Abstract
Chromatin-associated RNAs play key roles in various biological processes. However, both their repository and conjugation genomic loci and potential functions remain largely unclear. Here, we develop an effective method for mapping of chromatin-associated RNA–DNA interactions, followed by paired-end-tag sequencing (ChRD–PET) in rice. We present a comprehensive interaction map between RNAs and H3K4me3-marked regions based on H3K4me3 ChRD–PET data, showing three types of RNA–DNA interactions—local, proximal and distal. We further characterize the origin and composition of the RNA strand in R-loop RNA–DNA hybrids and identify that extensive cis and trans RNAs, including trans-non-coding RNAs, are prevalently involved in the R-loop. Integrative analysis of rice epigenome and three-dimensional genome data suggests that both coding and non-coding RNAs engage extensively in the formation of chromatin loops and chromatin-interacting domains. In summary, ChRD–PET is an efficient method for studying the features of RNA–chromatin interactions, and the resulting datasets constitute a valuable resource for the study of RNAs and their biological functions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The ChRD–PET, ATAC–seq, ssDRIP–seq and total RNA-seq data generated in this study have been deposited in the Gene Expression Omnibus (GEO) under accession no. GSE163119. The ChIP–seq, ChIA–PET, strand-specific RNA-seq and Whole Genome Bisulfite Sequencing (WGBS) data used in this study were published previously48 and are available at NCBI GEO under accession nos. GSE131202 and GSE142570. Source data are provided with this paper.
Code availability
Custom scripts for data analysis are available at https://github.com/fengchuiguo1994/ChRDPETpipeline.
References
Li, X. & Fu, X. D. Chromatin-associated RNAs as facilitators of functional genomic interactions. Nat. Rev. Genet. 20, 503–519 (2019).
Nozawa, R. S. & Gilbert, N. RNA: nuclear glue for folding the genome. Trends Cell Biol. 29, 201–211 (2019).
Preker, P. et al. RNA exosome depletion reveals transcription upstream of active human promoters. Science 322, 1851–1854 (2008).
Lam, M. T., Li, W., Rosenfeld, M. G. & Glass, C. K. Enhancer RNAs and regulated transcriptional programs. Trends Biochem. Sci. 39, 170–182 (2014).
Li, G. L. et al. Extensive promoter-centered chromatin interactions provide a topological basis for transcription regulation. Cell 148, 84–98 (2012).
Cho, W. K. et al. Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. Science 361, 412–415 (2018).
Tan, Y. et al. Dismissal of RNA polymerase II underlies a large ligand-induced rnhancer decommissioning program. Mol. Cell 71, 526–539 (2018).
Kim, D. H., Xi, Y. & Sung, S. Modular function of long noncoding RNA, COLDAIR, in the vernalization response. PLoS Genet. 13, e1006939 (2017).
Kim, D. H. & Sung, S. Vernalization-triggered intragenic chromatin loop formation by long noncoding RNAs. Dev. Cell 40, 302–312 (2017).
Liu, W., Yu, J., Ge, Y., Qin, P. & Xu, L. Pivotal role of LBD16 in root and root-like organ initiation. Cell. Mol. Life Sci. 75, 3329–3338 (2018).
Sun, Q. W., Csorba, T., Skourti-Stathaki, K., Proudfoot, N. J. & Dean, C. R-loop stabilization represses antisense transcription at the Arabidopsis FLC locus. Science 340, 619–621 (2013).
Lee, J. T. Lessons from X-chromosome inactivation: long ncRNA as guides and tethers to the epigenome. Genes Dev. 23, 1831–1842 (2009).
Wutz, A. Gene silencing in X-chromosome inactivation: advances in understanding facultative heterochromatin formation. Nat. Rev. Genet. 12, 542–553 (2011).
Jegu, T., Aeby, E. & Lee, J. T. The X chromosome in space. Nat. Rev. Genet. 18, 377–389 (2017).
Langdon, E. M. et al. mRNA structure determines specificity of a polyQ-driven phase separation. Science 360, 922–927 (2018).
