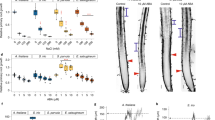
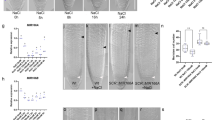
Abstract
Plant cells constantly alter their gene expression profiles to respond to environmental fluctuations. These continuous adjustments are regulated by multi-hierarchical networks of transcription factors. To understand how such gene regulatory networks (GRNs) have stabilized evolutionarily while allowing for species-specific responses, we compare the GRNs underlying salt response in the early-diverging and late-diverging plants Marchantia polymorpha and Arabidopsis thaliana. Salt-responsive GRNs, constructed on the basis of the temporal transcriptional patterns in the two species, share common trans-regulators but exhibit an evolutionary divergence in cis-regulatory sequences and in the overall network sizes. In both species, WRKY-family transcription factors and their feedback loops serve as central nodes in salt-responsive GRNs. The divergent cis-regulatory sequences of WRKY-target genes are probably associated with the expansion in network size, linking salt stress to tissue-specific developmental and physiological responses. The WRKY modules and highly linked WRKY feedback loops have been preserved widely in other plants, including rice, while keeping their binding-motif sequences mutable. Together, the conserved trans-regulators and the quickly evolving cis-regulatory sequences allow salt-responsive GRNs to adapt over a long evolutionary timescale while maintaining some consistent regulatory structure. This strategy may benefit plants as they adapt to changing environments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All experimental data, transcriptome data, R codes and genetic materials presented in this study are available on request. All transcriptome datasets collected in this study are openly available in the NCBI GEO repository (GSE153103, https://www.ncbi.nlm.nih.gov/geo/).
References
Yosef, N. & Regev, A. Impulse control: temporal dynamics in gene transcription. Cell 144, 886–896 (2011).
Marshall-Colón, A. & Kliebenstein, D. J. Plant networks as traits and hypotheses: moving beyond description. Trends Plant Sci. 24, 840–852 (2019).
Stergachis, A. B. et al. Conservation of trans-acting circuitry during mammalian regulatory evolution. Nature 515, 365–370 (2014).
Hickman, R. et al. Architecture and dynamics of the jasmonic acid gene regulatory network. Plant Cell 29, 2086–2105 (2017).
Ichihashi, Y. et al. Evolutionary developmental transcriptomics reveals a gene network module regulating interspecific diversity in plant leaf shape. Proc. Natl Acad. Sci. USA 111, E2616–E2621 (2014).
Gutiérrez, R. A. et al. Systems approach identifies an organic nitrogen-responsive gene network that is regulated by the master clock control gene CCA1. Proc. Natl Acad. Sci. USA 105, 4939–4944 (2008).
Varala, K. et al. Temporal transcriptional logic of dynamic regulatory networks underlying nitrogen signaling and use in plants. Proc. Natl Acad. Sci. USA 115, 6494–6499 (2018).
Wittkopp, P. J. & Kalay, G. Cis-regulatory elements: molecular mechanisms and evolutionary processes underlying divergence. Nat. Rev. Genet. 13, 59–69 (2012).
Liu, M. J. et al. Regulatory divergence in wound-responsive gene expression between domesticated and wild tomato. Plant Cell 30, 1445–1460 (2018).
Voordeckers, K., Pougach, K. & Verstrepen, K. J. How do regulatory networks evolve and expand throughout evolution? Curr. Opin. Biotechnol. 34, 180–188 (2015).
Bowman, J. L. et al. Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 171, 287–304 (2017).
Isayenkov, S. V. & Maathuis, F. J. M. Plant salinity stress: many unanswered questions remain. Front. Plant Sci. https://doi.org/10.3389/fpls.2019.00080 (2019).
Choi, W. G., Toyota, M., Kim, S. H., Hilleary, R. & Gilroy, S. Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc. Natl Acad. Sci. USA 111, 6497–6502 (2014).
Munns, R. & Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681 (2008).
Geng, Y. et al. A spatio-temporal understanding of growth regulation during the salt stress response in Arabidopsis. Plant Cell https://doi.org/10.1105/tpc.113.112896 (2013).
Dinneny, J. R. et al. Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science 320, 942–945 (2008).
