Abstract
Transition metal oxides (TMOs) exhibit fascinating physicochemical properties, which originate from the diverse coordination structures between the transition metal and oxygen atoms. Accurate determination of such structure-property relationships of TMOs requires to correlate structural and electronic properties by capturing the global parameters with high resolution in energy, real, and momentum spaces, but it is still challenging. Herein, we report the determination of characteristic electronic structures from diverse coordination environments on the prototypical anatase-TiO2(001) with (1 × 4) reconstruction, using high-resolution angle-resolved photoemission spectroscopy and scanning tunneling microscopy/atomic force microscopy, in combination with density functional theory calculation. We unveil that the shifted positions of O 2s and 2p levels and the gap-state Ti 3p levels can sensitively characterize the O and Ti coordination environments in the (1 × 4) reconstructed surface, which show distinguishable features from those in bulk. Our findings provide a paradigm to interrogate the intricate reconstruction-relevant properties in many other TMO surfaces.
Similar content being viewed by others
Introduction
Transition metal oxides (TMOs) are one of the most important materials due to their versatile physical and chemical properties, such as magnetism1,2, ferroelectricity3,4, superconductivity5,6, and inspire various applications including (photo)catalysis7,8. Tremendous research interest has been attracted to interrogate the complex structure-property relationships at the atomic scale, concerning how the coordination between the metal cation and oxygen anion can contribute to the electronic structures9,10. The local coordination environments are determined by the coordination number, bond order, length, and angle. However, the coordination environments suddenly change when the bulk structure terminates at a surface. Reconstructions usually take place at the TMOs’ surfaces to minimize the surface energy, accompanied by the rearrangement of electronic structures11. The surface region can usually confine 2D/3D electron gas, providing a playground for correlated electron-electron, electron-boson couplings, and spin-charge interconversion12,13. Furthermore, lower coordination could appear at the surface defects and step edges, which are recognized as the active center for catalytic reactions14,15. These fascinating phenomena ranging from strong correlation to surface catalysis require deep and comprehensive insights into the coordination environments and the corresponding electronic states.
The precise measurement of the coordination environment and electronic property relies on multiply high resolutions in energy, real and momentum spaces. Surface science characterization techniques have been widely used to measure the geometric and electronic structures of TMOs from different aspects, but each technique has its limitations. In real and momentum spaces, low-energy electron diffraction (LEED) and surface X-ray diffraction (SXRD) can characterize the surface structures16,17. Transmission electron microscopy (TEM) can image the cross-sectional atomic arrangements in bulk18. Non-contact atomic force microscopy (NC-AFM) with a qPlus sensor19 has achieved single-bond resolution20,21,22. Scanning tunneling microscopy (STM) has provided a powerful tool in detecting both the surface structures and local density of states23,24. However, STM is mainly sensitive to the electronic states in the vicinity of Fermi level (EF) at the near-surface region. The broadly distributed core level and valence band (VB) have usually been examined by X-ray and UV photoemission spectroscopies (XPS/UPS), respectively25,26. However, the lack of momentum resolution makes it difficult to identify the band dispersions and thus hard to assign the bands directly. Yet, angle-resolved photoemission spectroscopy (ARPES) has rarely been used to measure the deep valence band electronic structures of TMOs27,28.
Our study reports that the joint STM-AFM-XPS-UPS-ARPES, in combination with density functional theory (DFT), can provide a comprehensive determination of characteristic electronic structures from diverse coordination environments in the (1 × 4) reconstructed surface of anatase-TiO2(001). By analyzing the measured O 2p VB, O 2s semi-core level, and Ti 3d gap state (GS) and comparing with the calculated electronic structures on the basis of various structural models, the surface electronic states are distinguished from the bulk ones, and able to be correlated to the contributions from the different coordination environments. Such an approach by capturing high-resolution global parameters could be widely applied to other intricated TMO materials.
Results
Real-space imaging the surface structures of anatase-TiO2(001)-(1 × 4) using STM and AFM
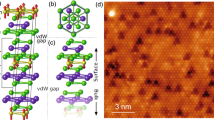
The bulk anatase-TiO2 consists of 6-fold coordinated Ti atoms and 3-fold coordinated O atoms (OBULK). Its terminating (001) surface involves the reduced coordination of 5-fold Ti atoms and the bridging 2-fold O atoms, endowing high surface energy for the well-studied catalytic applications29. However, surface science studies employ ultrahigh vacuum annealing that causes the release of (1 × 1) surface energy, leading to the (1 × 4) reconstruction at anatase-TiO2(001) surface30. The (1 × 4) superlattice consists of alternated terraces (OTERRACE) and hunching-up ridges, where the ridge involves different O rows at the side (OSIDE) and the top (OTOP) (Fig. 1a). There are two models, namely “ADM”31 and “AOM”32, that describe the possible structures of OTOP. The main difference is that the ADM ridge involves single OTOP row with 2-fold coordination (OTOP-ADM)31,33,34, while the AOM ridge involves double OTOP rows with 3-fold coordination (OTOP-AOM)32,35. The theory also predicts the possible coexistence of ADM and AOM ridge sites36,37 (sketched in Fig. 1a). In experiments, the STM images give some signature for their coexistence, for example, the contrast at ridge is quite nonuniform (Fig. 1b, c). However, the STM resolution is not enough to distinguish the ADM and AOM directly32, remaining a problem to be clarified.