Crossley, M. P., Bocek, M. & Cimprich, K. A. R-Loops as cellular regulators and genomic threats. Mol. Cell 73, 398–411 (2019).
Wahba, L., Gore, S. K. & Koshland, D. The homologous recombination machinery modulates the formation of RNA-DNA hybrids and associated chromosome instability. eLife 2, e00505 (2013).
Feretzaki, M. et al. RAD51-dependent recruitment of TERRA lncRNA to telomeres through R-loops. Nature 587, 303–308 (2020).
Ariel, F. et al. R-loop mediated trans action of the APOLO long noncoding RNA. Mol. Cell 77, 1055 (2020).
Hegazy, Y. A., Fernando, C. M. & Tran, E. J. The balancing act of R-loop biology: the good, the bad, and the ugly. J. Biol. Chem. 295, 905–913 (2020).
Xu, W. et al. The R-loop is a common chromatin feature of the Arabidopsis genome. Nat. Plants 3, 704–714 (2017).
Raju, S. K. K. The R-loop: an additional chromatin reature for gene regulation in Arabidopsis. Plant Cell 32, 785–786 (2020).
Conn, V. M. et al. A circRNA from SEPALLATA3 regulates splicing of its cognate mRNA through R-loop formation. Nat. Plants 3, 17053 (2017).
Yang, Z. et al. RNase H1 cooperates with DNA gyrases to restrict R-Loops and maintain genome integrity in Arabidopsis chloroplasts. Plant Cell 29, 2478–2497 (2017).
Shafiq, S. et al. DNA topoisomerase 1 prevents R-loop accumulation to modulate auxin-regulated root development in rice. Mol. Plant 10, 821–833 (2017).
Fang, Y. et al. Characterization of functional relationships of R-loops with gene transcription and epigenetic modifications in rice. Genome Res. 29, 1287–1297 (2019).
Sridhar, B. et al. Systematic mapping of RNA-chromatin interactions in vivo. Curr. Biol. 27, 602–609 (2017).
Yan, Z. M. et al. Genome-wide colocalization of RNA-DNA interactions and fusion RNA pairs. Proc. Natl Acad. Sci. USA 116, 3328–3337 (2019).
Bell, J. C. et al. Chromatin-associated RNA sequencing (ChAR-seq) maps genome-wide RNA-to-DNA contacts. eLife 7, e27024 (2018).
Li, X. et al. GRID-seq reveals the global RNA-chromatin interactome. Nat. Biotechnol. 35, 940–950 (2017).
Bonetti, A. & Castelo-Branco, G. RADICL-seq identifies cell-type specific RNA-chromatin interactions in mouse oligodendrocyte progenitor cells. Glia 67, E293–E294 (2019).
Simon, M. D. et al. The genomic binding sites of a noncoding RNA. Proc. Natl Acad. Sci. USA 108, 20497–20502 (2011).
Chu, C., Qu, K., Zhong, F. L., Artandi, S. E. & Chang, H. Y. Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Mol. Cell 44, 667–678 (2011).
Engreitz, J. M. et al. The Xist lncRNA exploits three-dimensional genome architecture to spread across the X chromosome. Science 341, 1237973 (2013).
Liu, X. et al. In situ capture of chromatin interactions by biotinylated dCas9. Cell 170, 1028–1043 (2017).
Zhang, S. L. et al. Profiling the long noncoding RNA interaction network in the regulatory elements of target genes by chromatin in situ reverse transcription sequencing. Genome Res. 29, 1521–1532 (2019).
Quinodoz, S. A. et al. RNA promotes the formation of spatial compartments in the nucleus. Cell 184, 5775–5790 (2021).
Liu, X. et al. A novel antisense long noncoding RNA, TWISTED LEAF, maintains leaf blade flattening by regulating its associated sense R2R3-MYB gene in rice. New Phytol. 218, 774–788 (2018).
