Abstract
In this post hoc analysis of the ASCENT study, we compared outcomes with sacituzumab govitecan (SG) vs single-agent chemotherapy in clinically important subgroups of patients with metastatic triple-negative breast cancer (mTNBC). Patients with mTNBC refractory to/relapsing after ≥2 prior chemotherapies (≥1 in the metastatic setting) were randomized 1:1 to receive SG or treatment of physician’s choice (TPC) until unacceptable toxicity/progression. The primary endpoint was progression-free survival (PFS) per RECIST 1.1 by central review in patients without brain metastases. Patients with brain metastases were allowed if metastases were stable ≥4 weeks. In the intention-to-treat (ITT) population, 19% of patients were age ≥65 years; 12% were Black, and 12% had brain metastases. SG improved PFS and overall survival (OS), respectively, vs TPC in patients age ≥65 years (7.1 vs 2.4 months and 14.7 vs 8.9 months), or of Black race (5.4 vs 2.2 months and 13.8 vs 8.5 months), consistent with outcomes in the ITT population. Patients with brain metastases had numerically higher median PFS with SG vs TPC, but median OS was similar between treatment groups. SG was well tolerated and had a manageable safety profile consistent with the full safety population across all subgroups; neutropenia and diarrhea were the most common treatment-emergent adverse events. These findings confirm the meaningful clinical benefit of SG vs standard chemotherapy in patient subgroups with high unmet needs. SG should be considered an effective and safe treatment option for patients with mTNBC eligible for second-line or later therapy. ClinicalTrials.gov Number: NCT02574455.
Similar content being viewed by others
Introduction
Triple-negative breast cancer (TNBC) is a heterogenous disease with an aggressive clinical course and poorer outcomes than other breast cancer subtypes1,2. Certain patient subgroups defined by age, race, or location of disease (e.g., brain metastases) within the TNBC subtype are associated with even worse outcomes. Approximately 20% of new TNBC diagnoses are in patients age ≥65 years, who may have a higher rate of comorbidities, complicating their ability to tolerate systemic treatment-related toxicities3,4,5,6. Incidence rates of metastatic TNBC (mTNBC) among Black women are double those of White women, with worse clinical outcomes, potentially due to healthcare disparities, comorbidities, and differences in disease biology7,8,9. Patients with mTNBC who have brain metastases can have debilitating neurologic symptoms and poor survival, and treatment options may be limited due to challenges with drug delivery across the blood–brain barrier10.
Though the treatment landscape for mTNBC has evolved, patients with later-line mTNBC, especially in these poor prognosis subgroups, exclusively relied on single-agent chemotherapy as the standard treatment option until recently. However, single-agent chemotherapy is associated with low response rates, short progression‑free survival (PFS), and dose-limiting, cumulative adverse events (AEs)11,12,13,14,15,16. As a result, these patients could benefit from more effective and well-tolerated novel agents.
Sacituzumab govitecan (SG) is an antibody-drug conjugate (ADC) composed of anti–trophoblast cell surface antigen 2 (Trop-2) antibody coupled to the well-characterized payload SN-38 via a proprietary, hydrolyzable linker. Trop-2 is highly expressed in all breast cancer subtypes and has been shown to be a viable target in TNBC17,18. SG is approved in multiple countries (including the United States) for patients with mTNBC who received ≥2 prior therapies (≥1 in the metastatic setting) and is also approved in the United States for patients with hormone receptor positive/human epidermal growth factor receptor (HER2) negative metastatic breast cancer who received endocrine-based therapy and ≥2 additional systemic therapies in the metastatic setting19,20,21. Approval for mTNBC was based on results from the global, open-label, phase 3 ASCENT study (NCT02574455), which demonstrated a significant survival improvement for SG vs single-agent chemotherapy, with a manageable safety profile in the second-line or later mTNBC setting22,23. Median PFS was 4.8 vs 1.7 months (hazard ratio [HR] 0.43; 95% confidence interval [CI] 0.35–0.54), and median overall survival (OS) was 11.8 vs 6.9 months (HR 0.51; 95% CI 0.41–0.62) in the intention-to-treat (ITT) population22.
Given the proven clinical benefit of SG vs single-agent chemotherapy in the mTNBC second-line or later setting, it is important to further understand whether specific patient subgroups could derive benefit from SG. Here, we present post hoc efficacy and safety results from the ASCENT study in patients with mTNBC by subgroups based on age, race, presence of previously treated brain metastases, and by chemotherapy treatment of physician’s choice (TPC) selected prior to randomization in the ITT population.
Results
Patients
In total, 529 patients enrolled in the study (ITT population) were randomly assigned to SG (n = 235) or TPC (n = 233; 54% eribulin, 20% vinorelbine, 13% capecitabine, or 12% gemcitabine); 468 patients had no known brain metastases at baseline22.
