., Bird, C., Holland, D., Joshi, S. B. & Volkin, D. B. Current and next-generation formulation strategies for inactivated polio vaccines to lower costs, increase coverage, and facilitate polio eradication. Hum. Vaccin Immunother. 18, 2154100 (2022).
Modlin, J. F., Bandyopadhyay, A. S. & Sutter, R. Immunization Against Poliomyelitis and the Challenges to Worldwide Poliomyelitis Eradication. J. Infect. Dis. 224, S398–S404 (2021).
Gray, E. J., Cooper, L. V., Bandyopadhyay, A. S., Blake, I. M. & Grassly, N. C. The Origins and Risk Factors for Serotype-2 Vaccine-Derived Poliovirus Emergences in Africa During 2016-2019. J. Infect. Dis. 228, 80–88 (2023).
Zhou, J. et al. Detection and Initial Response to a Type 2 Vaccine-Derived Poliovirus - Sichuan Province, China, 2019. China CDC Wkly 2, 172–175 (2020).
Zarocostas, J. WHO keeps polio on the international health emergency list. Lancet 392, 2425 (2018).
Falleiros-Arlant, L. H., Ayala, S. E. G., Domingues, C., Brea, J. & Colsa-Ranero, A. Current status of poliomyelitis in Latin America. Rev. Chil. Infectol. 37, 701–709 (2020).
Sutter, R. W. & Cochi, S. L. Inactivated Poliovirus Vaccine Supply Shortage: Is There Light at the End of the Tunnel? J. Infect. Dis. 220, 1545–1546 (2019).
Zipursky, S. et al. Lessons Learned From Managing the Planning and Implementation of Inactivated Polio Vaccine Introduction in Support of the Polio Endgame. J. Infect. Dis. 216, S15–S23 (2017).
WHO. Polio (Pol3) immunization coverage among 1-year-olds (%), https://www.who.int/data/gho/data/indicators/indicator-details/GHO/polio-(pol3)-immunization-coverage-among-1-year-olds-(-) (2022).
Haldar, P. et al. Fractional-dose inactivated poliovirus vaccine, India. Bull. World Health Organ 97, 328–334 (2019).
Anand, A., Molodecky, N. A., Pallansch, M. A. & Sutter, R. W. Immunogenicity to poliovirus type 2 following two doses of fractional intradermal inactivated poliovirus vaccine: A novel dose sparing immunization schedule. Vaccine 35, 2993–2998 (2017).
Saleem, A. F. et al. One-Year Decline of Poliovirus Antibodies Following Fractional-Dose Inactivated Poliovirus Vaccine. J. Infect. Dis. 223, 1214–1221 (2021).
WHO. Statement of the thirty-fourth Polio IHR Emergency Committee, https://www.who.int/news/item/02-02-2023-statement-of-the-thirty-fourth-polio-ihr-emergency-committee (2023).
Cooper, L. V. et al. Risk factors for the spread of vaccine-derived type 2 polioviruses after global withdrawal of trivalent oral poliovirus vaccine and the effects of outbreak responses with monovalent vaccine: a retrospective analysis of surveillance data for 51 countries in Africa. Lancet Infect. Dis. 22, 284–294 (2022).
Klapsa, D. et al. Sustained detection of type 2 poliovirus in London sewage between February and July, 2022, by enhanced environmental surveillance. Lancet 400, 1531–1538 (2022).
Yeh, M. T. et al. Engineering the Live-Attenuated Polio Vaccine to Prevent Reversion to Virulence. Cell Host Microbe 27, 736–751.e738 (2020).
Van Damme, P. et al. The safety and immunogenicity of two novel live attenuated monovalent (serotype 2) oral poliovirus vaccines in healthy adults: a double-blind, single-centre phase 1 study. Lancet 394, 148–158 (2019).
Macklin, G. R. et al. Enabling accelerated vaccine roll-out for Public Health Emergencies of International Concern (PHEICs): Novel Oral Polio Vaccine type 2 (nOPV2) experience. Vaccine 41, A122–A127 (2023).
Martin, J. et al. Genetic Characterization of Novel Oral Polio Vaccine Type 2 Viruses During Initial Use Phase Under Emergency Use Listing - Worldwide, March-October 2021. MMWR Morb. Mortal. Wkly Rep. 71, 786–790 (2022).
Acknowledgements
We thank the research staff of the Guangxi Province Center for Disease Control and Prevention, Liujiang City Center for Disease Control and Prevention and all clinical investigators for their contributions to the trial. We wish to thank the research staff of National Institutes for Food and Drug Control for serum neutralization antibody detection; the staff of Simurike pharmaceutical information consulting Co. Ltd for study supervision and data management services; the experts at the Center For Drug Evaluation, China Food and Drug Administration and the National Center for Disease Control and Prevention for their valuable opinions and suggestions, which made it possible to conduct a smooth clinical trial. This research was supported by the Applied Basic Research Foundation of Yunnan (grant number: 202101AT070293), CAMS Innovation Fund for Medical Sciences (grant number: 2021-I2M-1-043), the State Project for Essential Drug Research and Development (grant number: 2015ZX09101031), and Major Science and Technology Projects in Yunnan Province(grant number: 202302AA310003).
Author information
Authors and Affiliations
Contributions
The corresponding authors had full access to all the data generated in the present study and assumed full responsibility for the final submission of this manuscript for publication. T.Z, J.L, T.H, Z.Y, Y.C Contributed equally to this manuscript and are co-first author. J.Y., Z.M, C.L, Q.L and T.Z. designed the research study. T.Z., J.L., T.H., Z.Y., Y.C., Y.F., J.T, Q.Y, D.W, G.L, L.Y, Y.Z, H.C, J.W and R.J led the production and validation of the vaccine. L.Y, W.C, W.Y, M.X, Q.Y, J.P and L.S responsible for the sample collection and testing work. C.H, Y.D, L.C, J.Z, Y.W, H.L, W.H and led the clinical follow-up observation work. Y.C, Z.Z, T.Z and J.T analyzed the data. All authors contributed to data interpretation and report writing, and all approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, T., Li, J., Huang, T. et al. Immune persistence after different polio sequential immunization schedules in Chinese infants. npj Vaccines 9, 50 (2024). https://doi.org/10.1038/s41541-024-00831-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-024-00831-1