Ding, J. H. et al. A long noncoding RNA regulates photoperiod-sensitive male sterility, an essential component of hybrid rice. Proc. Natl Acad. Sci. USA 109, 2654–2659 (2012).
Jabnoune, M. et al. A rice cis-natural antisense RNA acts as a translational enhancer for its cognate mRNA and contributes to phosphate homeostasis and plant fitness. Plant Cell 25, 4166–4182 (2013).
Wang, Y. et al. Overexpressing lncRNA LAIR increases grain yield and regulates neighbouring gene cluster expression in rice. Nat. Commun. 9, 3516 (2018).
Song, X. W., Li, Y., Cao, X. F. & Qi, Y. J. MicroRNAs and their regulatory roles in plant-environment interactions. Annu. Rev. Plant Biol. 70, 489–525 (2019).
Banerjee, A. & Roychoudhury, A. The gymnastics of epigenomics in rice. Plant Cell Rep. 37, 25–49 (2018).
Li, W. et al. Integration analysis of small RNA and degradome sequencing reveals microRNAs responsive to Dickeya zeae in resistant rice. Int. J. Mol. Sci. 20, 222 (2019).
Miao, C. B. et al. The grain yield modulator miR156 regulates seed dormancy through the gibberellin pathway in rice. Nat. Commun. 10, 3822 (2019).
Ding, J. et al. RNA-directed DNA methylation is involved in regulating photoperiod-sensitive male sterility in rice. Mol. Plant 5, 1210–1216 (2012).
Hu, H. H. et al. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc. Natl Acad. Sci. USA 103, 12987–12992 (2006).
Zhao, L. et al. Integrative analysis of reference epigenomes in 20 rice varieties. Nat. Commun. 11, 2658 (2020).
Zhao, L. et al. Chromatin loops associated with active genes and heterochromatin shape rice genome architecture for transcriptional regulation. Nat. Commun. 10, 3640 (2019).
Jia, J. et al. Post-transcriptional splicing of nascent RNA contributes to widespread intron retention in plants. Nat. Plants 6, 780–788 (2020).
Li, Z. Y. et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 22, 256–264 (2015).
Hyman, A. A., Weber, C. A. & Juelicher, F. Liquid-liquid phase separation in biology. Annu. Rev. Cell Dev. Biol. 30, 39–58 (2014).
Banani, S. F., Lee, H. O., Hyman, A. A. & Rosen, M. K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Li, H. et al. The sequence alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Ramirez, F., Dundar, F., Diehl, S., Gruning, B. A. & Manke, T. deepTools: a flexible platform for exploring deep-sequencing data. Nucleic Acids Res. 42, W187–W187 (2014).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Magoc, T. & Salzberg, S. L. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963 (2011).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Li, G. et al. ChIA-PET tool for comprehensive chromatin interaction analysis with paired-end tag sequencing. Genome Biol. 11, R22 (2010).
Olson, S. A. EMBOSS opens up sequence analysis. European Molecular Niology Open Software Suite. Brief. Bioinform. 3, 87–91 (2002).
Pertea, M. et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 33, 290–295 (2015).
Zhou, Q., Lim, J. Q., Sung, W. K. & Li, G. An integrated package for bisulfite DNA methylation data analysis with Indel-sensitive mapping. BMC Bioinformatics 20, 47 (2019).
Tian, F., Yang, D. C., Meng, Y. Q., Jin, J. & Gao, G. PlantRegMap: charting functional regulatory maps in plants. Nucleic Acids Res. 48, D1104–D1113 (2020).
Acknowledgements
We thank G. Cao (Huazhong Agricultural University) and colleagues J. Dai and Z. Zhang for their help in performing the RNA FISH experiment. This work was funded by the National Natural Science Foundation of China (nos. 32070612 to X.L., 31771402 and 31970590 to G.L. and 31701115 to Y.Z.) and Fundamental Research Funds for the Central Universities, and was partly supported by open funds from the National Key Laboratory of Crop Genetic Improvement (no. K201906).