Baseline patient demographics and disease characteristics for the ITT population and by subgroup are shown in Table 1. In the ITT population, the median age was 54 years, and the median number of prior lines of systemic therapy was four. Of the 529 patients, 101 patients (19%) were age ≥65 years, 62 patients (12%) self-reported Black race, and 61 patients (12%) had known brain metastases at baseline.
In general, patient disease characteristics were similar between patients aged <65 years (n = 428) and ≥65 years (n = 101) with some exceptions. Patients aged <65 years had a higher rate (61%) of negative germline breast cancer gene (BRCA) mutations in those patients with known BRCA status than patients aged ≥65 years (35%). The most common prior chemotherapy treatment regimens were generally used at a higher frequency with patients aged <65 vs ≥65 years, including anthracyclines (86% vs 67%, respectively) and cyclophosphamide (85% vs 73%); differences were also found in the use of previous checkpoint inhibitors (30% vs 23%) and neoadjuvant systemic therapies (53% vs 24%) (Table 1).
Baseline characteristics between Black (n = 62) and Other race (n = 467) subgroups were similar except for missing information regarding BRCA1/2 mutation status (48% vs 35%, respectively), a higher rate of TNBC at initial diagnosis (79% vs 69%), and a higher frequency of axillary lymph node involvement (37% vs 24%). Additionally, there were some differences in the most common prior chemotherapy treatments between Black and Other subgroups, in particular for carboplatin (77% vs 67%). Black patients also had higher previous use of checkpoint inhibitors (37%) vs Other race patients (28%) (Table 1).
In general, patient disease characteristics were similar between patients without (n = 468) and with (n = 61) brain metastases with some exceptions. Patients with brain metastases had a higher rate of BRCA mutations (15%) than patients without brain metastases (7%). More patients with vs without brain metastases had TNBC at initial diagnosis (82% vs 69%, respectively), a greater number of prior anticancer regimens (median [range]: 4 [2–9]) vs 3 [1–16]), a higher rate of >3 prior chemotherapies (43% vs 30%), prior capecitabine therapy (79% vs 65%), and previous checkpoint inhibitory treatment (43% vs 27%) prior to enrollment. Patients with brain metastases also more commonly had major tumor locations in the lung (67% vs 44%) and bone (36% vs 22%), and less commonly in the liver (36% vs 43%) and axillary lymph nodes (11% vs 28%) (Table 1).
Demographics and baseline characteristics for SG and each TPC agent were balanced between treatment arms. Within the TPC arm, the most commonly used chemotherapy was eribulin (n = 139), followed by vinorelbine (n = 52), capecitabine (n = 33), and gemcitabine (n = 38) (Table 2).
In total, 258 patients in the SG group and 224 patients in the TPC group were treated. As of February 25, 2021, no patients remained on treatment in any subgroup. Across subgroups, disease progression was the most common reason for treatment discontinuation for SG and TPC (age ≥65 years, 88% and 75%; Black race, 79% and 74%; with brain metastases, 72% and 62% for SG and TPC, respectively). The median duration of follow-up for all ITT patients was 8.8 months. Median duration of SG treatment varied across the subgroups (Supplementary Table 1). Patients age ≥65 years generally had the longest exposure to SG treatment (median, 6.7 months) and patients with brain metastases had the shortest (median, 2.5 months). Patient disposition for the patient subgroups is presented in Fig. 1 and Supplementary Table 1.
aPatients in the TPC arms were randomized to eribulin (n = 139), vinorelbine (n = 52), gemcitabine (n = 38), or capecitabine (n = 33). Details of the trial profile for the BMNeg population have been published previously. Reasons the patients discontinued treatment in each subgroup are presented in Table S1 in the Supplemental Appendix. Other race subgroup includes any patient who did not self-identify as Black race. BMNeg brain metastases-negative, BMPos brain metastases-positive, ITT intention to treat, SG sacituzumab govitecan, TPC treatment of physician’s choice.
Efficacy outcomes
Age
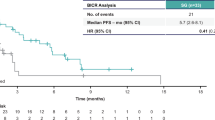
For patients <65 years (n = 428), median PFS for SG vs TPC was 4.2 vs 1.6 months (HR 0.45; 95% CI 0.35–0.57), and median OS was 10.8 vs 6.7 months (HR 0.54; 95% CI 0.43–0.66), respectively (Fig. 2). Objective response rate (ORR) was 28% vs 5%, and clinical benefit rate (CBR) was 37% vs 8%, respectively, in these patients (Table 3). In patients age ≥65 years (n = 101), median PFS for SG vs TPC was 7.1 vs 2.4 months (HR 0.25; 95% CI 0.14–0.43), and median OS was 14.7 vs 8.9 months (HR 0.47; 95% CI 0.29–0.75; Table 2 and Fig. 2). ORR by blinded independent central review (BICR) was 45% vs 0%, and CBR was 55% vs 8%, respectively (Table 3).
a Progression-free survival; b overall survival. Survival outcomes were assessed in the intention-to-treat population (all randomly assigned patients with and without brain metastases). Other race subgroup includes any patient who did not self-identify as Black race. PFS was determined by blinded independent central review according to Response Evaluation Criteria in Solid Tumors, version 1.1. OS overall survival, PFS progression-free survival, SG sacituzumab govitecan, TPC treatment of physician’s choice.