a Structural model of the (1 × 4) reconstructed surface. Upper panel: side view, lower panel: top view. The ball-and-stick model sketches three kinds of ridges. Left: the ADM ridge (OTOP-ADM), right: the AOM ridge (OTOP-AOM), middle: the mixed ADM and AOM ridge with a ratio of 1:1. The OTOP, OSIDE, OTERRACE, and OBULK atoms are correspondingly colored in upper and lower panels. An OTOP-AOM becomes an OTOP-ADM when it is missing an O atom marked by the red arrows. The surface OV defects at terrace (OV-TERRACE and OV-3C) and ridge (OV-RIDGE) sites are marked by the black arrows. b The empty-state STM images of high bias (3.0 V, 350 pA) and high-resolution (1.2 V, 350 pA) with the same area, measured at 80 K. High-resolution STM image exhibits clear terrace and ridge structures. The white arrow indicates an intrinsic dark point defect32. c Line profiles extracted from the corresponding colored lines in b.The red curve from high bias STM image shows the nonuniform electronic distributions, and the cyan one from the high-resolved image presents two different heights. d A set of in-situ empty-state STM (1.5 V, 10 pA) and the NC-AFM images at a frequency shift of −29 Hz within the same area, measured at 5 K, using a W tip. e Line profiles extracted from the corresponding colored lines in d. The red curve from STM image shows the nonuniform electronic distributions, and the blue one from AFM presents two distinct contrasts, labeled as ADM (blue arrow) and AOM (yellow arrow), according to the relative height in their models. f Left panel: a representative XPS spectrum in the energy range 0–30 eV measured at excitation with light energy of hυ = 200 eV, sample kept at 20 K, showing the main features of GSs, VBs, and semi-core levels. Right panel: schematic diagram of the main contributions from Ti 3d, O 2p, and O 2s.
We perform in situ STM and AFM experiments to characterize the structures at a high resolution. The empty-state STM image mainly integrates the Ti electronic state on the ridge and terrace (Fig. 1d, left panel), while the AFM image (given in inverted contrast because of attraction force regime in imaging) mainly reflect the contrasts of O atoms along the ridges (Fig. 1d, right panel). The two groups of O atoms can be distinguished from their relative contrasts, as labeled by ADM and AOM according to the relative heights in their structural models32,38 (Fig. 1e). Both the STM and the AFM images indicate the coexistence of the ADM and AOM structures on the ridge.
Actually, oxygen vacancy (OV) defects can appear on the surface at either terrace or ridge sites, which act as the reactive centers and introduce metallic states and GSs39,40. In Fig. 1a, these coordination environments for O atoms in the anatase-TiO2(001)-(1 × 4) surface are labeled by OBULK, OTERRACE, OSIDE, OTOP-ADM, OTOP-AOM, OV at ridge (OV-RIDGE), and at terrace (OV-TERRACE and OV-3C). They together contribute to the complex electronic states, as shown by the measured XPS spectrum (Fig. 1f), which presents a weak Ti 3d GS between the Fermi level (EF) and VB maximum (VBM), the intense VB mainly consisting of O 2p levels, and a broad peak consisting of O 2s semi-core levels. We now attempt to correlate these electronic states with their coordination environments.
Distinguishing the surface bands from momentum-resolved O 2p spectra
The anatase-TiO2 has a body-centered-tetragonal (bct) structure with the space group I41/amd41. The bulk bct Brillouin zone (BZ) of primitive cell (shaded in light blue) is depicted in Fig. 2a. A simple tetragonal (st) BZ of unit cell (shaded in orange) is usually applied to examine the band structures along the high symmetric axes42, which is projected to a surface BZ with (1 × 4) reconstruction along the high symmetric points of \(\bar{\Gamma }\), \(\bar{{{{{{\rm{M}}}}}}}\) and \(\bar{{{{{{\rm{X}}}}}}}\). As known for most TMOs, the VB of anatase-TiO2(001) involves the main contribution from the O 2p states and a minor contribution from the Ti 3d states43, as shown in the spectrum in Fig. 1f. However, such momentum-integrated spectrum cannot clearly resolve the exact bands. We present the 2D energy-momentum [E(k||)] resolved VB map along the high-symmetry \(\bar{\Gamma }-\bar{{{{{{\rm{X}}}}}}}\) and \(\bar{\Gamma }-\bar{{{{{{\rm{M}}}}}}}\) directions, with the binding energy (Eb) mainly distributing from ~3.0 to 9.0 eV below EF and the energy distribution curve (EDC) at \(\bar{\Gamma }\) point (Fig. 2b). Using its second derivative map44, the contrasts of the band structure in the ARPES map can be highly improved (Fig. 2c). To assign the observed bands, the bulk electronic bands have been calculated by using both the primitive cell and the unit cell41,42, as well as different kz (see calculation details in Supplementary Fig. 1). The calculated bulk VBs can overall reproduce the experimental results, except for the two at Eb ~ 6.5 eV and 7.1 eV, as labeled by S1 and S2, respectively (Fig. 2c).
a Upper panel: The bulk BZ and the projected surface BZ. Blue shade indicates the bulk bct structure of primitive cell. Orange shade indicates the bulk st structure of unit cell. The top orange shade is the projected surface 2D structure. Lower panel: surface (1 × 4) reconstructed BZ. b The raw ARPES cut excited by hυ = 21.2 eV along the high-symmetry axes and the corresponding EDC at \(\bar{\Gamma }\) point. c The second derivative cut from b, overlaid with the calculated bulk VBs at the corresponding kz (\({{{{{\rm{\delta }}}}}}^{\prime} \sim 0.5\)) (yellow dashed lines, see details in Supplementary Fig. 1). The calculated VBs have been shifted to maximize the overlap with the experimental results. White arrows: surface levels of S1, S2 bands. d The calculated pDOSs of OBLUK, OTERRACE, OSIDE, OTOP-ADM and OTOP-AOM, based on the ADM (solid lines) and AOM (dotted lines) models. e The extracted electronic states of S1, S2 and VBM bands of the ADM model at \(\bar{\Gamma }\) point, exhibiting localized spatial distributions at the OTERRACE, OSIDE, and OTOP-ADM atoms, respectively. f Calculated VB dispersion according to the weightings of surface OTERRACE and OSIDE sites, respectively. The OTERRACE bands involve the main feature of S1 and the OSIDE involve the main feature of S2, respectively.