Author information
Authors and Affiliations
Contributions
X.L. and G.L. conceived the project. Q.X. generated data with assistance from Y.Z., W.X., Y.Y., Q.Z., Z.H. and Q.S. X.H. performed data analysis with help from F.X. X.L., G.L., Q.X. and X.H. interpreted data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Chang Liu, Julia Chekanova and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 ChRD-PET data analysis pipeline and reproducibility.
a, Main steps of ChRD-PET experiment are shown in the boxes. DNA fragment analysis for relevant steps is included on the right of the main steps. b, Analysis pipeline for ChRD-PET data. c, Bar charts showing the percentage of sequence reads with no linker (grey), one linker (purple), and more than one linker (green). Reads with one linker entered the downstream analysis. d, Bar charts showing the percentage of DNA and RNA reads that are unmapped (grey), uniquely mapped and mapped quality >= 20 (purple), uniquely mapped and mapped quality < 20 (green), and multi-mapped (light grey) in RNA-DNA interaction PETs. e, Scatter plots of DNA coverage signals between ChRD-PET replicates, with spearman correlation coefficient r = 0.990. Bin size: 10kb. f, Scatter plots of RNA coverage signals between ChRD-PET replicates, with spearman correlation coefficients r = 0.891. Bin size: the transcribed region of a gene. g, Scatter plot showing two replicates of ChRD-PET data. 2D contact map with Pearson correlation coefficient: r = 0.963. Bin size: 10kb. h, Scatter plot showing two replicates of ChRD-PET data. Loop frequency with Pearson correlation coefficient r = 0.987.
Extended Data Fig. 2 Characterization of H3 ChRD-PET and H3K4me3 ChRD-PET.
a, Venn diagram showing the peak overlap between H3K4me3 ChRD-PET DNA and H3K4me3 ChIP-seq. 24,028 ChIP-seq peaks overlapped with 23,686 ChRD-PET DNA peaks. p value was calculated using one-sided Fisher’s exact test. b, Length distribution of DNA and RNA reads in H3K4me3 ChRD-PET. c, d, Location analysis of uniquely mapped H3K4me3 ChRD-PET DNA reads (c) and RNA reads (d) compared with the expected genomic distribution. e, f, Location analysis of H3K4me3 ChRD-PET DNA peaks(e) and RNA peaks (f) compared with the expected genomic distribution. g, h, i, Simulated relationships between interaction cluster numbers and ChRD-PET sequence depths. The total sequencing data was first divided into 10 equal parts, a bin ð‘– ð‘¡ℎ was generated by merging the first ð‘– ð‘¡ℎ parts into the ð‘– ð‘¡ℎ bin, where ð‘– = 1, 2, 3 …, 10. the x-axis represents the bins of 1, 2, …,10 with increasing sequence depths. (g) Y-axis: DNA peak number (red), RNA peak number (blue) (h) Y-axis: the interaction cluster counts. (i) Y-axis: local interaction cluster number (purple), proximal interaction cluster number (grey) and distal interaction cluster number (green). j, Global view of H3K4me3 ChRD-PET interactions, along with epigenetic and transcriptional features. k, Global view of H3K4me3 ChRD-PET interactions, along with epigenetic and transcriptional features on chromosome 1. l, Pairwise correlation analysis (with gene expression: FPKM) between H3 ChRD-PET RNA, H3K4me3 ChRD-PET RNA and Total RNA-seq. m, Length distribution of H3 ChRD-PET DNA and RNA reads. n, Genomic distributions of uniquely mapped H3 ChRD-PET DNA reads (left) and RNA reads (right). o, Genome-wide contact heatmap at 1Mb resolution of H3 ChRD-PET. X-axis: H3 ChRD-PET RNA. Y-axis: H3 ChRD-PET DNA.
