Abstract
Clinical efficacy of intranasal administration of oxytocin is increasingly explored in autism spectrum disorder, but to date, the biological effects of chronic administration regimes on endogenous oxytocinergic function are largely unknown. Here exploratory biological assessments from a completed randomized, placebo-controlled trial showed that children with autism (n = 79, 16 females) receiving intranasal oxytocin for four weeks (12 IU, twice daily) displayed significantly higher salivary oxytocin levels 24 hours after the last oxytocin nasal spray administration, but no longer at a four-week follow up session. Regarding salivary oxytocin receptor gene (OXTR) epigenetics (DNA-methylation), oxytocin-induced reductions in OXTR DNA-methylation were observed, suggesting a facilitation of oxytocin receptor expression in the oxytocin compared to the placebo group. Notably, heightened oxytocin levels post-treatment were significantly associated with reduced OXTR DNA-methylation and improved feelings of secure attachment. These findings indicate that four weeks of chronic oxytocin administration stimulated the endogenous oxytocinergic system in children with autism.
Similar content being viewed by others
Introduction
Autism spectrum disorder is a neurodevelopmental condition, characterized by repetitive and restrictive behaviours and socio-communicative difficulties for which limited therapeutic options exist to date1. In the past decade, intranasal administration of oxytocin has been increasingly explored as a new approach to facilitate social development and reduce disability associated with autism2. To date, two main mechanistic frameworks on oxytocin’s role in regulating social behaviour have been proposed. First, the anxiolytic account suggests that oxytocin primarily regulates stress and social anxiety responses, thereby promoting social approach behaviour during interactions3,4,5,6. Secondly, the social salience hypothesis proposes that oxytocin enhances attention to and perception of social cues by prioritizing neural resources for processing these cues7. However, while initial single-dose administration studies yielded promising acute effects of oxytocin on pro-social behaviour2, subsequent multiple-dose, chronic administration studies (i.e. administrating the oxytocin nasal spray over a course of multiple weeks) have yielded a more mixed pattern of results, with some studies demonstrating beneficial outcomes, while others did not8,9,10,11,12 (see13 for a full review on chronic oxytocin administration trials).
In light of the growing number of studies demonstrating a limited bioavailability of naturally occurring oxytocin in autistic children14,15,16, it is crucial to gain deeper insights into how exogenous administration can impact the functioning of the endogenous oxytocin system. This is important, considering that biological changes likely underlie oxytocin-induced behavioural-clinical outcomes17 and therefore allow delineating possible biological mechanisms of inter-individual variation in clinical treatment responses.
To date, insights into how exogenous administration impacts endogenous oxytocinergic signalling have predominantly emerged from single-dose administration studies, showing elevated salivary oxytocin levels up to 2 and even 7 hours after administration in autistic and non-autistic populations18,19,20,21,22,23,24. Considering that the half-life of oxytocin is only a few minutes in blood plasma and 20 minutes in cerebrospinal fluid25, sustained high levels of salivary oxytocin likely reflect an upregulation of endogenous oxytocin release induced by its acute exogenous administration. This notion is supported by a recent chronic administration study, examining the effect of a four-week course of daily oxytocin administrations on salivary oxytocin levels in autistic adult men26. Here, elevated salivary oxytocin levels were shown up to four weeks after cessation of the nasal spray administration period, indicating a self-perpetuating elevation of oxytocin levels through a feed-forward triggering of its own release, in line with the notion of a ‘positive spiral of oxytocin release’ as suggested before by De Dreu (2012)27. More research is needed, however, to understand the impact of chronic oxytocin administration on its endogenous production, especially in autistic children, considering that oxytocin can preferably exert its therapeutic potential within early life developmental windows.
Aside assessments of circulating oxytocin, also variations in (epi)genetic modifications of the oxytocin receptor gene (OXTR) are considered important markers of endogenous oxytocinergic function. DNA methylation (DNAm) is one of the most extensively studied epigenetic mechanisms involved in the regulation of gene transcription, with increased DNAm frequency found to be associated to decreased gene transcription, and therefore less receptor expression and availability28. While initial studies have linked increased OXTR DNAm to social difficulties and traits associated with autism29, insight in whether (chronic) oxytocin administration impacts OXTR DNAm and whether these changes relate to altered oxytocin levels remains currently unexplored.