Black race
In Black patients (SG, n = 28; TPC, n = 34), median PFS for SG vs TPC was 5.4 vs 2.2 months (HR 0.44; 95% CI 0.24–0.80), and median OS was 13.8 vs 8.5 months (HR 0.62; 95% CI 0.34–1.11). ORR by BICR was 32% vs 6%, and CBR was 43% and 15%, respectively. In the SG group, one patient (4%) achieved a complete response (CR), and eight patients (29%) achieved a partial response (PR). In Other race patients (SG, n = 239; TPC n = 228), median PFS for SG vs TPC was 4.6 vs 1.6 months (HR 0.40; 95% CI 0.32–0.51), and median OS was 11.7 vs 6.9 months (HR 0.51; 95% CI 0.41–0.62). ORR by BICR was 31% vs 4%, and CBR was 40% vs 7%, respectively. In the SG group, 9 patients (4%) achieved a CR, and 65 patients (27%) achieved a PR (Table 3).
Brain metastases
In patients with stable brain metastases at study entry (SG, n = 32; TPC, n = 29), median PFS for SG vs TPC was 2.8 vs 1.6 months (HR 0.68; 95% CI 0.38–1.23). Median OS was 7.0 vs 7.5 months (HR 0.96; 95% CI 0.55–1.68; Table 3). ORR by BICR was 3% vs 0%, and CBR was 9% and 3%, respectively (Table 3).
In patients without brain metastases at study entry (SG, n = 235; TPC, n = 233), median PFS for SG vs TPC was 5.5 vs 1.7 months (HR 0.35; 95% CI 0.28–0.44). Median OS was 12.1 vs 6.7 months (HR 0.48; 95% CI 0.38–0.59). ORR by BICR was 35% vs 5%, and CBR was 45% vs 9%, respectively (Table 3).
Individual TPC agents
Treatment with SG (n = 267) resulted in longer median PFS vs eribulin (n = 139), vinorelbine (n = 52), capecitabine (n = 33), or gemcitabine (n = 38), with a median of 4.8 vs 2.1, 1.5, 1.6, and 2.4 months, respectively, as well as OS (median 11.8 vs 7.2, 5.6, 5.2, and 8.4 months) and ORR (31% vs 4%, 4%, 6%, and 3%, respectively) (Table 4).
Safety outcomes
In general, the safety profile of SG in the overall safety population was similar to that for TPC and was manageable. Grade ≥3 treatment-emergent AEs (TEAEs) occurred in 73% of patients treated with SG and 65% of patients treated with TPC. Serious AEs occurred in 27% and 29% of patients treated with SG compared with TPC, respectively. TEAEs leading to dose reduction occurred in 22% of patients treated with SG compared with 26% of patients treated with TPC, and 5% of patients in both arms experienced TEAEs leading to study drug discontinuation (Table 5).
Overall, a similar AE profile was seen for SG among the age, race, and brain metastases subgroups. In the SG arm, the frequency of grade ≥3 TEAEs was higher in patients with brain metastases vs patients without brain metastases (80% vs 72%). Patients of Black race in the TPC arm were more likely to have an AE leading to dose reduction (35% vs 25%) or interruption (48% vs 37%) compared with Other race patients. Patients age ≥65 years were more likely to undergo dose reduction due to TEAEs vs patients age <65 years (37% vs 19%). The incidence of TEAEs leading to SG treatment discontinuation was generally low across subgroups (age ≥65 years, 2%; of Black race, 4%; with brain metastases, 7%) and consistent with that of the overall safety population (5%). Serious AEs occurred less frequently in patients of Black race compared with the overall safety population (20% vs 27%).
The most common TEAEs of any grade with SG vs TPC in the overall safety population included diarrhea (65% vs 17%), neutropenia (64% vs 44%), nausea (62% vs 30%), and fatigue (52% vs 40%). These were also the most common TEAEs of any grade across the patient subgroups, all of which occurred at a higher frequency in the SG vs TPC arm: neutropenia (age ≥65 years, 59% vs 44%; Black race, 64% vs 61%; with brain metastases, 63% vs 52%, respectively), diarrhea (age ≥65 years, 74% vs 19%; Black race, 72% vs 19%; with brain metastases, 50% vs 13% respectively), nausea (age ≥65 years, 51% vs 29%; Black race, 52% vs 35%; with brain metastases, 43% vs 26%, respectively), and fatigue (aged ≥65 years, 53% vs 50%; Black race, 52% vs 45%; with brain metastases, 63% vs 52%, respectively) (Supplementary Results and Supplementary Tables 2–4).