We turn to consider the S1 and S2 as the VBs contributed by surface O atoms. To verify their surface nature, we carried out the experiment by depositing potassium (K) atoms to the surface because the alkali atoms can strongly modify the surface levels but have little influence on the bulk bands. It is clear that the S1 and S2 can be quenched by K atoms adsorption and recovered after K desorption, similar to behaviors of the H2 adsorption on Ir surface45, while the bulk bands remain unchanged (Supplementary Fig. 2). Different from the bulk VBs with broadly distributed E(k||) dispersions, the S1 and S2 are nearly flat near \(\bar{\Gamma }\) point. Such unique features imply the S1 and S2 may be contributed by certain specific O sites in the surface. We calculated the surface electronic structures using the slab models by including the geometries of ADM and AOM models, respectively. The projected density of states (pDOSs) of each O atoms of OTERRACE, OSIDE, OTOP-ADM and OTOP-AOM are extracted to distinguish their contributions (Fig. 2d). For OTERRACE and OSIDE atoms, it is found that both ADM (solid lines) and AOM (dotted lines) models give approximate electronic structures within a broadly hybridized energy range of Eb ~ 3–8 eV. But, the OTERRACE atoms dominate at Eb ~ 6.8 eV (green shaded) and the OSIDE atoms dominate at Eb ~ 7.2 eV (orange shaded) with well-defined spatial distributions (Fig. 2e), which could be readily assigned to the observed S1 and S2 bands, respectively. Furthermore, we plot the band dispersion of each O atoms calculated using the ADM model (Supplementary Fig. 3). The weightings of surface O atoms show localized features relative to the that of bulk O atoms. The weightings of S1 and S2 indicate near flat character in the vicinity of \(\bar{\Gamma }\) point (Fig. 2f), in good agreement with those observed in the experimental E(k||) map. For OTOP atoms, it is known that they mainly contribute to the VBM46,47 with localized features (Fig. 2e). The calculated pDOS of OTOP-ADM and OTOP-AOM with ADM and AOM models, respectively, dominate at VBM, but with slight energy difference (Fig. 2d). From the O 2p spectra, it is still difficult to distinguish OTOP-ADM and OTOP-AOM, we thus search for more evidence from the O 2s spectra in the following sections.
Recognizing the surface semi-core levels from O 2 s spectra
The O 1s XPS spectrum of anatase-TiO2(001)-(1 × 4) exhibits only a single peak, as widely observed in the crystalline TiO2 surfaces48,49. By contrast, even under excitation by a high photon energy (610 eV), the O 2s spectrum of anatase-TiO2(001)-(1 × 4) presents a tail spreading to a low Eb region, where the tail could be from the contributions of the surface O atoms (Supplementary Fig. 4). Distinctly different features can be observed in comparison with the results from a simpler rutile-TiO2(110)-(1 × 1) surface at excitations using lower photon energies, which can be more sensitive to the surface states (Supplementary Fig. 5a, b). By tuning the excitation photon energies (hυ) from 39 eV to 200 eV, the evolution of these peaks can be more clearly shown (Fig. 3a). According to the universal curve for the electron inelastic mean free path (IMFP) as a function of the kinetic energy50, it can be expected that the spectra excited with hυ > 100 eV could mainly detect the bulk information, while those excited with hυ ~ 30–100 eV are sensitive to surface region. The spectra excited with hυ = 120 and 200 eV show a strong peak at Eb ~ 22.7 eV, which could be assigned to the contributions of OBULK atoms (Fig. 3a), similar to the results from the rutile TiO2(110) surface51. As the hυ reduced to below 100 eV, the intensity of the peak at Eb ~ 22.7 eV decreased dramatically, and meanwhile, several new peaks at lower Eb arise. Three selected spectra with hυ = 39, 43 and 46 eV excitation are presented in Fig. 3b–d, which can be decomposed into four peaks by the best XPS fitting (Supplementary Fig. 6). Except for the peak at Eb ~ 22.7 eV that is contributed by OBULK atoms (gray shaded), the three peaks at Eb ~ 21.2, 20.1, and 18.7 eV could be contributed by surface O atoms, as a result of the chemical shifts52 of the surface semi-core levels. It is noted the intensity of these distinguishable peaks are changing dramatically: for the one at Eb ~ 22.7 eV of OBULK atoms, the enhanced intensity could be reasonably attributed to the increased IMFP with larger hυ; for the other three peaks of surface O atoms, we need to consider some other possible factors, like the photoelectron diffraction and the resonant photoemission processes. We can exclude the effect of the photoelectron diffraction due to the possible diffraction-caused enhanced angle-dependent intensity variations (Supplementary Fig. 7). The angle distribution curves (ADCs) at each photon energy give overall Gaussian-like shape for the integrated intensity distributions against θ, obviously no diffraction-enhanced intensity with ±15°. The EDCs show nearly the same feature at each excitation photon energy, showing the angle-independent O 2s spectra. These analyses can exclude the possibility of the diffraction effect by the reconstructed surface structure. While, by measuring the spectra using the tunable excitation phonon energy in the range of hυ ~ 39–55 eV, the resonant photoemission processes were observed to occur at around hυ ~ 43–46 eV (Supplementary Fig. 5c), which could be assigned to the Ti 3p → 3d optical transition at anatase-TiO2(001) surface53,54. Such resonant photoemission processes could enhance the photoemission intensities of the surface semi-core levels at certain excitation photon energy, and make the peaks more distinguishable in the O 2s spectra. Nevertheless, this effect does not contribute any additional peak and much easily be recognized according to the analysis of our results.
a Evolution of O 2s semi-core level spectra with increasing excitation photon energy from 39 eV to 200 eV. b–d Peak fitting of O 2s spectra with BG subtracted for 39, 43, and 46 eV excitation, respectively. Four peaks at 22.7, 21.2, 20.1, and 18.7 eV can be extracted with relative intensity changes. e The calculated O 2s semi-core level pDOSs of each O atom. f Plot of the OTOP-ADM (red triangle) and OTOP-AOM (red inverted triangle) contributed peak area ratio in the left axis and the corresponding Ti 3d GS intensity (blue circle) in the right axis as a function of sample reduction. The as-grown sample was gradually reduced by increasing Ar+ sputtering (each cycle 2 min) and annealing at 650 °C.