Here exploratory biological assessments from a completed double-blind, randomized, placebo-controlled study were performed to examine the effects of a four-week course of chronic oxytocin administration (12 IU, twice daily), or placebo, on oxytocinergic function as assessed using salivary oxytocin levels and OXTR DNAm in school-aged children with autism. To do so, salivary oxytocin and DNA samples were collected at baseline, prior to nasal spray administration (T0); immediately after the four-week administration period, at least 24 hours after the last nasal spray administration (T1); and at a follow-up session, four weeks after cessation of the daily administrations (T2).
Importantly, the exploratory biological salivary collections were part of a larger protocol, additionally including the assessment of oxytocin-induced changes on clinical-behavioural scales, as outlined in more detail in Daniels et al. (2023)12. Overall, Daniels et al. 12 showed that - compared to children receiving placebo - the group of children receiving oxytocin did not display stronger improvements in parent-reported social functioning (assessed as primary behavioural outcome using the Social Responsiveness Scale) or repetitive behaviours, attachment or anxiety, although the combination of oxytocin administration with psychosocial treatment might suggest a synergetic effect12. To understand possible inter-individual variations in clinical treatment outcomes, we here explored whether individual variation in oxytocin-induced effects on endogenous oxytocinergic function may relate to variation in clinical effects in terms of social function, repetitive behaviours, attachment and anxiety.
Results
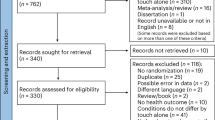
Children with autism participating in this double-blind, randomized, placebo-controlled study, were randomly allocated to receive four weeks of intranasal oxytocin administration (12IU twice daily) or placebo (see Fig. 1 for the CONSORT flow diagram visualizing the number of participants randomized and Table 1 for the number of participants analyzed).
Participants were assessed for eligibility prior to the baseline session (T0) and after the session allocated to receive either oxytocin or placebo nasal sprays for a four week period of twice daily nasal spray administration. Saliva oxytocin (morning and afternoon) and DNA samples were collected at baseline (T0), immediately after the four-week administration period (T1; at least 24 hours after the last nasal spray administration), and at a follow-up session, four weeks after cessation of the daily administrations (T2). As outlined, for some participants, saliva samples were missing at one or more assessment sessions due to participant being unable or forgetful to bring the sample or due to discontinuation of the study by the participant.
Effect of chronic oxytocin administration on salivary oxytocin levels
Exploratory assessments of salivary oxytocin levels were acquired before (T0) and after the four-week nasal spray administration period (post: T1, follow-up: T2). At each assessment, both a morning and an afternoon sample were acquired.
Morning oxytocin levels
Mixed-effect analyses with the fixed factors ‘nasal spray’ (oxytocin, placebo) and ‘assessment session’ (T1, T2) (and the inclusion of baseline T0 scores as covariate) revealed a significant main effect of ‘nasal spray’ (F(1,74) = 15.50; p < 0.001; ηp2 = 0.18) and ‘assessment session’ (F(1,74) = 10.15; p = .002; ηp2 = .13). Yet, these main effects need to be interpreted in the context of the significant ‘nasal spray x assessment session’ interaction effect, indicating that morning oxytocin levels were significantly augmented in the oxytocin group, compared to the placebo group (F(1,74) = 12.19; p < 0.001; ηp2 = 0.15), but only at the T1 postassessment (pBonferroni < 0.001), not at the T2 four-week follow-up (pBonferroni > 0.05), see Fig. 2A.
Salivary oxytocin levels are visualised for each nasal spray group (oxytocin and placebo) at each assessment session: baseline (T0), ≥24 hours post-nasal spray (T1) and four weeks post-nasal spray (T2). Panel A shows the morning oxytocin levels (AM sample; T0: noxytocin = 39, nplacebo = 39; T1: noxytocin = 39, nplacebo = 36; T2: noxytocin = 38, nplacebo = 34) and panel B the afternoon oxytocin levels (PM sample; T0: noxytocin = 38, nplacebo = 39; T1: noxytocin = 39, nplacebo = 39; T2: noxytocin = 38, nplacebo = 37). Panel C visualises the salivary OXTR DNAm levels at CpG −924 for each nasal spray group (oxytocin and placebo) at each assessment session (T0: noxytocin = 38, nplacebo = 39; T1: noxytocin = 37, nplacebo = 38; T2: noxytocin = 38, nplacebo = 37). Data are presented as mean values, vertical bars denote standard errors. Panel D visualises the association of salivary oxytocin levels (averaged over the morning and afternoon samples) with the salivary OXTR DNAm levels in CpG −924 in the oxytocin group at T1. Source data are provided as a Supplementary Data Source file.