The most common grade ≥3 TEAEs in the SG arm vs TPC arm in the overall safety population were neutropenia (52% vs 34%) and diarrhea (12% vs <1%). The same grade ≥3 TEAEs were the most common across the patient subgroups, and occurred at a higher frequency in the SG vs TPC arm: neutropenia (age ≥65 years, 47% vs 40%; Black race, 48% vs 42%; with brain metastases, 60% vs 26%, respectively) and diarrhea (age ≥65 years, 12% vs 0%; Black race, 4% vs 0% with brain metastases, 7% vs 0%, respectively) (Supplementary Results and Supplementary Tables 2–4). In the SG arm, concomitant growth factor support and other supportive measures were used for AE management as previously described22. No interstitial lung disease was observed in any subgroup.
SG and TPC
Grade ≥3 TEAEs with SG vs eribulin or vs vinorelbine, capecitabine, and gemcitabine combined were primarily hematological. Discontinuation rates due to TEAE were generally similar between groups (Table 6 and Supplemental Table 5).
Discussion
Due to the significant clinical benefit observed in the phase 3 ASCENT study, SG was approved for use and recommended by major guidelines for the treatment of patients with mTNBC who received ≥2 prior therapies (≥1 in the metastatic setting)19,20,21,24. Additional subgroup analyses for specific patient populations with pretreated mTNBC that have high unmet need and present particular challenges provide valuable insight into SG outcomes for treatment decision making. In these post hoc analyses of the ASCENT study, SG demonstrated improved outcomes compared with single-agent chemotherapy among patients who are age ≥65 years, of Black race, or with brain metastases, with a safety profile consistent with the original ITT population22. Furthermore, SG showed consistent efficacy benefit over each TPC chemotherapy agent individually including median PFS, OS, and ORR.
Comorbidities and functional impairment can predispose patients to a higher rate of chemotherapy-related toxicities, especially in frail older patients with TNBC3,4,25. In ASCENT, patients age ≥65 years who received SG had a significant improvement in outcomes compared to TPC with respect to PFS (median, 7.1 vs 2.4 months), OS (median, 15.3 vs 8.2 months), and ORR (50% vs 0%). This efficacy benefit together with longer time to treatment discontinuation for SG vs TPC in the ITT population26, supports a favorable risk/benefit profile for SG vs TPC in patients with mTNBC age ≥65 years.
Black patients with mTNBC have poor outcomes but historically constituted a low percentage of breast cancer clinical trial participants (3%–5%)7,8,9. As a result, data related to optimal treatment of these patients are often lacking, though efforts are ongoing to improve representation in clinical trials27. In total, 62 (12%) patients enrolled in the ASCENT study self-identified as Black. Black patients derived a similar clinical benefit from SG over TPC in PFS (Black: 5.4 vs 2.2 months; Other: 4.6 vs 1.6 months) and OS (Black: 13.8 vs 8.5 months; Other: 11.7 vs 6.9 months) as seen in the Other race population, suggesting SG is an effective treatment option for these patients.
Clinical trials in patients with breast cancer often exclude patients with brain metastases due to their poor prognosis and the limited ability of systemic agents to cross the blood–brain barrier; as such, limited clinical data are available for these patients10. Translational data suggest that SN-38 can cross the blood–brain barrier28,29. Furthermore, SG has shown activity in the CNS in clinical trials and real-world evidence studies30,31,32,33,34. In this post hoc analysis of 61 patients with stable treated brain metastases from the ASCENT study, SG showed numerically better outcomes than TPC for median PFS (2.8 vs 1.6 months) and tumor responses (3% vs 0%) but showed similar median OS to TPC. Though this represents only an incremental improvement in efficacy outcomes, whether SG is active across the blood–brain barrier in patients with progressive brain metastases remains unknown and is currently being investigated35.
These post hoc analyses in patients with age ≥65 years, Black race, or with brain metastases demonstrated that the safety profile of SG was consistent with the known AEs associated with SG in the ITT population22. The most commonly reported TEAEs across all patient subgroups were neutropenia and diarrhea, which were manageable with supportive care and dose reductions. Additionally, no cases of interstitial lung disease were reported with SG in this trial, an AE of concern with other classes of ADC agents commonly used to treat this patient population36. Treatment discontinuation due to TEAEs was generally low and there were no treatment-related deaths across patient subgroups. The safety profiles for individual TPC agents (eribulin, vinorelbine, capecitabine, and gemcitabine) were consistent with that of TPC overall22.