To understand these O 2s semi-core level peaks, we analyzed the calculated pDOS in Fig. 3e. The pDOS of OBULK has a broad spectral weight in the range Eb ~ 21–23 eV, which corresponds well to the peak of Eb ~ 22.7 eV in the experiment. The pDOS of OSIDE and OTERRACE sites overlap in the energy range of Eb ~ 20.8–21.4 eV (Fig. 3e). These two together could be probably correlated to the peak of Eb ~ 21.2 eV in the experiment. More interestingly, the pDOS of OTOP-ADM and OTOP-AOM atoms are clearly separated at Eb ~ 20.1 eV and 18.7 eV, respectively, which are in excellent agreement with the two lower energy peaks in the experiment (red shaded in Fig. 3b–d). Based on the above assignments, the existence of the two peaks at Eb ~ 20.1 eV and 18.7 eV implies the coexistence of ADM and AOM ridge structures at the anatase-TiO2(001)-(1×4) surface, in line with the two distinct contrast of ADM and AOM in the AFM measurements (Fig. 1d).
The quantitative analysis of the mixed ADM and AOM ratio is difficult, because the absolute areas of the two peaks are sensitive to the excitation hυ and rely on how the background signal are subtracted. Qualitatively, we investigated the change of ADM and AOM ratio at the excitation hυ = 40.8 eV using slightly reduced surface through treatments of sputtering and annealing cycles (Supplementary Fig. 8). The reduction procedure is monitored by the increase of Ti 3d GS intensities (blue circle) in Fig. 3f. It can be seen that the OTOP-ADM component (red triangle) is increasing while the OTOP-AOM component (red inverted triangle) is decreasing during the sample reduction procedure, which strongly supports our assignments. To further examine the stability of mixed ADM-AOM structure of anatase TiO2(001)-(1×4), we calculate the surface energy correlation with the oxygen chemical potential and the phase diagram (Supplementary Fig. 9), in consistent with the similar phase diagrams for AOM and ADM in previous stusies47,55,56. Our study provides additional information for the mixed ADM-AOM structures with tunable ratios. The results indicate that the mixed ADM-AOM structure could be a stable phase during sample growth under a relatively low temperature and pressure conditions.
Identifying the O defect states from Ti 3p and 3d spectra
The O 2p and 2s spectra above have successfully identified the different coordination environments of O atom at the reconstructed anatase-TiO2(001)-(1 × 4) surface. In addition, the OV defects commonly exist on oxides’ surface causing excess electron doping at the surface. The electron doping could introduce two features: (i) a delocalized electron gas at near-surface region confined by the band bending potential, which forms a metallic state (MS) just below EF (refs. 39,40); (ii) localized electron denotation to nearby Ti atoms, which reduces Ti4+ to Ti3+ and forms Ti 3d GSs32,39,40. The OV defects could be produced by either sputtering and annealing in vacuum32, or light irradiation with a high flux radiation beam39. We here present the evolution of Ti 3p and 3d spectra with OV formation under light irradiation. By increasing the light irradiation time, the intensity of Ti4+ state gradually decreases while the intensity of Ti3+ state arises in the Ti 3p spectra (Fig. 4a). Meanwhile, both MS and GSs arise in the Ti 3d spectra (Fig. 4b). The intensities of GSs increase gradually, while that of MS looks rapidly saturating at EF. This is because the MS is formed due to the lowered Ti 3d band below EF, which has a saturated electron density that leads to the saturated intensity near the EF (ref. 39). Two distinct GSs appear at Eb ~ 1.1 and 1.8 eV in the Ti 3d spectra, as denoted by GS1 and GS2, respectively (Fig. 4b). Different from the rutile-TiO2(110)−1 × 1 surface, where the OV defects can only produce one GS at Eb ~ 0.9 eV57, the emergence of two GSs on anatase-TiO2(001)-(1 × 4) surface is unique, which had been observed in previous studies39,40 but have not been understood yet. Bigi et al. found the adsorption of O2 at defective anatase-TiO2(001)-(1 × 4) surface can quench both the GS1 and GS2, and thus demonstrate their origin should be from surface defects rather than from bulk defects40. We extract the intensities of the two GSs separately from the best fitting (Supplementary Fig. 10). It is found that although the intensities of Ti3+ state (Fig. 4c) and the total GSs (Fig. 4d) are increasing meanwhile as a function of light irradiation time, the intensities of GS1 and GS2 are changing asynchronously (Fig. 4d), implying the GS1 and GS2 must have different origins from surface defects. In our previous experiments, we have detected the point defect induced GS at Eb ~ 0.9 eV at ridge sites by scanning tunneling spectroscopy32, and the hydroxyl groups induced GS at Eb ~ 1.6 eV at terrace sites by UPS49. Considering the approximated energies, we assign the observed GS1 at Eb ~ 1.1 eV and GS2 at Eb ~ 1.8 eV to the electronics states of OV defects at ridge and terrace sites, respectively.
a, b Ti 3p core level spectra obtained by hυ = 80 eV and the in-gap UPS spectra excited by hυ = 44 eV (synchrotron radiation light), respectively. c, d Evolution of the Ti3+/(Ti3++Ti4+) ratio, and the intensities of GS1, GS2 and total of (GS1 + GS2), as a function of light irradiation time. e–g Calculated charge density contours of excess electrons and DOSs of total (gray) and 1–3 layer (yellow: spin up, blue: spin down) with an OV-RIDGE at ridge e, an OV-3C or OV-TERRACE at terrace f and multiple Ov defects at ridge and terrace g, respectively. h, i The in-gap UPS spectra obtained after 30 min UV irradiation (He lamp, 21.2 eV) with CH3OH adsorption at 160 and 220 K, respectively. In i, the UPS spectra are fitted after background subtraction with one peak at 1.20 eV for the spectrum at 220 K, and double peaks at 1.20 and 1.75 eV for the one at 160 K. A spectrum measured at the bare surface is used for reference (gray).