Afternoon oxytocin levels
An overall similar pattern of results was identified for afternoon oxytocin levels, indicating a significant augmentation in the oxytocin group compared to the placebo group (F(1,71) = 7.06; p = 0.010; ηp2 = 0.09), at the T1 post assessment (pBonferroni = 0.001), not at the T2 follow-up (p > 0.05), see Fig. 2B.
Effect of chronic oxytocin administration on salivary OXTR DNAm
Epigenetic salivary samplings were performed at each assessment session (T0, T1, T2) and variations in OXTR DNAm were assessed at three CpG sites of the OXTR gene, previously shown to be impacted in autism (i.e., -934, −924 and -914, see29).
CpG site −924
A significant main effect of nasal spray was identified (F(1,70) = 7.76; p = 0.007; ηp2 = 0.10), indicating that DNAm of CpG site −924 was significantly reduced in the oxytocin group, compared to the placebo group, see Fig. 2C. The absence of a spray x assessment session interaction (F(1,70) = 0.90; p = 0.346; ηp2 = 0.01) indicates that the main effect of nasal spray was evident across both the T1 post and T2 follow-up assessment. The significant main effect of assessment session indicates overall higher DNAm at the T2 assessment compared to the T1 assessment (F(1,70) = 6.76; p = 0.011; ηp2 = 0.085).
CpG site -914 and -934
No significant main nor interaction effects were identified for CpG site -914 and -934 (all p > 0.05), indicating no differential modulation of DNAm at these sites after oxytocin or placebo nasal spray administration, see Fig. S1.
Associations of oxytocin levels with OXTR DNAm
Notably, in the oxytocin group, a significant association was evident between salivary oxytocin levels post-nasal spray, at T1, and DNAm of CpG site −924 (ρ = -0.39; p = 0.018), indicating that children of the oxytocin group displaying higher oxytocin levels at T1 also showed a stronger reduction in DNAm of CpG site -924 (reflecting higher oxytocin-receptor expression), see Fig. 2D. Note that the relationship was only evident at the T1 assessment session - at which treatment-induced elevations in salivary oxytocin levels were observed – but no longer at the T2 follow-up session (ρ = -0.21; p = 0.214). Also no significant association was evident at the T0 session (ρ = 0.15; p = 0.371), indicating no significant relationship between baseline oxytocin levels and baseline DNAm of CpG site -924.
Associations with behavioural-clinical reports
A significant relationship was identified between salivary oxytocin levels assessed from the oxytocin group at T1 (at which treatment-induced elevations were evident) and self-reports of secure attachment towards peers (ASCQ), indicating that children of the oxytocin group displaying higher oxytocin levels at T1 also showed higher reports of secure attachment at T1 (ρ = 0.35; p = 0.030), see Fig. 3A. Moderate (but nonsignificant) opposite relationships were evident for self-reported attachment avoidance (ρ = -0.30; p = 0.070, Fig. 3B),parent-reports of social difficulties (SRS-2; ρ = -0.31; p = 0.062, Fig. 3C) and anxiety (SCARED-NL; ρ = -0.32; p = 0.053, Fig. 3D) all indicating that improved clinical presentations at T1 were associated with higher T1 oxytocin levels. No significant associations were evident between oxytocin levels and clinical-behavioural scales at the follow-up session T2 (all p > 0.208), or at the baseline T0 assessment (all p > 0.170).
The association of salivary oxytocin levels with questionnaire scores at the postoxytocin nasal sprays session (T1) are visualised. Panel A shows the association with secure-attachment (Attachment Style Classification Questionnaire, ASCQ). Panel B shows the association with avoidant-attachment (ASCQ). Panel C shows the association with social responsiveness (Social Responsiveness Scale, SRS). Panel D shows the association with anxiety scores (Screen for Child Anxiety Related Emotional Disorders, SCARED). Source data are provided as a Supplementary Data Source file.
Also no significant associations were identified between levels of DNAm of CpG site -924 and the abovementioned clinical scales, either at T1, T2 or T0 (all p > 0.108).
Discussion
This study examined the impact of a four-week chronic oxytocin administration regime on endogenous oxytocinergic function in autistic children. Salivary oxytocin levels were reliably increased 24 hours after the last oxytocin nasal spray, but no longer at the follow-up session, four weeks after cessation of the oxytocin administrations. Reduced OXTR DNAm levels were also observed, suggesting a facilitation in oxytocin-receptor expression, as previously evidenced28,30, in the oxytocin, compared to the placebo group, up to four weeks after the last nasal spray administration. Increased oxytocin levels were significantly associated with reduced OXTR DNAm levels, as well as with improved clinical presentations on self-reported feelings of secure peer attachment. These findings provide evidence that the chronic exogenous administration induced stimulation of endogenous oxytocinergic system in children with autism.