Data interpretation in these subgroup analyses is limited by the small sample size, therefore it can be difficult to draw firm conclusions from specific patient populations, especially patients with brain metastases. As all subgroup analyses were post hoc, no adjustment was made for multiple testing in the current analysis.
In conclusion, in these subgroup analyses from ASCENT, SG improved efficacy outcomes vs TPC in patient groups with mTNBC and poor prognosis in the second-line or later setting. In patients age ≥65 years, SG is safe and effective demonstrating improved clinical benefit over single-agent chemotherapy. In Black patients, a population historically known to have poor outcomes, SG offers an effective treatment option that improves survival outcomes over single-agent chemotherapy in this largest study of an ADC in patients with mTNBC to date. Efficacy outcomes across these subgroups and safety were consistent with that of the ITT population22. Although SG did not improve outcomes for patients with brain metastases compared with the ITT population, numerical PFS clinical benefit was observed in this extremely high-risk and difficult-to-treat population. These data also indicate that for patients with poorer survival outcomes, SG appears to have a greater benefit than TPC. Additionally, SG showed superior efficacy compared with each individual TPC chemotherapy. Taken together, SG should be considered an effective treatment option for patients with mTNBC eligible for second-line or later therapy.
Methods
Patients
Eligibility for the phase 3 ASCENT study (NCT02574455) has been reported previously22. Briefly, eligible patients had mTNBC (according to the standard American Society of Clinical Oncology/College of American Pathologists criteria; HER2 immunohistochemistry 0, 1, or 2/in situ hybridization negative; estrogen receptor/progesterone receptor <1%37) that was relapsed/refractory to ≥2 prior standard chemotherapy regimens (≥1 in the metastatic setting; no upper limit) for unresectable, locally advanced/metastatic disease. Patients were required to have received a prior taxane (any setting). Patients with known brain metastases (capped at 15%) were eligible if their central nervous system disease was stable for ≥4 weeks by MRI defined as ≥2 weeks from discontinuation of antiseizure medication and corticosteroid dose (≤20 mg prednisone equivalent) that was stable or decreasing for ≥2 weeks before randomization. Brain MRIs were required at each restaging throughout the study for these patients.
Trial design and treatment
Details of the study design have been previously described22. Briefly, patients were randomized 1:1 to either SG (10 mg/kg intravenously on days 1 and 8, every 21 days) or TPC (capecitabine, eribulin, vinorelbine, or gemcitabine) until disease progression, unacceptable toxicity, study withdrawal, or death, whichever occurred first. No crossover to the SG arm was allowed upon progression with chemotherapy. Stratifications factors at randomization included number of prior chemotherapy regimens for advanced disease (2–3 vs >3) and presence of known brain metastases at baseline (yes vs no).
Trial oversight
The study was approved by national regulatory authorities and each investigational site’s institutional review/ethics committee (see Supplement for full list of institutions) before implementation and was compliant with the Declaration of Helsinki and International Council for Harmonisation Good Clinical Practice Guidelines. As described in the originally reported study22, all patients provided written informed consent.
Endpoints
The primary endpoint was PFS per BICR (using Response Evaluation Criteria in Solid Tumors [RECIST] v1.138) in patients without known brain metastases. Secondary endpoints included PFS per investigator assessment, PFS in the ITT population (patients with and without brain metastases) by BICR, ORR, OS, and safety.
Assessments
Tumor responses were assessed by imaging scans (computed tomography or MRI) obtained every 6 weeks for 36 weeks, then every 9 weeks thereafter, until disease progression requiring treatment discontinuation. Scans to confirm responses were required 4–6 weeks after initial assessment. During long-term follow-up, survival data were collected every 4 weeks.
Safety and tolerability were evaluated in all treated patients, with the severity of AEs graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03, and coded per the Medical Dictionary for Regulatory Activities, version 22.1. Premedication/concomitant medications and supportive measures allowed and recommended for patients during the study were previously described22.
Subgroup analyses
Post hoc analyses described herein include subgroups defined by baseline demographics of age (age <65 years and ≥65 years), Black race, and brain metastases from the ITT population. The analysis of patients with no known brain metastases at baseline was prespecified in the study protocol. Analysis of outcomes with SG or TPC treatment were prespecified. Analysis by TPC selected prior to randomization was not prespecified per the protocol.
Statistical analysis
Subgroup analyses were conducted using the statistical approach similar to the primary analysis22. Kaplan–Meier estimates were used to analyze PFS and OS in each treatment group, with medians and corresponding 95% CIs determined according to the Brookmeyer and Crowley method with log-log transformation (one-sided). The magnitude of the PFS and OS benefit was measured by HRs and their 95% CIs estimated from unstratified Cox proportional-hazards models. Response rate in each treatment group was reported together with the corresponding 95% CI based on the exact method.
