Abstract
The balance between linear electron transport (LET) and cyclic electron transport (CET) plays an essential role in plant adaptation and protection against photo-induced damage. This balance is largely maintained by phosphorylation-driven alterations in the PSII–LHCII assembly and thylakoid membrane stacking. During the dark-to-light transition, plants shift this balance from CET, which prevails to prevent overreduction of the electron transport chain and consequent photo-induced damage, towards LET, which enables efficient CO2 assimilation and biomass production. Using freeze-fracture cryo-scanning electron microscopy and transmission electron microscopy of Arabidopsis leaves, we reveal unique membrane regions possessing characteristics of both stacked and unstacked regions of the thylakoid network that form during this transition. A notable consequence of the morphological attributes of these regions, which we refer to as ‘stacked thylakoid doublets’, is an overall increase in the proximity and connectivity of the two photosystems (PSI and PSII) that drive LET. This, in turn, reduces diffusion distances and barriers for the mobile carriers that transfer electrons between the two PSs, thereby maximizing LET and optimizing the plant’s ability to utilize light energy. The mechanics described here for the shift between CET and LET during the dark-to-light transition are probably also used during chromatic adaptation mediated by state transitions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data are available in the article, Extended Data Figs. 1–3 and Extended Data Tables 1 and 2. The images are deposited at figshare (https://doi.org/10.6084/m9.figshare.24942420). Source data are provided with this paper.
Code availability
The MATLAB code is available in Supplementary Code 1.
References
Bussi, Y. et al. Fundamental helical geometry consolidates the plant photosynthetic membrane. Proc. Natl Acad. Sci. USA 116, 22366–22375 (2019).
Shimoni, E., Rav-hon, O., Ohad, I., Brumfeld, V. & Reich, Z. Three-dimensional organization of higher-plant chloroplast thylakoid membranes revealed by electron tomography. Plant Cell 17, 2580–2586 (2005).
Nevo, R., Charuvi, D., Tsabari, O. & Reich, Z. Composition, architecture and dynamics of the photosynthetic apparatus in higher plants. Plant J. 70, 157–176 (2012).
Daum, B., Nicastro, D., Austin, J., Richard McIntosh, J. & Kühlbrandt, W. Arrangement of photosystem II and ATP synthase in chloroplast membranes of spinach and pea. Plant Cell 22, 1299–1312 (2010).
Kirchhoff, H. et al. Structural and functional self-organization of photosystem II in grana thylakoids. Biochim. Biophys. Acta Bioenerg. 1767, 1180–1188 (2007).
Rantala, M., Rantala, S. & Aro, E. M. Composition, phosphorylation and dynamic organization of photosynthetic protein complexes in plant thylakoid membrane. Photochem. Photobiol. Sci. 19, 604–619 (2020).
Tikkanen, M., Nurmi, M., Kangasjärvi, S. & Aro, E. M. Core protein phosphorylation facilitates the repair of photodamaged photosystem II at high light. Biochim. Biophys. Acta Bioenerg. 1777, 1432–1437 (2008).
Puthiyaveetil, S. et al. Compartmentalization of the protein repair machinery in photosynthetic membranes. Proc. Natl Acad. Sci. USA 111, 15839–15844 (2014).
Koochak, H., Puthiyaveetil, S., Mullendore, D. L., Li, M. & Kirchhoff, H. The structural and functional domains of plant thylakoid membranes. Plant J. 97, 412–429 (2019).
Anderson, J. M. The grana margins of plant thylakoid membranes. Physiol. Plant. 76, 243–248 (1989).
Albertsson, P. Å. A quantitative model of the domain structure of the photosynthetic membrane. Trends Plant Sci. 6, 349–354 (2001).
Armond, P. A., Staehelin, L. A. & Arntzen, C. J. Spatial relationship of photosystem I, photosystem II, and the light-harvesting complex in chloroplast membranes. J. Cell Biol. 73, 400–418 (1977).