Shiu, S.-H., Uygun, S. & Azodi, C. B. Cis-regulatory code for predicting plant cell-type transcriptional response to high salinity. Plant Physiol. https://doi.org/10.1104/pp.19.00653 (2019).
Julkowska, M. M. & Testerink, C. Tuning plant signaling and growth to survive salt. Trends Plant Sci. 20, 586–594 (2015).
Song, L. et al. A transcription factor hierarchy defines an environmental stress response network. Science 354, aag1550 (2016).
Golldack, D., Lüking, I. & Yang, O. Plant tolerance to drought and salinity: stress regulating transcription factors and their functional significance in the cellular transcriptional network. Plant Cell Rep. 30, 1383–1391 (2011).
Jiang, Y. & Deyholos, M. K. Functional characterization of Arabidopsis NaCl-inducible WRKY25 and WRKY33 transcription factors in abiotic stresses. Plant Mol. Biol. 69, 91–105 (2009).
Liu, S., Kracher, B., Ziegler, J., Birkenbihl, R. P. & Somssich, I. E. Negative regulation of ABA signaling by WRKY33 is critical for Arabidopsis immunity towards Botrytis cinerea 2100. eLife 4, e07295 (2015).
Zheng, Z., Mosher, S. L., Fan, B., Klessig, D. F. & Chen, Z. Functional analysis of Arabidopsis WRKY25 transcription factor in plant defense against Pseudomonas syringae. BMC Plant Biol. 7, 2 (2007).
Xu, X., Chen, C., Fan, B. & Chen, Z. Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. Plant Cell 18, 1310–1326 (2006).
Li, H. & Johnson, A. D. Evolution of transcription networks—lessons from yeasts. Curr. Biol. 20, R746–R753 (2010).
Phukan, U. J., Jeena, G. S. & Shukla, R. K. WRKY transcription factors: molecular regulation and stress responses in plants. Front. Plant Sci. 7, 760 (2016).
Teichmann, S. A. & Babu, M. M. Gene regulatory network growth by duplication. Nat. Genet. 36, 492–496 (2004).
Khraiwesh, B. et al. Genome-wide expression analysis offers new insights into the origin and evolution of Physcomitrella patens stress response. Sci. Rep. 5, 17434 (2015).
Keshishian, E. A. et al. Salt and oxidative stresses uniquely regulate tomato cytokinin levels and transcriptomic response. Plant Direct 2, e00071 (2018).
Gerstein, M. B. et al. Architecture of the human regulatory network derived from ENCODE data. Nature 489, 91–100 (2012).
Erwin, D. H. & Davidson, E. H. The evolution of hierarchical gene regulatory networks. Nat. Rev. Genet. 10, 141–148 (2009).
Khoueiry, P. et al. Uncoupling evolutionary changes in DNA sequence, transcription factor occupancy and enhancer activity. eLife 6, e28440 (2017).
Paris, M. et al. Extensive divergence of transcription factor binding in Drosophila embryos with highly conserved gene expression. PLoS Genet. 9, e1003748 (2013).
Borneman, A. R. et al. Divergence of transcription factor binding sites across related yeast species. Science 317, 815–819 (2007).
Inukai, S., Kock, K. H. & Bulyk, M. L. Transcription factor–DNA binding: beyond binding site motifs. Curr. Opin. Genet. Dev. 43, 110–119 (2017).
Yamasaki, K. et al. Structural basis for sequence-specific DNA recognition by an Arabidopsis WRKY transcription factor. J. Biol. Chem. 287, 7683–7691 (2012).
Cheng, X. et al. Structural basis of dimerization and dual W-box DNA recognition by rice WRKY domain. Nucleic Acids Res. 47, 4308–4318 (2019).
Hendler, A. et al. Gene duplication and co-evolution of G1/S transcription factor specificity in fungi are essential for optimizing cell fitness. PLoS Genet. 13, e1006778 (2017).
Hoang, X. L. T., Nhi, D. N. H., Thu, N. B. A., Thao, N. P. & Tran, L.-S. P. Transcription factors and their roles in signal transduction in plants under abiotic stresses. Curr. Genomics 18, 483–497 (2017).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Ernst, J., Nau, G. J. & Bar-Joseph, Z. Clustering short time series gene expression data. Bioinformatics 21, i159–i168 (2005).