We calculate the configurations and formation energies for possible surface OV defects at ridge and terrace sites using spin-polarized DFT (Supplementary Fig. 11). From the formation energies, it is found that the missing of one OTOP is the most possible OV defect at the ridge (OV-RIDGE, Fig. 4e), and the missing of a bridging O atom along [100] direction or along [010] direction could be the OV defect at terrace (OV-3C and OV-TERRACE, Fig. 4f). The calculated electronic structures with PBE + U functional (U = 3.9 eV)40 show that each OV defect can contribute to a GS with its excess electron redistributing to adjacent Ti atoms (Fig. 4e, f). Further ab initio molecular dynamics (AIMD) simulations indicate the excess electrons are stable at the adjacent Ti atoms (Supplementary Fig. 12). The charge redistribution and the distortion of the lattice in the vicinity imply the formation of small electron polaron, similar as the small polaron of OV at rutile-TiO2(110) surface58. But, from the pDOS with either antiferromagnetic59 or ferromagnetic states (Supplementary Fig. 13), the energies of different OV defects are not separated clearly, making the assignment difficult. Such energy inaccuracy is possibly because of the self-interaction error in DFT, which usually leads to an underestimated bandgap of TiO2. In particular, when multiple OV defects are considered (Supplementary Fig. 14), the GSs show very close energies within the underestimated bandgap of 2.5 eV (Fig. 4g).
It is difficult to create OV defects at either ridge or terrace in a controllable manner for verifying the assignment of GS1 and GS2. Alternatively, we design an experiment to create hydroxyl groups at ridge and terrace sites from methanol (CH3OH) dissociation, by considering the hydroxyl groups can provide surface states similar to those of the OV defects owing to the induced excess electrons that contribute to the GSs. Methanol has similar adsorption behavior as water49, that the molecular CH3OH only adsorbs at ridge sites at T > 190 K, but adsorbs at both ridge and terrace sites at T < 190 K. Therefore, at T > 190 K, a single GS is expected to appear for ridge sites after CH3OH dissociation, while at T < 190 K, double GSs for ridge and terrace sites are observed. Figure 4h compares the spectra obtained after CH3OH dissociation with samples prepared at 160 K and 220 K, respectively. Clearly, for the spectrum at 220 K, only one GS peak at ~1.20 eV can be detected; for the spectrum at 160 K, the peak is broadened to higher energies. The best fit can distinguish two peaks at about Eb ~ 1.20 eV and 1.75 eV (Fig. 4i), which could be assigned to the hydroxyl groups induced GSs at ridge and terrace sites, respectively. Such similarity strongly suggests that the excess electron doping by either OV defects or hydroxyl groups can form a GS at Eb ~ 1.1–1.3 eV at the ridge and a GS at Eb ~ 1.6–1.8 eV at the terrace.
Discussion
The above measurements unveil the surface electronic states in the intricated reconstructed surface of anatase-TiO2(001), which presents diverse O coordination environments. Using the multi-technique approach, we distinguish the bands of OTERRACE and OSIDE using the energy-momentum-resolved O 2p spectra, the electronic states of OBULK, OTOP-ADM and OTOP-AOM using the O 2s spectra, the defect electronic states at ridge and terrace sites through measuring the gap-state Ti 3d spectra. These global parameters together establish the correlation of the surface electronic states with the (1 × 4) reconstructed structure of anatase-TiO2(001), and provide the deeper understanding of the mixed ADM-AOM configurations36,37. The revealed coordination (structure)-electronic (property) relationships could pave the way to understand the widely concerned surface catalysis and correlation phenomena of anatase-TiO2. For example, the main focus in photocatalytic water splitting on TiO2 is to understand the energy level alignment between the TiO2 VBs and the highest occupied molecular orbitals (HOMOs) of water at their interface60, which requires an insight into the intricate coordination environments and surface electronic structures. Distinguishing the surface VBs of OTOP, OTERRACE and OSIDE can directly locate and determine how the water HOMOs can hybridize with the TiO2 VBs with energy-momentum-site specificities; regarding the correlation phenomena, the anatase-TiO2 can provide a platform to tune the electron-phonon and electron-plasmon couplings through the excess charge by OV defect39,61 and photo-excitation62,63, and can support an enhanced electron-phonon coupling interface of FeSe/anatase-TiO2 to induce high-Tc superconductivity64. Distinguishing the origins of the doped electrons from different GSs can provide prerequisites to tune the electron-boson couplings in the complicate many-body interactions. In summary, our results provide the comprehensive understanding of the complex coordination and the corresponding electronic structures of the prototypical anatase-TiO2(001), and beyond, may benefit to other TMO materials.
Methods
Sample preparation
The anatase-TiO2(001) thin films were epitaxially grown on 0.7 wt% Nb-doped SrTiO3(001) substrates by pulse laser deposition (PLD) method39. During deposition, The O2 pressure was kept at 1.5 × 10−3 Pa and the substrate temperatures were kept at a 650 °C. To obtain high quality 1 × 4 reconstructed surface, the anatase-TiO2 films were grown slowly on SrTiO3 substrate with deposition rate of ~1 nm/h. The typical thickness of the thin films was 20–30 nm. The as-grown anatase-TiO2(001) samples were transferred between the PLD, STM and ARPES systems without exposure to air, via a portable ultrahigh vacuum transfer chamber with a battery powered ion pump to maintain a pressure better than 1 × 10−9 mbar.
STM, XPS, and ARPES measurements
The STM measurements were conducted in a low temperature STM (Omicron LT, Matrix) at 80 K with a tungsten tip. High-resolved in situ STM and AFM experiments with a qPlus sensor were conducted at 5 K. The photoelectrons in XPS and ARPES measurements at 90 K were excited by the Mg Kα radiation (1253.6 eV) and the resonance He lamp (21.2 eV and 40.8 eV), respectively. The hυ-dependent XPS measurements were performed at the beam line 09U of Shanghai Synchrotron Radiation Facility (SSRF), with the photon energy varying from 39 to 610 eV with horizontally polarized light at 20 K. Hemispherical energy analyzer (VG Scienta, DA30L) was used for all measurements. Energy resolution of instrument was better than 5 meV and the angular resolution was better than 0.1°.