Dekker, J. P. & Boekema, E. J. Supramolecular organization of thylakoid membrane proteins in green plants. Biochim. Biophys. Acta Bioenerg. 1706, 12–39 (2005).
Wietrzynski, W. et al. Charting the native architecture of Chlamydomonas thylakoid membranes with single-molecule precision. eLife 9, e53740 (2020).
Puthiyaveetil, S., Van Oort, B. & Kirchhoff, H. Surface charge dynamics in photosynthetic membranes and the structural consequences. Nat. Plants 3, 17020 (2017).
Barber, J. Influence of surface charges on thylakoid structure and function. Annu. Rev. Plant Physiol. 33, 261–295 (1982).
Anderson, J. M., Horton, P., Kim, E. H. & Chow, W. S. Towards elucidation of dynamic structural changes of plant thylakoid architecture. Phil. Trans. R. Soc. B 367, 3515–3524 (2012).
Chow, W. S., Kim, E.-H., Horton, P. & Anderson, J. M. Granal stacking of thylakoid membranes in higher plant chloroplasts: the physicochemical forces at work and the functional consequences that ensue. Photochem. Photobiol. Sci. 4, 1081–1090 (2005).
Kirchhoff, H. Architectural switches in plant thylakoid membranes. Photosynth. Res. 116, 481–487 (2013).
Fridlyand, L. E., Backhausen, J. E., Holtgrefe, S., Kitzmann, C. & Scheibe, R. Quantitative evaluation of the rate of 3-phosphoglycerate reduction in chloroplasts. Plant Cell Physiol. 38, 1177–1186 (1997).
Robinson, S. P. & Walker, D. A. The control of 3-phosphoglycerate reduction in isolated chloroplasts by the concentrations of ATP, ADP and 3-phosphoglycerate. Biochim. Biophys. Acta Bioenerg. 545, 528–536 (1979).
Horton, P. in Photosynthetic Mechanisms and the Environment (eds Barber, J. & Baker, N. R.) 135–187 (Elsevier, 1985).
Hepworth, C. et al. Dynamic thylakoid stacking and state transitions work synergistically to avoid acceptor-side limitation of photosystem I. Nat. Plants 7, 87–98 (2021).
Li, Z., Wakao, S., Fischer, B. B. & Niyogi, K. K. Sensing and responding to excess light. Annu. Rev. Plant Biol. 60, 239–260 (2009).
Miyake, C. Molecular mechanism of oxidation of p700 and suppression of ROS production in photosystem I in response to electron-sink limitations in C3 plants. Antioxidants 9, 230 (2020).
Murata, N., Takahashi, S., Nishiyama, Y. & Allakhverdiev, S. I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta Bioenerg. 1767, 414–421 (2007).
Fristedt, R., Granath, P. & Vener, A. V. A protein phosphorylation threshold for functional stacking of plant photosynthetic membranes. PLoS ONE 5, e10963 (2010).
Tikkanen, M. et al. Phosphorylation-dependent regulation of excitation energy distribution between the two photosystems in higher plants. Biochim. Biophys. Acta Bioenerg. 1777, 425–432 (2008).
Wood, W. H. J. et al. Dynamic thylakoid stacking regulates the balance between linear and cyclic photosynthetic electron transfer. Nat. Plants 4, 116–127 (2018).
Chuartzman, S. G. et al. Thylakoid membrane remodeling during state transitions in Arabidopsis. Plant Cell 20, 1029–1039 (2008).
Johnson, G. N. Physiology of PSI cyclic electron transport in higher plants. Biochim. Biophys. Acta Bioenerg. 1807, 384–389 (2011).
Joliot, P. & Joliot, A. Cyclic electron transfer in plant leaf. Proc. Natl Acad. Sci. USA 99, 10209–10214 (2002).
Slovacek, R. E., Crowther, D. & Hind, G. Relative activities of linear and cyclic electron flows during chloroplast CO2-fixation. Biochim. Biophys. Acta Bioenerg. 592, 495–505 (1980).