Katoh, K. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059–3066 (2002).
Guindon, S. et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321 (2010).
Vercruysse, J. et al. Comparative transcriptomics enables the identification of functional orthologous genes involved in early leaf growth. Plant Biotechnol. J. 18, 553–567 (2020).
Wu, H. W. et al. A noncoding RNA transcribed from the AGAMOUS (AG) second intron binds to CURLY LEAF and represses AG expression in leaves. New Phytol. 219, 1480–1491 (2018).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Nicol, J. W., Helt, G. A., Blanchard, S. G., Raja, A. & Loraine, A. E. The Integrated Genome Browser: free software for distribution and exploration of genome-scale datasets. Bioinformatics 25, 2730–2731 (2009).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Yu, G., Wang, L. G. & He, Q. Y. ChIP seeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 31, 2382–2383 (2015).
Bailey, T. L. et al. MEME suite: tools for motif discovery and searching. Nucleic Acids Res. 37, W202–W208 (2009).
Bartlett, A. et al. Mapping genome-wide transcription-factor binding sites using DAP-seq. Nat. Protoc. 12, 1659–1672 (2017).
Langfelder, P. & Horvath, S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinform. 9, 559 (2008).
Huynh-Thu, V. A., Irrthum, A., Wehenkel, L. & Geurts, P. Inferring regulatory networks from expression data using tree-based methods. PLoS ONE 5, e12776 (2010).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Morrissey, E. R., Juárez, M. A., Denby, K. J., Burroughs, N. J. & Ideker, T. On reverse engineering of gene interaction networks using time course data with repeated measurements. Bioinformatics 26, 2305–2312 (2010).
Schwarz, B., Azodi, C. B., Shiu, S.-H. & Bauer, P. Putative cis-regulatory elements predict iron deficiency responses in Arabidopsis roots. Plant Physiol. https://doi.org/10.1104/pp.19.00760 (2020).
Wu, T.-Y., Gruissem, W. & Bhullar, N. K. Targeting intracellular transport combined with efficient uptake and storage significantly increases grain iron and zinc levels in rice. Plant Biotechnol. J. 17, 9–20 (2019).
Liang, Y. et al. A nondestructive method to estimate the chlorophyll content of Arabidopsis seedlings. Plant Methods 13, 26 (2017).
Ma, C., Xin, M., Feldmann, K. A. & Wang, X. Machine learning-based differential network analysis: a study of stress-responsive transcriptomes in Arabidopsis. Plant Cell 26, 520–537 (2014).
Acknowledgements
This work was financially supported by the Singapore-MIT Alliance for Research and Technology Program (SMART) and by Industry Alignment Fund—Prepositioning Program (IAF-PP).
Author information
Authors and Affiliations
Contributions
T.Y.W. led the project design, the data collection and interpretation and the drafting of manuscript. C.A. and M.J.L. performed cis-regulatory element analysis and wrote the manuscript. D.U. conceived the project and wrote the manuscript. H.G. and S.K. prepared genetic mutants and collected experimental data. All authors contributed to finalizing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Plants thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–14.
Supplementary Data 1
Supporting dataset for A. thaliana.
Supplementary Data 2
Supporting dataset for M. polymorpha.
Rights and permissions
About this article
Cite this article
Wu, TY., Goh, H., Azodi, C.B. et al. Evolutionarily conserved hierarchical gene regulatory networks for plant salt stress response. Nat. Plants 7, 787–799 (2021). https://doi.org/10.1038/s41477-021-00929-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-021-00929-7
This article is cited by
-
Genome-wide identification of WRKY transcription factors in Casuarina equisetifolia and the function analysis of CeqWRKY11 in response to NaCl/NaHCO3 stresses
BMC Plant Biology (2024)
-
Liquid in vitro culture system allows gradual intensification of osmotic stress in Solanum tuberosum through sorbitol
Plant Cell, Tissue and Organ Culture (PCTOC) (2024)
-
Cross-stress gene expression atlas of Marchantia polymorpha reveals the hierarchy and regulatory principles of abiotic stress responses
Nature Communications (2023)
-
Environmental gradients reveal stress hubs pre-dating plant terrestrialization
Nature Plants (2023)