Efficacy analyses were conducted in the patient population specified for each subgroup analysis. Efficacy in older and Black patients has previously been reported22. Safety was analyzed in patients who received one or more dose of study drug.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Gilead Sciences shares anonymized individual patient data upon request or as required by law or regulation with qualified external researchers based on submitted curriculum vitae and reflecting non conflict of interest. The request proposal must also include a statistician. Approval of such requests is at Gilead Science’s discretion and is dependent on the nature of the request, the merit of the research proposed, the availability of the data, and the intended use of the data. Data requests should be sent to datarequest@gilead.com.
References
Kohler, B. A. et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J. Natl Cancer Inst. 107, djv048 (2015).
Plasilova, M. L. et al. Features of triple-negative breast cancer: analysis of 38,813 cases from the national cancer database. Medicine 95, e4614 (2016).
Kaplan, H. G., Malmgren, J. A. & Atwood, M. K. Triple-negative breast cancer in the elderly: prognosis and treatment. Breast J. 23, 630–637 (2017).
Kim, J. & Hurria, A. Determining chemotherapy tolerance in older patients with cancer. J. Natl Compr. Cancer Netw. 11, 1494–1502 (2013).
Steinmeyer, Z. et al. Low lean mass and chemotherapy toxicity risk in the elderly: the Fraction study protocol. BMC Cancer 19, 1153 (2019).
Bhatt, V. R. Cancer in older adults: understanding cause and effects of chemotherapy-related toxicities. Future Oncol. 15, 2557–2560 (2019).
Cho, B. et al. Evaluation of racial/ethnic differences in treatment and mortality among women with triple-negative breast cancer. JAMA Oncol. 7, 1016–1023 (2021).
DeSantis, C. E., Miller, K. D., Goding Sauer, A., Jemal, A. & Siegel, R. L. Cancer statistics for African Americans, 2019. CA Cancer J. Clin. 69, 211–233 (2019).
Dietze, E. C., Sistrunk, C., Miranda-Carboni, G., O’Regan, R. & Seewaldt, V. L. Triple-negative breast cancer in African-American women: disparities versus biology. Nat. Rev. Cancer 15, 248–254 (2015).
Kadamkulam Syriac, A., Nandu, N. S. & Leone, J. P. Central nervous system metastases from triple-negative breast cancer: current treatments and future prospective. Breast Cancer (Dove Med. Press) 14, 1–13 (2022).
Brufsky, A. et al. Second-line bevacizumab-containing therapy in patients with triple-negative breast cancer: subgroup analysis of the RIBBON-2 trial. Breast Cancer Res. Treat. 133, 1067–1075 (2012).
Park, I. H. et al. Randomized open label phase III trial of irinotecan plus capecitabine versus capecitabine monotherapy in patients with metastatic breast cancer previously treated with anthracycline and taxane: PROCEED trial (KCSG BR 11-01). Cancer Res. Treat. 51, 43–52 (2019).
Perez, E. A., Patel, T. & Moreno-Aspitia, A. Efficacy of ixabepilone in ER/PR/HER2-negative (triple-negative) breast cancer. Breast Cancer Res. Treat. 121, 261–271 (2010).
Pivot, X. et al. Pooled analyses of eribulin in metastatic breast cancer patients with at least one prior chemotherapy. Ann. Oncol. 27, 1525–1531 (2016).
Twelves, C. et al. Efficacy of eribulin in women with metastatic breast cancer: a pooled analysis of two phase 3 studies. Breast Cancer Res. Treat. 148, 553–561 (2014).
Li, C. H., Karantza, V., Aktan, G. & Lala, M. Current treatment landscape for patients with locally recurrent inoperable or metastatic triple-negative breast cancer: a systematic literature review. Breast Cancer Res. 21, 143 (2019).
Ambrogi, F. et al. Trop-2 is a determinant of breast cancer survival. PLoS ONE 9, e96993 (2014).
Vidula, N., Yau, C. & Rugo, H. S. Trop2 gene expression (Trop2e) in primary breast cancer (BC): correlations with clinical and tumor characteristics. J. Clin. Oncol. 35, 1075–1075 (2017).
Trodelvy. Prescribing information. Gilead Sciences, Inc., Foster City, CA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761115s000lbl.pdf (2023).
Trodelvy. Summary of product characteristics. Gilead Sciences Ireland UC, County Cork, Ireland https://www.ema.europa.eu/en/documents/product-information/trodelvy-epar-product-information_en.pdf (2021).
Michaleas, S. et al. The European Medicines Agency review of sacituzumab govitecan for the treatment of triple-negative breast cancer. ESMO Open 7, 100497 (2022).
Bardia, A. et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N. Engl. J. Med. 384, 1529–1541 (2021).
Carey, L. A. et al. Sacituzumab govitecan as second-line treatment for metastatic triple-negative breast cancer-phase 3 ASCENT study subanalysis. NPJ Breast Cancer 8, 72 (2022).