Hertle, A. P. et al. PGRL1 is the elusive ferredoxin-plastoquinone reductase in photosynthetic cyclic electron flow. Mol. Cell 49, 511–523 (2013).
Munekage, Y. et al. Cyclic electron flow around photosystem I is essential for photosynthesis. Nature 429, 579–582 (2004).
Suorsa, M. et al. PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 24, 2934–2948 (2012).
Tikkanen, M., Grieco, M., Kangasjärvi, S. & Aro, E. M. Thylakoid protein phosphorylation in higher plant chloroplasts optimizes electron transfer under fluctuating light. Plant Physiol. 152, 723–735 (2010).
Telfer, A., Hodges, M., Millner, P. A. & Barber, J. The cation-dependence of the degree of protein phosphorylation-induced unstacking of pea thylakoids. Biochim. Biophys. Acta Bioenerg. 766, 554–562 (1984).
Höhner, R. et al. Plastocyanin is the long-range electron carrier between photosystem II and photosystem I in plants. Proc. Natl Acad. Sci. USA 117, 15354–15362 (2020).
Pribil, M., Pesaresi, P., Hertle, A., Barbato, R. & Leister, D. Role of plastid protein phosphatase TAP38 in LHCII dephosphorylation and thylakoid electron flow. PLoS Biol. 8, e1000288 (2010).
Kirchhoff, H. et al. Dynamic control of protein diffusion within the granal thylakoid lumen. Proc. Natl Acad. Sci. USA 108, 20248–20253 (2011).
Johnson, M. P. & Wientjes, E. The relevance of dynamic thylakoid organisation to photosynthetic regulation. Biochim. Biophys. Acta Bioenerg. 1861, 148039 (2020).
Staehelin, L. A. in Photosynthesis III (eds Staehelin, L. A. & Arntzen, C. J.) 1–84 (Springer Berlin, 1986); https://doi.org/10.1007/978-3-642-70936-4_1
Pesaresi, P., Pribil, M., Wunder, T. & Leister, D. Dynamics of reversible protein phosphorylation in thylakoids of flowering plants: the roles of STN7, STN8 and TAP38. Biochim. Biophys. Acta Bioenerg. 1807, 887–896 (2011).
Tikkanen, M. & Aro, E. M. Thylakoid protein phosphorylation in dynamic regulation of photosystem II in higher plants. Biochim. Biophys. Acta Bioenerg. 1817, 232–238 (2012).
Longoni, P., Samol, I. & Goldschmidt-Clermont, M. The kinase STATE TRANSITION 8 phosphorylates light harvesting complex II and contributes to light acclimation in Arabidopsis thaliana. Front. Plant Sci. 10, 1156 (2019).
Bellaflore, S., Barneche, F., Peltler, G. & Rochalx, J. D. State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433, 892–895 (2005).
Samol, I. et al. Identification of a photosystem II phosphatase involved in light acclimation in Arabidopsis. Plant Cell 24, 2596–2609 (2012).
Rochaix, J.-D. et al. Protein kinases and phosphatases involved in the acclimation of the photosynthetic apparatus to a changing light environment. Phil. Trans. R. Soc. B 367, 3466–3474 (2012).
Shapiguzov, A. et al. The PPH1 phosphatase is specifically involved in LHCII dephosphorylation and state transitions in Arabidopsis. Proc. Natl Acad. Sci. USA 107, 4782–4787 (2010).
Charuvi, D., Nevo, R., Kaplan-Ashiri, I., Shimoni, E. & Reich, Z. Studying the supramolecular organization of photosynthetic membranes within freeze-fractured leaf tissues by cryo-scanning electron microscopy. J. Vis. Exp. https://doi.org/10.3791/54066 (2016).
Branton, D. Fracture faces of frozen membranes: 50th anniversary. Mol. Biol. Cell 27, 421–423 (2016).