Although the exact physiological mechanisms remain unclear, prior research showed that oxytocin-receptor binding promotes secretion of additional oxytocin and affects DNA transcriptional activation/repression25,31,32. Particularly, an auto-excitatory mechanism of somato-dendritic release of oxytocin from hypothalamic cells has been described, indicating that auto-binding of oxytocin to its G protein-coupled receptors is sufficient to elevate intracellular Ca2+ levels from internal storage, in turn triggering a feed-forward of dendritic oxytocin exocytosis (i.e. release)25,33.
With regard to the regulation of OXTR expression and receptor availability, prior rodent research showed mixed results, with some studies showing a receptor downregulation upon chronic application of oxytocin in mice (10 ng/h, over a period of 14 days)34,35, while others showed opposite effects36. For example, in oxytocin knockout mice, the expression of the OXTR gene was shown to be reduced in hippocampal tissue, due to unavailability of the ligand36. In the current human sample, the chronic oxytocin administration induced a predominant decrease in OXTR methylation, previously shown to be reflective of increased receptor expression28,30. It is likely, however, that the effects on gene regulation of (chronic) oxytocin administration are dependent on the administered dose and frequency, with some dosing schemes inducing facilitation, while others might induce downregulation of receptor availability. In a recent study by Le et al. (2022), the possibility of an intermittent dosing scheme - administering oxytocin intranasally every other day for a period of 6 weeks to avoid pharmacological overstimulation - was explored in a paediatric sample of young autistic children, and although no direct assessments of epigenetic changes were reported, the administration regime yielded strong beneficial effects on various clinical-behavioural outcomes assessing social function10. It should be interesting for future studies to further explore optimal dosing schema’s for yielding maximal endogenous stimulation of the oxytocinergic system. Since epigenetic mechanisms constitute a direct pathway by which environmental changes (e.g. increased oxytocin availability) may impact DNA transcriptional activation/repression, the observed changes in DNAm of OXTR likely reflect an important biological pathway through which chronic oxytocin administration regimes may induce long-lasting neurobiological and possibly associated clinical-behavioural effects. In short, the elevated oxytocin availability (through its exogenous administration) may have induced a feed-forward mechanism of the endogenous oxytocinergic function by increasing its own release, as well as prompting more sustained epigenetic OXTR modifications. The stimulating effect of chronic oxytocin administration on peripheral oxytocin levels in autistic children, observed at T1 but not at T2, is partly in line with prior observations in adults with autism26. In the latter study, increases in endogenous oxytocin levels persisted up to four weeks after cessation of the chronic nasal spray administrations. Previously, developmental differences of the oxytocinergic system have been evidenced, indicating lower endogenous oxytocin levels in autistic children, but not adults14. Perhaps in autistic children, more so than in adults, homeostatic responses upon cessation of the chronic oxytocin administration occur faster, thereby reducing heightened levels of circulating oxytocin more rapidly to initial baseline levels. Alternatively, adults (as compared to children) might adapt their social interactive behaviour more readily upon receiving oxytocin administrations, i.e., through increasingly engaging in and positively experiencing of socially stimulating contexts. According to the positive spiral of oxytocin release, this increase in (positive) social experiences may be pivotal for further facilitating oxytocin’s endogenous release, also in the long-term, as observed up to four-weeks after cessation of the nasal spray administration regime in adults26, but not children in the current cohort. In light of potential treatment applications, these observations highlight the importance of combining oxytocin administration with social stimulation (e.g. psychosocial therapy) to perpetuate the endogenous oxytocin feedforward-loop27. This aligns with emerging evidence indicating that oxytocin administration as stand-alone treatment may be suboptimal37,38, and that combinatory approaches, combining the chronic oxytocin administrations to concomitant psychosocial training interventions will be pivotal for maximizing its full therapeutic potential and clinical efficacy10,12.
It is noteworthy that higher levels of endogenous oxytocin observed post-treatment were linked to better self-reported feelings of secure attachment. Additionally, there was a trend towards a reduced self-reported attachment avoidance, as well as parent-reported social difficulties and anxiety. A large body of literature links variations in circulating oxytocin to variations in behavioural manifestations, including prosociality and attachment-related constructs39,40,41. Over the past decades, the role of the oxytocinergic system in inter-personal bonding and formation and maintenance of secure attachment bonds was highlighted. Our study provides evidence of the link between elevated oxytocin levels and a better clinical presentation of secure attachment in autistic children, supporting the notion that also in autistic children heightened oxytocin may facilitate a sense of (social) security and safety42,43.