DFT calculation
All the calculations are performed with the Vienna Ab-initio Simulation Package (VASP). Electron-nuclei interactions are described by the projector-augmented wave pseudopotentials65. The Perdew-Burke-Ernzerhof (PBE) exchange-correlation functions are employed in all calculation66. The long-range van der Vaals interactions are corrected with Grimme’s D2 method67. The Kohn-Sham wave functions are expanded in plane waves up to 500 eV. The structures are relaxed until the atomic forces are less than 0.01 eV/atom and total energies are converged to 10−5 eV. A 15 Å thick area of vacuum in the z-direction and dipole correction are employed to avoid non-physical interaction between neighboring slabs.
Band calculation
The 3D BZs are sampled with a grid of 13 × 13 × 13 k-points according to the Monkhorst-Pack procedure for primitive cell and unit-cell of bulk anatase-TiO2, respectively68. The anatase-TiO2 (001)-(1 × 4) surface was modelled using a slab of four O-Ti-O trilayers based on ADM and AOM model, with the bottom O-Ti-O trilayer being fixed and 3 × 9 × 1 k-points are used for this surface. To assign the three new peaks in detail, we calculate the electronic structures using DFT with the hybrid HSE06 functional.
Surface energy and phase diagram calculation
Using the (4 × 4) supercell, we investigate different oxidized surfaces for ADM: AOM corresponding to ADM (not oxidation), 3:1 (25% oxidation), 1:1 (50% oxidation), 1:3 (75% oxidation) and AOM (all oxidation). The stability of these structures in different environments can be discussed by calculating the free energy correlation with the oxygen chemical potential. The chemical potentials must satisfy the following boundary conditions: (i) \({\mu }_{{{{{{\rm{O}}}}}}}\le \frac{1}{2}{E}_{{{{{{{\rm{O}}}}}}}_{2}}\); (ii) \({\mu }_{{{{{{\rm{Ti}}}}}}}\le {\mu }_{{{{{{\rm{Ti}}}}}}}^{{{{{{\rm{bulk}}}}}}}\) and (iii) \({\mu }_{{{{{{\rm{Ti}}}}}}}+2{\mu }_{{{{{{\rm{O}}}}}}}={E}_{{{{{{{\rm{TiO}}}}}}}_{2}}\), in which \({E}_{{{{{{{\rm{TiO}}}}}}}_{2}}\) is the internal energy of the bulk TiO2 per unit cell. The stability of surface energy can thus be written as:
According to the constraints of the chemical potential, the range of \({{{{{{\rm{\mu }}}}}}}_{{{{{{\rm{O}}}}}}}\) is set as \({\mu }_{{{{{{{\rm{O}}}}}}}_{2}}/2\ge {\mu }_{{{{{{\rm{O}}}}}}}\ge ({E}_{{{{{{{\rm{TiO}}}}}}}_{2}}-{\mu }_{{{{{{\rm{Ti}}}}}}})/2\). In equilibrium with O2 gas, \({\mu }_{{{{{{\rm{O}}}}}}}\) is expressed as:
where \({k}_{{{{{{\rm{B}}}}}}}\), \({P}^{0}\) and \(P\) are the Boltzmann constant, standard atmospheric pressure, and oxygen partial pressure, respectively. \({E}_{{{{{{{\rm{O}}}}}}}_{2}}\) is the total energy of the O2 obtained from a spin-polarized calculation. \({\Delta H}_{{{{{{{\rm{O}}}}}}}_{2}}(T,{P}^{0})\) and \({T\Delta S}_{{{{{{{\rm{O}}}}}}}_{2}}(T,{P}^{0})\) are taken from a thermodynamic database69. The last term is the contribution coming from the partial pressure of oxygen.
OV defects calculation
The geometry optimizations and electronic states of OV defects at the ridge and terrace sites are simulated in 4 × 3 supercell, which supplies enough room to distort the lattice for electron. We perform the spin-polarized DFT calculation using the PBE method to calculate the configurations and formation energies of possible surface OV configurations. Due to the self-interaction error70, the orbitals are too delocalized in DFT calculation to locate an electron to an individual Ti atom. To overcome this problem, we perform the spin-polarized DFT calculation using the PBE + U method with U = 3.9 eV40 to calculate pDOS and electronic charge distribution. After geometry optimization, we use velocity rescaling to bring the temperature of the system to 300 K. An 8 ps microcanonical ab initio molecular dynamics (AIMD) trajectory is generated.
Data availability
All data needed to support the conclusions in the study are available within the article and/or the supplementary files. Data underlying Figs. 1–4 and Supplementary Figs. 1–14 are provided in the source data file with this paper. Source data are provided in this paper.
References
Heron, J. T. et al. Deterministic switching of ferromagnetism at room temperature using an electric field. Nature 516, 370–373 (2014).
Brinkman, A. et al. Magnetic effects at the interface between non-magnetic oxides. Nat. Mater. 6, 493–496 (2007).
Choi, K. J. et al. Enhancement of ferroelectricity in strained BaTiO3 thin films. Science 306, 1005–1009 (2004).
Haeni, J. H. et al. Room-temperature ferroelectricity in strained SrTiO3. Nature 430, 758–761 (2004).
Damascelli, A., Hussain, Z. & Shen, Z. X. Angle-resolved photoemission studies of the cuprate superconductors. Rev. Mod. Phys. 75, 473–541 (2003).
Reyren, N. et al. Superconducting interfaces between insulating oxides. Science 317, 1196–1199 (2007).
Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 48, 53–229 (2003).
Fujishima, A., Zhang, X. & Tryk, D. A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 63, 515–582 (2008).
Waroquiers, D. et al. Statistical analysis of coordination environments in oxides. Chem. Mater. 29, 8346–8360 (2017).
Leng, M. et al. A new perspective on the composition-structure-property relationships on Nb/Mo/Cr-doped O3-type layered oxide as cathode materials for sodium-ion batteries. Chem. Eng. J. 413, 127824 (2021).