Staehelin, L. A. Reversible particle movements associated with unstacking and restacking of chloroplast membranes in vitro. J. Cell Biol. 71, 136–158 (1976).
Wollman, F. A., Olive, J., Bennoun, P. & Recouvreur, M. Organization of the photosystem II centers and their associated antennae in the thylakoid membranes: a comparative ultrastructural, biochemical, and biophysical study of Chlamydomonas wild type and mutants lacking in photosystem II reaction centers. J. Cell Biol. 87, 728–735 (1980).
Staehelin, L. A. & van der Staay, G. W. M. in Oxygenic Photosynthesis: The Light Reactions (eds Ort, D. R. et al.) 11–30 (Springer Netherlands, 1996); https://doi.org/10.1007/0-306-48127-8_2
Armond, P. A. & Arntzen, C. J. Localization and characterization of photosystem II in grana and stroma lamellae. Plant Physiol. 59, 398–404 (1977).
Hankamer, B., Barber, J. & Boekema, E. J. Structure and membrane organization of PSII in green plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 641–671 (1997).
Fristedt, R. et al. Phosphorylation of photosystem II controls functional macroscopic folding of photosynthetic membranes in Arabidopsis. Plant Cell 21, 3950–3964 (2009).
Armbruster, U. et al. Arabidopsis CURVATURE THYLAKOID1 proteins modify thylakoid architecture by inducing membrane curvature. Plant Cell 25, 2661–2678 (2013).
Kirchhoff, H. Diffusion of molecules and macromolecules in thylakoid membranes. Biochim. Biophys. Acta Bioenerg. 1837, 495–502 (2014).
Kirchhoff, H., Schöttler, M. A., Maurer, J. & Weis, E. Plastocyanin redox kinetics in spinach chloroplasts: evidence for disequilibrium in the high potential chain. Biochim. Biophys. Acta Bioenerg. 1659, 63–72 (2004).
Wood, W. H. J. & Johnson, M. P. Modeling the role of LHCII–LHCII, PSII–LHCII, and PSI–LHCII interactions in state transitions. Biophys. J. 119, 287–299 (2020).
Trissl, H. W. & Wilhelm, C. Why do thylakoid membranes from higher plants form grana stacks? Trends Biochem. Sci. 18, 415–419 (1993).
Anderson, J. M. Insights into the consequences of grana stacking of thylakoid membranes in vascular plants: a personal perspective. Aust. J. Plant Physiol. 26, 625–639 (1999).
Pribil, M., Labs, M. & Leister, D. Structure and dynamics of thylakoids in land plants. J. Exp. Bot. 65, 1955–1972 (2014).
Wood, W. H. J., Barnett, S. F. H., Flannery, S., Hunter, C. N. & Johnson, M. P. Dynamic thylakoid stacking is regulated by LHCII phosphorylation but not its interaction with PSI. Plant Physiol. 180, 2152–2166 (2019).
Tsabari, O. et al. Differential effects of ambient or diminished CO2 and O2 levels on thylakoid membrane structure in light-stressed plants. Plant J. 81, 884–894 (2015).
Barber, J. An explanation for the relationship between salt-induced thylakoid stacking and the chlorophyll fluorescence changes associated with changes in spillover of energy from photosystem II to photosystem I. FEBS Lett. 118, 1–10 (1980).
Briantais, J. M., Vernotte, C., Olive, J. & Wollman, F. A. Kinetics of cation-induced changes of photosystem II fluorescence and of lateral distribution of the two photosystems in the thylakoid membranes of pea chloroplasts. Biochim. Biophys. Acta Bioenerg. 766, 1–8 (1984).
Yokono, M., Takabayashi, A., Akimoto, S. & Tanaka, A. A megacomplex composed of both photosystem reaction centres in higher plants. Nat. Commun. 6, 6675 (2015).