Extended Data Fig. 3 Features of RNA-DNA interactions in H3K4me3 ChRD-PET.
a, Three types of ChRD-PET interactions of exon RNA and intron RNA. b, Screenshot showing introns of MH05g0031500 (chr5: 1,278,000-1292000) participating in local interactions, along with the epigenomic and transcriptomic features. Top: gene (track 1), ChRD-PET RNA (red, track 2), ChRD-PET DNA (blue, track 3). Bottom: local interactions, ChRD-PET DNA (blue), ChRD-PET intron RNA (green), linker (black). c, Three types of ChRD-PET interactions of RNAs from single- and multi-exon gene. Y-axis: average number of interacting DNA on each RNA. d, Gene Ontology of hub RNAs (top 5% RNAs) in ChRD-PET. p value was calculated using one-sided Fisher’s exact test. p values are corrected for multiple comparison using the Yekutieli_FDR correction. e, Network showing the targets of SNAC1 RNA (red). ChRD-PET DNAs: yellow and blue, blue: DNAs showed in Fig. 2h. Gene Ontology of SNAC1’s targets are shown on the Bottom. p value was calculated using one-sided hypergeometric distribution test. p value is corrected for multiple comparison using the Benjamini-Hochberg correction. f, The motifs of ATAC-seq related to SNAC1 RNA targets. g, Three types of ChRD-PET interactions of transcription factor RNA and non-transcription factor RNA. Y-axis: average number of interacting DNA on each RNA.
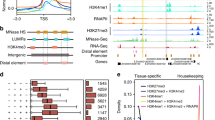
Extended Data Fig. 4 Coding and non-coding RNA in H3K4me3 ChRD-PET.
a, Number of interactions of coding RNA, lncRNA, snRNA/snoRNA, and microRNA in ChRD-PET. Total RNAs: coding RNAs were from coding genes, while lncRNA, snRNA/snoRNA, and microRNA were predicted. RNAs in ChRD-PET interactions: RNAs from total RNAs and involved in ChRD-PET interactions. ChRD-PET interactions: ChRD-PET interactions related to each type of RNAs. b, c, Scatter plots of RNA reads detected by ChRD-PET in comparison with RNA-seq (b) and total RNA-seq (c) Bin size: the transcribed region of a gene. d, Histogram showing the number of DNA/coding RNA numbers in each coding RNA/DNA category. Bottom left: histogram showing the number of DNA regions interacting with different number of coding RNAs. For the second pillar, there are 5496 DNA peaks, and each peak interacting with 2 coding RNAs. Upper right: histogram showing the number of RNAs in each DNA peak category. For the second pillar, there are 4956 RNAs, and each RNA interacted with 2 DNA peaks. e, Cluster analysis of the pairwise correlation between the associated DNA regions of 8 types of snoRNA in ChRD-PET. Bin size: 25kb. f, Top: genomic loci, ChIP-seq, ChRD-PET and transcriptome information for lncRNA MSTRG.20623. Bottom: circlet plot of H3K4me3 ChRD-PET interactions related to lncRNA MSTRG.20623. g, Top: genomic loci, ChIP-seq, ChRD-PET and transcriptome information for microRNA osa-MIR2106. Bottom: circlet plot of H3K4me3 ChRD-PET interactions related to osa-MIR2106. h, Top: genomic loci, ChIP-seq, ChRD-PET and transcriptome information for the cluster of snoRNAs. Bottom: circlet plot of H3K4me3 ChRD-PET interactions related to the cluster of snoRNAs. i, j, k, l, m, Circlet plot of ChRD-PET interactions in which 5 types of snRNA were involved. U1(i), U2 (j), U4 (k), U5 (l), U6atac (m).
Extended Data Fig. 5 Intra-chromosome H3K4me3 ChRD-PET interactions.
a, b, c, d, RNAs engaged in intra-chromosome interactions on 12 chromosomes. Coding RNA (a), lncRNA (b), snRNA/snoRNA (c), microRNA (d).