Polo-Garzon, F., Bao, Z., Zhang, X., Huang, W. & Wu, Z. Surface reconstructions of metal oxides and the consequences on catalytic chemistry. ACS Catal. 9, 5692–5707 (2019).
Plumb, N. C. & Radović, M. Angle-resolved photoemission spectroscopy studies of metallic surface and interface states of oxide insulators. J. Phys.: Condens. Matter 29, 433005 (2017).
Trier, F. et al. Oxide spin-orbitronics: spin-charge interconversion and topological spin textures. Nat. Rev. Mater. 7, 258–274 (2022).
Gong, X.-Q., Selloni, A., Batzill, M. & Diebold, U. Steps on anatase TiO2(101). Nat. Mater. 5, 665–670 (2006).
Pastor, E. et al. Electronic defects in metal oxide photocatalysts. Nat. Rev. Mater. 7, 503–521 (2022).
Bliem, R. et al. Subsurface cation vacancy stabilization of the magnetite (001) surface. Science 346, 1215–1218 (2014).
Hussain, H. et al. Structure of a model TiO2 photocatalytic interface. Nat. Mater. 16, 461–466 (2017).
Su, D. S., Zhang, B. & Schlögl, R. Electron microscopy of solid catalysts—transforming from a challenge to a toolbox. Chem. Rev. 115, 2818–2882 (2015).
Giessibl, F. J. High-speed force sensor for force microscopy and profilometry utilizing a quartz tuning fork. Appl. Phys. Lett. 73, 3956–3958 (1998).
Gross, L. et al. Bond-order discrimination by atomic force microscopy. Science 337, 1326–1329 (2012).
Xu, J. et al. Determining structural and chemical heterogeneities of surface species at the single-bond limit. Science 371, 818–822 (2021).
Wagner, M., Meyer, B., Setvin, M., Schmid, M. & Diebold, U. Direct assessment of the acidity of individual surface hydroxyls. Nature 592, 722–725 (2021).
Bonnell, D. A. Scanning tunneling microscopy and spectroscopy of oxide surfaces. Prog. Surf. Sci. 57, 187–252 (1998).
Setvín, M., Wagner, M., Schmid, M., Parkinson, G. S. & Diebold, U. Surface point defects on bulk oxides: atomically-resolved scanning probe microscopy. Chem. Soc. Rev. 46, 1772–1784 (2017).
Parmigiani, F. & Sangaletti, L. Fine structures in the X-ray photoemission spectra of MnO, FeO, CoO, and NiO single crystals. J. Electron. Spectrosc. Relat. Phenom. 98-99, 287–302 (1999).
Ye, Y. et al. X-ray spectroscopies studies of the 3d transition metal oxides and applications of photocatalysis. MRS Commun. 7, 53–66 (2017).
Emori, M., Sugita, M., Ozawa, K. & Sakama, H. Electronic structure of epitaxial anatase TiO2 films: angle-resolved photoelectron spectroscopy study. Phys. Rev. B 85, 035129 (2012).
Sánchez-Sánchez, C. et al. Valence band electronic structure characterization of the rutile TiO2(110)-(1×2) reconstructed surface. Surf. Sci. 608, 92–96 (2013).
Vittadini, A., Selloni, A., Rotzinger, F. & Grätzel, M. Structure and energetics of water adsorbed at TiO2 anatase (101) and (001) surfaces. Phys. Rev. Lett. 81, 2954 (1998).
Fang, K. et al. An environmental transmission electron microscopy study of the stability of the TiO2(1×4) reconstructed (001) surface. J. Phys. Chem. C 123, 21522–21527 (2019).
Lazzeri, M. & Selloni, A. Stress-driven reconstruction of an oxide surface: The anatase TiO2(001)-(1×4) surface. Phys. Rev. Lett. 87, 266105 (2001).
Wang, Y. et al. Role of point defects on the reactivity of reconstructed anatase titanium dioxide (001) surface. Nat. Commun. 4, 2214 (2013).
Vittadini, A., Casarin, M. & Selloni, A. Chemistry of and on TiO2-anatase surfaces by DFT calculations: a partial review. Theor. Chem. Acc. 117, 663–671 (2007).
Gong, X.-Q., Selloni, A. & Vittadini, A. Density functional theory study of formic acid adsorption on anatase TiO2(001): geometries, energetics, and effects of coverage, hydration, and reconstruction. J. Phys. Chem. B 110, 2804–2811 (2006).
Tang, H. et al. Understanding the intrinsic chemical activity of anatase TiO2(001)-(1×4) surface. J. Phys. Chem. C 121, 1272–1282 (2017).
Shi, Y. et al. Role of surface stress on the reactivity of anatase TiO2(001). J. Phys. Chem. Lett. 8, 1764–1771 (2017).
Shi, Y. et al. Structures of defects on anatase TiO2(001) surfaces. Nanoscale 9, 11553–11565 (2017).
Liu, J. et al. In-situ measurements of reconstructed anatase TiO2(001) surface by variable-temperature STM. Chin. J. Chem. Phys. 36, 125–131 (2023).
Ma, X. et al. Formation of plasmonic polarons in highly electron-doped anatase TiO2. Nano Lett. 21, 430–436 (2021).
Bigi, C. et al. Distinct behavior of localized and delocalized carriers in anatase TiO2 (001) during reaction with O2. Phys. Rev. Mater. 4, 025801 (2020).
Calatayud, M. et al. Quantum-mechanical analysis of the equation of state of anatase TiO2. Phys. Rev. B 64, 184113 (2001).
Asahi, R., Taga, Y., Mannstadt, W. & Freeman, A. J. Electronic and optical properties of anatase TiO2. Phys. Rev. B 61, 7459–7465 (2000).
Landmann, M., Rauls, E. & Schmidt, W. G. The electronic structure and optical response of rutile, anatase and brookite TiO2. J. Phys.: Condens. Matter 24, 195503 (2012).