Järvi, S., Suorsa, M., Paakkarinen, V. & Aro, E. M. Optimized native gel systems for separation of thylakoid protein complexes: novel super- and mega-complexes. Biochem. J. 439, 207–214 (2011).
Grieco, M., Suorsa, M., Jajoo, A., Tikkanen, M. & Aro, E. M. Light-harvesting II antenna trimers connect energetically the entire photosynthetic machinery—including both photosystems II and II. Biochim. Biophys. Acta Bioenerg. 1847, 607–619 (2015).
Rantala, S. & Tikkanen, M. Phosphorylation-induced lateral rearrangements of thylakoid protein complexes upon light acclimation. Plant Direct 2, e00039 (2018).
Rantala, M. et al. Chloroplast acetyltransferase GNAT2 is involved in the organization and dynamics of thylakoid structure. Plant Cell Physiol. https://doi.org/10.1093/pcp/pcac096 (2022).
Rozak, P. R., Seiser, R. M., Wacholtz, W. F. & Wise, R. R. Rapid, reversible alterations in spinach thylakoid appression upon changes in light intensity. Plant Cell Environ. 25, 421–429 (2002).
Nevo, R. et al. in Lipids in Photosynthesis: Essential and Regulatory Functions (eds Wada, H. & Murata, N.) Dordrecht Springer-Verlag Vol. 30, 295–328 (2009).
Li, M. et al. Measuring the dynamic response of the thylakoid architecture in plant leaves by electron microscopy. Plant Direct 4, e00280 (2020).
Allen, J. F. Protein phosphorylation in regulation of photosynthesis. Biochim. Biophys. Acta Bioenerg. 1098, 275–335 (1992).
Anderson, J. M. Consequences of spatial separation of photosystem 1 and 2 in thylakoid membranes of higher plant chloroplasts. FEBS Lett. 124, 1–10 (1981).
Anderson, J. M. The significance of grana stacking in chlorophyll B containing chloroplasts. Photobiochem. Photobiophys. 3, 225–241 (1982).
Suorsa, M. et al. Light acclimation involves dynamic re-organization of the pigment–protein megacomplexes in non-appressed thylakoid domains. Plant J. 84, 360–373 (2015).
Bag, P. et al. Direct energy transfer from photosystem II to photosystem I confers winter sustainability in Scots pine. Nat. Commun. 11, 6388 (2020).
Kramer, D. M., Johnson, G., Kiirats, O. & Edwards, G. E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Biol. Chem. 79, 209–218 (2004).
Walther, P. & Müller, M. Double-layer coating for field-emission cryo-scanning electron microscopy—present state and applications. Scanning 19, 343–348 (1997).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Berg, S. et al. Ilastik: interactive machine learning for (bio)image analysis. Nat. Methods 16, 1226–1232 (2019).
R Core Team. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing (2020).
Wickham, H. et al. Welcome to the Tidyverse. J. Open Source Softw. 4, 1686 (2019).
Wientjes, E., Van Amerongen, H. & Croce, R. Quantum yield of charge separation in photosystem II: functional effect of changes in the antenna size upon light acclimation. J. Phys. Chem. B 117, 11200–11208 (2013).
Acknowledgements
This work is dedicated to the memory of Eyal Shimoni, who passed away in July 2023. This work was supported by grants from the Israel Science Foundation (no. 1082/17 to Z.R. and R.N.; no. 1377/18 to D.C.) and the National Science Foundation United States–Israel Binational Science Foundation Molecular and Cellular Biosciences Program (no. 1616982 to H.K.; no. 2019695 to Z.R. and R.N.; no. 2015839 to Z.R.; no. 1953570 to H.K.).
Author information
Authors and Affiliations
Contributions
Z.R., H.K. and R.N. designed the research. Y.G., S.L.-Z. and E.S. performed the experiments. Y.G., Y.B., D.C. and R.N. analysed the data. Y.B., D.C., Z.R., H.K. and R.N. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Anjali Pandit and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Representative cryo-SEM images of freeze-fractured leaf samples from dark- and light-adapted plants.