Extended Data Fig. 6 Characterization of H3K4me3 peaks overlapping with R-loops.
a, Venn diagram showing the peak overlap between H3K4me3 ChIP-seq, H3K4me3 ChRD-PET DNA, and ssDRIP-seq. b, Density distribution of H3K4me3 ChIP-seq, H3K4me3 ChRD-PET DNA, and ssDRIP-seq at transcription start site. c, The percentage of S9.6 foci colocalized/non-colocalized with H3K4me3 foci in 29 rice nuclei. Averagely, 91% S9.6 foci were colocalized with H3K4me3 foci, 9% non-colocalized. Biological replicates, n=29. p value was calculated using two-sided Wilcoxon rank-sum test. Centre line represents the median, box extent ranges from 25th to 75th percentile, and whiskers extend at most to 1.5× the interquartile range (distance between the first and third quartiles). d, Number of ChRD-PET interactions for coding RNA, lncRNA, snRNA/snoRNA and, microRNA in the overlap and non-overlap portion of ChRD-PET DNA and ssDRIP-seq peaks. e, Histogram showing the number of R-loop derived RNA-DNA interactions related to ncRNAs in H3K4me3 ChRD-PET filtered by RNase H treated H3K4me3 ChRD-PET and 8-bp RNA-DNA complementary pairing test. f, Histogram showing the number of R-loops in different categories of RNA. g, Histogram showing the number of RNAs in different categories of R-loops.
Extended Data Fig. 7 Analysis of RNase H treated H3K4me3 ChRD-PET interactions.
a, Density distribution of RNase H treated H3K4me3 ChRD-PET DNAs around the H3K4me3 peak center. b, Density distribution of RNase H treated H3K4me3 ChRD-PET sense (red) and antisense (grey) RNA at the annotated genes region. c, Length distribution of RNase H treated H3K4me3 ChRD-PET DNA and RNA reads. d, Genomic distributions of uniquely mapped RNase H treated H3K4me3 ChRD-PET DNA (left) and RNA reads (right). e, Genome-wide contact heatmap at 1Mb resolution of RNase H treated H3K4me3 ChRD-PET. X-axis: RNase H treated H3K4me3 ChRD-PET RNA. Y-axis: RNase H treated H3K4me3 ChRD-PET DNA. f, Histogram showing the number fraction of R-loop derived RNA-DNA interactions and non R-loop derived RNA-DNA interactions in local, proximal, distal interactions. g, Screenshot showing osa-MIR2106 mediated R-loop formation in trans. Track 1: ChRD-PET RNA coverage signals. Arrows point out the trans location of RNA strand involved in R-loop. Track 2: ssDRIP-seq coverage signals and peak in the forward strand. Track 3: ssDRIP-seq coverage signals and peak in the reverse strand. Track 4: RNase H treated ssDRIP-seq. Track 5: ChRD-PET DNA coverage signals. Track 6: H3K4me3 ChIP-seq coverage signals and peak. Track 7: R-loop formation site. Track 8: annotation genes. Track 9: sequences and R-loop structure, RNA (red), DNA (blue).
Extended Data Fig. 8 R-loop distribution and epigenetic features of ChRD-PET.
a, Density distribution of H3K4me3 ChRD-PET DNA at H3K4me3 peak center. Three categories of H3K4me3 ChRD-PET DNA related to R-loop were analysed: H3K4me3 anchors overlapping with hybrid RNA: DNAs, H3K4me3 anchors overlapping with R-loop and associating with proximal RNA, and H3K4me3 anchors not overlapping with R-loop. b, Density distribution of H3K4me3 ChIP-seq at H3K4me3 peaks center in different categories as same as classification in a. c, Conservation analysis of ChRD-PET RNA in different categories of interacting DNA with same classification as a. d, Methylation ratios of peaks with H3K4me3 modification in different categories as same as classification in a. Random: regions on the genome randomly generated by BEDTools. e, Methylation ratios of ChRD-PET RNA in different categories of interacting DNA with same classification as a. Random: regions on the genome randomly generated by BEDTools. f, Density distribution and heatmap of H3K4me3 ChIP-seq and ATAC-seq for three types of peaks classified in Fig. 5c. g, DNA methylation level of three types of peaks classified in Fig. 5c.