Gennari, A. et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 32, 1475–1495 (2021).
Repetto, L. Greater risks of chemotherapy toxicity in elderly patients with cancer. J. Support. Oncol. 1, 18–24 (2003).
Loibl, S. et al. Assessment of health-related quality of life by clinical response from the phase 3 ASCENT study in metastatic triple-negative breast cancer (mTNBC). Cancer Res. 82, Abstract P5-16-01 (2022).
Oyer, R. A. et al. Increasing racial and ethnic diversity in cancer clinical trials: an American Society of Clinical Oncology and Association of Community Cancer Centers joint research statement. J. Clin. Oncol. 40, 2163–2171 (2022).
Mohammad, A. S. et al. Liposomal irinotecan accumulates in metastatic lesions, crosses the blood-tumor barrier (BTB), and prolongs survival in an experimental model of brain metastases of triple negative breast cancer. Pharm. Res. 35, 31 (2018).
Vejjasilpa, K. et al. Antitumor efficacy and intratumoral distribution of SN-38 from polymeric depots in brain tumor model. Exp. Biol. Med. 240, 1640–1647 (2015).
Balinda, H. et al. CTNI-10. Sacituzumab govitecan for breast cancer brain metastasis. Neuro-oncology 25, v75 (2023).
Kelly, W. et al. CTNI-36. Sacituzumab govitecan for recurrent glioblastoma. Neuro-oncology 25, v82 (2023).
Alaklabi, S., Elijah, J., Roy, A. M., Groman, A. & Gandhi, S. 3 Real-world outcomes of sacituzumab govitecan in metastatic breast cancer patients: a single institution experience. Oncology 37, 9–10 (2023).
Hanna, D. et al. Real-world study of sacituzumab govitecan in metastatic triple-negative breast cancer in the United Kingdom. ESMO Open 8, Abstract 232P (2023).
Loirat, D. et al. Sacituzumab govitecan in metastatic triple-negative breast cancer: efficacy -with a focus on brain metastases- and toxicity in a real-world cohort. ESMO Open 8, Abstract 216P (2023).
ClinicalTrials.gov. ClinicalTrials.gov: a phase II trial of sacituzumab govitecan (IMMU-132) (NSC #820016) for patients with HER2-negative breast cancer and brain metastases. https://classic.clinicaltrials.gov/ct2/show/NCT04647916.
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 387, 9–20 (2022).
Allison, K. H. et al. Estrogen and progesterone receptor testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Guideline Update. Arch. Pathol. Lab. Med. 144, 545–563 (2020).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Acknowledgements
We would like to thank the patients, their caregivers, and their families for their participation and commitment to clinical research. Thank you to the clinical trial investigators and their team members, without whom this work would not have been possible. The study was sponsored by Gilead Sciences, Inc. and was designed through a collaboration of the sponsor and the lead investigators. Medical writing and editorial assistance were provided by Team 9 Science and Gwendolyn F. Elphick, PhD, at Parexel and was funded by Gilead Sciences, Inc. This study was sponsored by Gilead Sciences, Inc.
Author information
Authors and Affiliations
Contributions
S.A.H.: conceptualization of the manuscript; review of data, manuscript writing or review and final approval to submit. A.B.: enrolled patients, review of data, manuscript writing or review and final approval to submit. K.P.: enrolled patients, review of data, manuscript writing or review and final approval to submit. K.K.: enrolled patients, review of data, manuscript writing or review and final approval to submit. L.A.C.: enrolled patients, review of data, manuscript writing or review and final approval to submit. H.S.R.: enrolled patients, review of data, manuscript writing or review and final approval to submit. V.D.: enrolled patients, review of data, manuscript writing or review and final approval to submit. S.P.: conceptualization of the manuscript, review of data, manuscript writing or review and final approval to submit. R.D.: conceptualization of the manuscript, review of data, manuscript writing or review and final approval to submit. Y.Z.: review of data, manuscript writing or review and final approval to submit. S.M.T.: enrolled patients, review of data, manuscript writing or review and final approval to submit.