The exoplasmic fracture faces of unstacked membranes (EFu) are outlined. Scale bars: 100 nm. Images shown are representative images of the experiment described in Fig. 2.
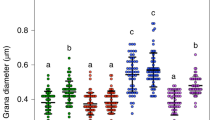
Extended Data Fig. 2 Representative thin-section TEM images of chloroplasts (means ± SE) and a box plot of grana widths from dark- and light-adapted leaves of WT and of the stn7/stn8 and pph1/pbcp mutants.
N = number of grana probed per genotype and condition: WT - 291 (D), 232 (L); stn7/stn8 - 160 (D), 126 (L); pph1/pbcp - 140 (D), 140 (L). For each genotype and condition, samples were obtained from two different plants. In the box plots, the box indicates the interquartile area, whiskers are drawn down to the 5th percentile and up to the 95th, with small black squares in the middle representing the means. Scale bars: 500 nm.
Extended Data Fig. 3 Simulation of PSII to PSI nearest neighbor distance using empirical (PSII) and calculated (PSI) densities in unstacked membranes in WT.
For the obtained PSI:PSII ratios, of 5.1 for dark (D) and 3.1 for light (L) (gray lines), the analysis showed that the PSII-PSI nearest neighbor distances were ˂2 nm. For the simulation, PSI and PSII complexes were represented by disks with areas approximated from their PDB structures (PSI [2WSC]; PSII [7OUI]). PSII particles were randomly placed in a 1 µm2 grid with the densities observed in dark (D) and light (L) conditions (522 particles/µm2 and 773 particles/µm2, respectively, see Fig. 2). PSI particles were randomly placed in unoccupied positions (not allowing for particle overlap) until either no more particles could be added randomly or the PSI density reached our estimated values (2685 particles/µm2 [D] or 2410 particles/µm2 [L])*. Using the PSII and PSI densities resulted in PSI:PSII ratios of 5.1 for D (dark grey) and 3.1 for L (light grey). The simulation was carried out 100 times for different PSI:PSII ratios, three examples for ratios of 1:1; 2:1; 3:1 in the dark condition are shown with PSII colored in green and PSI in magenta. The plot depicts the PSII-PSI nearest neighbor distance (from particle edge to edge) for D (blue) and L (red), with points representing the mean of the means and error bars showing the mean of the standard deviations for the distances in the 100 replicates (N = 100; Data are presented as means of means ± means of SDs). For both D and L, at the calculated PSI:PSII ratios (5.1 [D] and 3.1 [L]) when the maximum PSI particles were placed in the grid randomly, the extrapolated PSII-PSI nearest neighbor distances were ˂2 nm. The simulation (Garty_at_al_Supplementary_information_2.m) was carried out using MATLAB version: 9.13.0 (R2022b), Natick, Massachusetts: The MathWorks Inc.; 2022. *Values of PSI densities were estimated using a PSII:PSI ratio of 1.3 for the whole thylakoid fraction89. We then calculated the ratios of stacked/unstacked membranes, from the observed thylakoid fractions (Fig. 3f), as \(\frac{\frac{1}{2}{f}_{GSL}+{f}_{G}}{\frac{1}{2}{f}_{GSL}+{f}_{SL}}\). The values obtained, 1.94 in dark and 1.56 in light, were used to calculate the PSI density, which was similar to published AFM data29.
Supplementary information
Supplementary Code 1
Code for Extended Data Fig. 3.
Source data
Source Data Fig. 1
Statistical source data for Figs. 1–5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Garty, Y., Bussi, Y., Levin-Zaidman, S. et al. Thylakoid membrane stacking controls electron transport mode during the dark-to-light transition by adjusting the distances between PSI and PSII. Nat. Plants 10, 512–524 (2024). https://doi.org/10.1038/s41477-024-01628-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-024-01628-9