In the current study, salivary sampling was adopted for both oxytocin level and OXTR DNAm characterizations, as these assessments were most feasible in a paediatric population. While correlations have been demonstrated between salivary and central (cerebrospinal fluid) oxytocin levels44, salivary oxytocin may constitute only a proxy of central oxytocinergic function. Future studies are therefore warranted to examine whether the currently reported effects - both in terms of salivary oxytocin and epigenetic modification – would hold also for other biological material, including blood and cerebrospinal fluid. Moreover, the OXTR DNAm results were only found at CpG site -924, even though all three investigated sites fall within the same region in the CpG island of the OXTR, shown to regulate the expression of the oxytocin receptor28. Future studies should aim to clarify this differential effect, and specifically, how altered methylation at CpG site -924 might have impacted oxytocin receptor gene expression. Further, considering the tight age range of our school-aged children with autism, and a predominant sampling from autistic boys, it should be explored whether the observed effects will generalize across different age ranges and in samples with a larger representation of girls.
To conclude, four weeks of chronic oxytocin administration was shown to stimulate the endogenous oxytocinergic system in autistic children, as evidenced by increased salivary oxytocin levels and associated decreased OXTR DNAm levels (previously shown to reflect increased oxytocin-receptor expression28,30). Furthermore, elevated oxytocin levels post-treatment were associated with a better clinical presentation, indicating an important mechanistic link between oxytocin’s chronic biological and clinical-behavioural effects.
Methods
All study procedures and consent forms were approved by the local Ethics Committee at the KU Leuven (S61358) in accordance with Declaration of Helsinki. Parents/legal guardians of the participants provided informed consents and participants provided informed assents, prior to the start of the study. The trial was registered at the European Clinical Trial Registry (EudraCT 2018-000769-35) and the Belgian Federal Agency for Medicines and Health products (see Supplementary methods).
Study design
A double-blind, randomized, placebo-controlled study with parallel design was conducted at the Leuven University Hospital (Belgium) to assess the effects of four weeks of intranasal oxytocin administration (12IU twice daily) on endogenous levels of salivary oxytocin and OXTR DNAm in school-aged children with autism (see Fig. 1 for the CONSORT flow diagram visualizing the number of biological samples collected and analysed for each assessment session). Saliva oxytocin and DNA samples were collected at baseline, prior to nasal spray administration (T0); immediately after the four-week administration period, at least 24 hours after the last nasal spray administration (T1); and at a follow-up session, four weeks after cessation of the daily administrations (T2). This follow-up timing was similar to a prior chronic oxytocin administration study showing elevated oxytocin levels after a 4-week follow-up in autistic adults26.
Saliva samples were collected as exploratory, secondary endpoints in the context of a larger protocol, including clinical-behavioural12 and neural45,46 assessments as primary outcomes, as registered at EudraCT 2018-000769-35.
Participants
Children with a formal diagnosis of autism were recruited through the Leuven Autism Expertise Centre of the between July 2019 and January 2021 (see Fig. 1 for the CONSORT flow diagram visualizing the number of included participants). All (travel) costs associated with the experimental procedures were reimbursed and children were given a toy of their choosing at the end of each assessment session. Participants randomized to receive oxytocin or placebo nasal sprays did not differ in terms of baseline symptom characteristics (Autism Diagnostic Observation Schedule, ADOS-247; Social Responsiveness Scale-Children, SRS-248), intelligence quotients (IQ; WISC-V-NL49), biological sex or age, see Table 1.
A total of 80 participants (40 in each treatment arm) participated in the trial, allowing to detect a medium effect size (d = 0.60) with α = 0.05 and 80% power, corresponding to effect sizes previously reported in a four-week oxytocin trial with school-aged children. Main inclusion criteria comprised a clinical diagnosis of ASD, age (8-12 years old), IQ above 70, no prior oxytocin administrations and native Dutch speaker. The ASD diagnosis was established by a multidisciplinary team (child psychiatrist, psychologist, speech/language pathologist, physiotherapist) based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 5th edition1.