Zhang, P. et al. A precise method for visualizing dispersive features in image plots. Rev. Sci. Instrum. 82, 043712 (2011).
Van der Veen, J., Himpsel, F. & Eastman, D. Structure-dependent 4f—core-level binding energies for surface atoms on Ir (111), Ir (100)-(5×1), and metastable Ir (100)-(1×1). Phys. Rev. Lett. 44, 189 (1980).
Xiong, F. et al. Surface reconstruction-induced site-specific charge separation and photocatalytic reaction on anatase TiO2(001) surface. J. Phys. Chem. C. 121, 9991–9999 (2017).
Jin, F. & Zhao, Z. Reactivity of anatase (001) surface from first-principles many-body Green’s function theory. RSC Adv. 12, 28178–28184 (2022).
Blomquist, J., Walle, L. E., Uvdal, P., Borg, A. & Sandell, A. Water dissociation on single crystalline anatase TiO2(001) studied by photoelectron spectroscopy. J. Phys. Chem. C 112, 16616–16621 (2008).
Ma, X. et al. Hydrogen-bond network promotes water splitting on the TiO2 surface. J. Am. Chem. Soc. 144, 13565–13573 (2022).
Seah, M. P. & Dench, W. Quantitative electron spectroscopy of surfaces: a standard data base for electron inelastic mean free paths in solids. Surf. Interface Anal. 1, 2–11 (1979).
Sayago, D. I., Serrano, P., Bhme, O., Goldoni, A. & Martinn-Gago, J. A. A photoemission study of the SO2 adsorption on TiO2(110) surfaces. Surf. Sci. 482–485, 9–14 (2001).
Nelin, C. J. et al. Surface core-level binding energy shifts for MgO(100). Phys. Chem. Chem. Phys. 16, 21953–21956 (2014).
Thomas, A. et al. Resonant photoemission of anatase TiO2 (101) and (001) single crystals. Phys. Rev. B 67, 035110 (2003).
Thomas, A. G. et al. Comparison of the electronic structure of anatase and rutile TiO2 single-crystal surfaces using resonant photoemission and x-ray absorption spectroscopy. Phys. Rev. B 75, 035105 (2007).
Yuan, W. et al. Real-time observation of reconstruction dynamics on TiO2(001) surface under oxygen via an environmental transmission electron microscope. Nano Lett. 16, 132–137 (2015).
Xu, M., Wang, S. & Wang, H. A reconstructed anatase (001)-1 × 4 surface and its reactivity. Phys. Chem. Chem. Phys. 19, 16615–16620 (2017).
Yim, C., Pang, C. & Thornton, G. Oxygen vacancy origin of the surface band-gap state of TiO2(110). Phys. Rev. Lett. 104, 036806 (2010).
Setvin, M. et al. Direct view at excess electrons in TiO2 rutile and anatase. Phys. Rev. Lett. 113, 086402 (2014).
Yang, K., Dai, Y., Huang, B. & Feng, Y. P. Density-functional characterization of antiferromagnetism in oxygen-deficient anatase and rutile TiO2. Phys. Rev. B 81, 033202 (2010).
Sun, H. et al. Comparing quasiparticle H2O level alignment on anatase and rutile TiO2. ACS Catal. 5, 4242–4254 (2015).
Caruso, F., Verdi, C., Poncé, S. & Giustino, F. Electron-plasmon and electron-phonon satellites in the angle-resolved photoelectron spectra of n-doped anatase TiO2. Phys. Rev. B 97, 165113 (2018).
Lian, M., Wang, Y.-C., Peng, S. & Zhao, Y. Photo-induced ultrafast electron dynamics in anatase and rutile TiO2: effects of electron-phonon interaction. Chin. J. Chem. Phys. 35, 270–280 (2022).
Park, S. H. et al. Direct and real-time observation of hole transport dynamics in anatase TiO2 using X-ray free-electron laser. Nat. Commun. 13, 2531 (2022).
Ding, H. et al. High-temperature superconductivity in single-unit-cell FeSe films on Anatase TiO2(001). Phys. Rev. Lett. 117, 067001 (2016).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953 (1994).
Perdew, J. P. et al. Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 46, 6671 (1992).
Grimme, S. Semiempirical GGA‐type density functional constructed with a long‐range dispersion correction. J. Comput. Chem. 27, 1787–1799 (2006).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188 (1976).
Marinopoulos, A. G. et al. Anisotropy and interplane interactions in the dielectric response of graphite. Phys. Rev. Lett. 89, 076402 (2002).
Mori-Sánchez, P., Cohen, A. J. & Yang, W. Localization and delocalization errors in density functional theory and implications for band-gap prediction. Phys. Rev. Lett. 100, 146401 (2008).
Acknowledgements
This work is supported by the Innovation Program for Quantum Science and Technology (2021ZD0303302), the CAS Project for Young Scientists in Basic Research (YSBR-054) and the CAS Strategic Priority Research Program (XDB36020200), National Natural Science Foundation of China (21972129, 11904349), Anhui Initiative in Quantum Information Technologies (AHY090300), and the New Cornerstone Science Foundation. X.M. acknowledges the support of the National Natural Science Foundation of China (22302188). X.S. acknowledges the support of the National Natural Science Foundation of China (12074361).
Author information
Authors and Affiliations
Contributions
B.W. and S.T. designed and supervised the research; J.Z. and X.S. supervised the calculations; X.M. contributed to the sample preparation. X.M., Z.C. and Z.G. performed the UPS, XPS, and ARPES experiments; Y.S. and X.L. performed the theoretical calculations; J.L. and X.C. performed the STM and AFM experiments; X.M., X.C., S.T. and B.W. contributed to the data analysis. S.T. and X.M. generated the figures and wrote the manuscript; All authors discussed the results and commented on the manuscript at all stages.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ma, X., Shi, Y., Cheng, Z. et al. Unveiling diverse coordination-defined electronic structures of reconstructed anatase TiO2(001)-(1 × 4) surface. Nat Commun 15, 2326 (2024). https://doi.org/10.1038/s41467-024-46570-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-46570-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.