Corresponding author
Ethics declarations
Competing interests
S.A.H. has contracted research with Ambrx, Amgen, AstraZeneca, Arvinas, Bayer, Daiichi Sankyo, Genentech/Roche, Gilead, GSK, Immunomedics, Lilly, MacroGenics, Novartis, Pfizer, OBI Pharma, Pieris, PUMA, Radius, Sanofi, Seattle Genetics, Dignitana, Zymeworks, and Phoenix Molecular Designs; and stock options with NK Max. A.B. received grants from Genentech, Novartis, Pfizer, Merck, Sanofi, Radius Health, and Immunomedics; grants and personal fees from Biotheranostics; and personal fees from Pfizer, Novartis, Genentech, Merck, Radius Health, Immunomedics, Taiho, Sanofi, Daiichi Sankyo Pharma/AstraZeneca, Puma, Phillips, Eli Lilly, and Foundation Medicine. K.P. received honoraria for consultancy/advisory board functions and speaker fees from AstraZeneca, Eli Lilly, Exact Sciences, Focus Patient, Gilead Sciences, MSD, Novartis, Roche, and Seagen; support for attending meetings and/or travel from AstraZeneca, Novartis, Pfizer, PharmaMar, and Roche; and equipment, materials, drugs, medical writing, gifts or other services from MSD and Gilead Sciences. He has participated on a Data Safety Monitoring Board or Advisory Board of Eli Lilly, Gilead Sciences, MSD, Novartis, Pierre Fabre, Roche, Teva, and Vifor Pharma; is a board member of the Belgian Society of Medical Oncology; and is a member of the ESMO Young Oncologists Committee and the ESMO Resilience Task Force committee. His institution has received research grants from or had contracts with Sanofi and MSD; consulting fees from AstraZeneca, Gilead Sciences, MSD, Novartis, Pfizer, and Roche; and payment or honoraria from AstraZeneca, Eli Lilly, Gilead Sciences, MSD, Mundi Pharma, Novartis, Pfizer, and Roche. K.K. received contracts from Incyte, Novartis, Genentech, Lilly, Pfizer, Calithera, Immunomedics, Acetylon, Seattle Genetics, Amgen, Zeno, and CytomX; consulting fees from Lilly, Pfizer, Novartis, Eisai, AstraZeneca, Genentech, Immunomedics, Merck, Seattle Genetics, Cyclacel, and OncoSec Medical; payment/honoraria from Lilly; and support for attending meetings and/or travel from Lilly, AstraZeneca, and Pfizer. He is a member of steering committees at Immunomedics, AstraZeneca, and Genentech. His other financial/non-financial interests include having a spouse who has employment with Grail, Array, and Pfizer. L.A.C. participated on a Data Safety Monitoring Board or Advisory Board of Sanofi Aventis, Novartis, Genentech/Roche, GSK, AstraZeneca/Daiichi Sankyo, and Aptitude Health. Her other financial or non-financial interests include having a spouse who has served on the board of Falcon Therapeutics and who has had involvement in a neural stem cell therapy patent. H.S.R. received grants from Pfizer, Merck, Novartis, Lilly, Genentech, OBI, Odonate, Daiichi Sankyo, Seattle Genetics, Eisai, MacroGenics, Sermonix, Immunomedics, and AstraZeneca; non-financial support from Daiichi Sankyo, Mylan, Pfizer, Merck, Novartis, AstraZeneca, and MacroGenics; and personal fees from Mylan, Puma, and Samsung. A.B. reports grants from Genentech, Novartis, Pfizer, Merck, Sanofi, Radius Health, and Immunomedics; grants and personal fees from Biotheranostics; and personal fees from Pfizer, Novartis, Genentech, Merck, Radius Health, Immunomedics, Taiho, Sanofi, Daiichi Sankyo Pharma/AstraZeneca, Puma, Phillips, Eli Lilly, and Foundation Medicine. V.D. received honoraria from and consulting/advisory roles with Daiichi Sankyo, Gilead Sciences, MSD, Pierre Fabre Oncologie, Roche/Genentech, Novartis, Lilly, Pfizer, AstraZeneca, Eisai, AbbVie, and Seagen; and participated in speakers’ bureaus and support for travel, accommodation, and expenses from Gilead, Roche, Novartis, Pfizer, Lilly, AstraZeneca, Daiichi Sankyo, and Seagen. S.P. has stock/stock options from Gilead as an employee. R.D. has stock/stock options from Gilead as an employee. Y.Z. has stock/stock options from Gilead as an employee. S.M.T. reports grants and personal fees from Immunomedics/Gilead, AstraZeneca, Eli Lilly, Merck, Nektar, Novartis, Pfizer, Genentech/Roche, Exelixis, Bristol Myers Squibb, Eisai, NanoString, Sanofi, Odonate, and Immunomedics/Gilead; personal fees from Puma, Celldex, Seattle Genetics, Silverback Therapeutics, G1 Therapeutics, AbbVie, Athenex, OncoPep, Kyowa Kirin Pharmaceuticals, Daiichi Sankyo, CytomX, Samsung Bioepis Inc., Certara, Mersana Therapeutics; and grants from Cyclacel.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hurvitz, S.A., Bardia, A., Punie, K. et al. Subgroup analyses from the phase 3 ASCENT study of sacituzumab govitecan in metastatic triple-negative breast cancer. npj Breast Cancer 10, 33 (2024). https://doi.org/10.1038/s41523-024-00635-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-024-00635-5