Extended Data Fig. 9 Analysis of ChIA-PET and ChRD-PET at chromatin loop level.
a, Table for ChRD-PET DNA and RNA from peak or anchor and the number of ChRD-PET interactions. DNA peak: DNA with the H3K4me3 ChIP-seq peak (Dp). DNA anchor: DNA with the H3K4me3 ChIP-seq peak and in ChIA-PET interaction (Da). Peak RNA: RNA transcript from DNA peak (Rp). Anchor RNA: RNA transcript from DNA anchor (Ra). Rest RNA: RNA transcript from a region that is neither DNA peak nor DNA anchor (Rr). b, Histogram showing ChRD-PET interaction number for six kinds of PETs. c, Right: Venn diagram showing the overlap of three group RNAs: RNAs interacting with basal peaks, RNAs interacting with anchors, RNAs interacting with both basal peaks and anchors. Left: expression level of cognate genes of three group RNAs. RNA without interaction, n=36,040. p value was calculated using two-sided Wilcoxon rank-sum test. Centre line represents the median, box extent ranges from 25th to 75th percentile, and whiskers extend at most to 1.5× the interquartile range (distance between the first and third quartiles). d, Similar example to Fig. 6g showing the interactions of model 3. Six anchors and four RNAs were in the complex of ChIA-PET and ChRD-PET interactions. Among them, anchor 802 and anchor 804 in the ChIA-PET interactions loops and both interacting with 4 ChRD-PET RNAs of MH01g0150100, MH01g0150300, MH01g0150400 and MH01g0150700. e, Screenshot showing the details of d. f, Similar example to Fig. 6g showing the interactions of model 3. Three anchors and six RNAs were in the complex of ChIA-PET and ChRD-PET interactions. Among them, anchor 13131 and anchor 13133 in the ChIA-PET interactions loops and both interacting with 4 ChRD-PET RNAs of MH02g0618900, MH03g0317500, MH03g0317700 and MH03g0318400. In addition, non-coding RNA MH02gsn00210 (U1) and MSTRG.12745 interact with anchor 13133.
Extended Data Fig. 10 Analysis of ChIA-PET and ChRD-PET at CID level.
a, Heatmap showing chromatin-enriched RNA across whole chromatin interaction domain (CID) in MH63. Y-axis: chromatin-enriched RNA. X-axis: MH63 chromatin interaction domain (CID). b, Number of ChRD-PET RNAs inside/outside the CID. c, Histogram showing the number of coding RNAs and ncRNAs related ChRD-PET interactions in or out of the CID. d, Density distribution of ChRD-PET DNA and ChIP-seq around CID boundary. e, Number of ChRD-PET interactions in A/B compartment. A/B compartment, which is a type of high-order 3D genome architecture unit composed of multiple interconnected CIDs (chromatin interacting domains). In the DNA-DNA interaction matrix heatmap, each chromosome is partitioned into mega base-scale gene-rich and gene-poor chromatin blocks corresponding to transcriptionally active “A” and inactive “B” compartments, respectively. AA: RNA from A compartment, DNA from A compartment. AB: DNA from A compartment, RNA from B compartment. BA: DNA from B compartment, RNA from A compartment. BB: RNA from B compartment, DNA from B compartment. f, Gene Ontology analysis of CID (614) hub RNAs.
Supplementary information
Supplementary Table 1
Contains 13 tables with peak results, ChRD–PET interaction results, GO enrichment analysis results and more.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
About this article
Cite this article
Xiao, Q., Huang, X., Zhang, Y. et al. The landscape of promoter-centred RNA–DNA interactions in rice. Nat. Plants 8, 157–170 (2022). https://doi.org/10.1038/s41477-021-01089-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-021-01089-4
This article is cited by
-
An efficient CRISPR–Cas12a promoter editing system for crop improvement
Nature Plants (2023)
-
RNA-DNA interactomes of three prokaryotes uncovered by proximity ligation
Communications Biology (2023)