Main exclusion criteria comprised a history of any neurological disorder (stroke, concussion, epilepsy etc.), any physical disorder (liver, renal, cardiac pathology) or significant hearing or vision impairments. Only premenarchal girls were included. Seventeen participants had a co-occurring diagnosis of attention-deficit/hyperactivity disorder and one of obsessive-compulsive disorder. Twenty participants used stimulant medication (e.g., methylphenidate), 13 anti-psychotics (e.g. risperidone), 4 anti-depressants (e.g., sertraline) and 35 used other medication (e.g., sleeping aids, gastro-intestinal medication or nutritional supplements).
Nasal spray administration
Participants received oxytocin (Syntocinon®, Sigma-tau) or placebo nasal sprays for four weeks (28 days), administered twice daily with 12 IU (3 puffs of 2 IU in each nostril) in the morning and 12 IU in the afternoon (after school), resulting in a daily dose of 24 IU, in accordance with prior studies assessing chronic oxytocin administration effects in children50,51 and adults with autism9.
Using permuted-block randomization, participants were randomly assigned to receive oxytocin or placebo nasal sprays, administered in identical blinded amber 10 ml glass bottles with a white nasal pump (0.05 ml or 2 IU /puff). Preparation, packaging and blinding of the nasal sprays was performed by Heidelberg University Hospital, Germany. Placebo sprays contained all the ingredients used in the active solution except the oxytocin compound. Participants were randomly assigned in a 1:1 ratio, with age, IQ and biological sex balanced across treatment arms (see Table 1). Children and their parents received clear instructions on how to administer the nasal spray52 through a demonstration together with the experimenter. Two daily doses of 12 IU oxytocin nasal spray or placebo equivalent (3 puffs of 2 IU in each nostril) were administered: 12 IU in the morning and 12 IU in the afternoon, during 28 consecutive days. The last day before the post-nasal spray administration assessment session (T1), participants withheld the afternoon spray, to allow a window of 24 hours between the last nasal spray and the salivary sampling, thereby mitigating the potential for acute (single-dose) oxytocin effects. All research staff conducting the trial, participants and their parents were blinded to nasal spray allocation.
Compliance was monitored by weighing the disposed and returned nasal sprays, no differences were reported in the amount of administered fluid between the oxytocin and placebo group12. Potential adverse events were tracked using weekly parent reports and daily parent and child diaries. These revealed no evidence of nasal spray-specific adverse events (for a full report on compliance and adverse events see Daniels et al., (2023)12).
Assessment of salivary oxytocin levels
Oxytocin levels were assessed via saliva samples acquired at each assessment session (T0, T1, T2), at two-time points: (i) a morning sample, acquired at home, within 30 min after awakening and before breakfast; and (ii) an afternoon sample, acquired at the Leuven University hospital. Salivary samples were collected using Salivette cotton swabs (Sarstedt AG & Co., Germany) and analysed using a commercial enzyme immunoassay oxytocin ELISA kit (Enzo Life Sciences, Inc., USA) in accordance with the manufacturer’s instructions. Sample concentrations (100 µl/well) were calculated conform plate-specific standard curves.
Assessment of OXTR DNA methylation levels
To assess variations in DNAm of OXTR (hg19, chr3:8,810,729-8,810,845), salivary samples were obtained via the Oragene DNA sample collection kit (DNA Genotek Inc., Canada) at the Leuven University hospital at T0, T1, and T2. Next, extracted DNA (200 ng) was bisulfite-converted following the manufacturer’s protocol (EZ-96 DNAm Kit, Zymo Research, Irvine, USA) and stored at −80 °C. DNAm levels were determined at three CpG sites (−934, −924 and −914, relative to the translation start site) using Pyrosequencing (Qiagen, Germany) and analysed using Pyromark Q96 software, in accordance with manufacturer’s protocols53. Protocols for the PCR amplification and Pyrosequencing analysis were adopted from54. To amplify the DNA fragment of interest, within the OXTR, including the CpG sites -934, −924 and -914, the following PCR primers were used: [OXTR Forward: TTG AGT TTT GGA TTT AGA TAA TTA AGG ATT; OXTR Reverse: /5Biosg/AC TTA ACA TCA CAT TAA ATA CAA CC]. The following PCR amplification steps were used: Step 1: (95 °C/15 min)/1 cycle, Step 2: (94 °C/30 s, 58 °C/30 s, 72 °C/30 s)/50 cycles, Step 3: (72 °C/10 min)/1 cycle, Step 4: 4 °C hold]. The following sequencing primer was used to read the DNA sequence (OXTR Sequencing: AGA AGT TAT TTT ATA ATT TT).
Data handling and statistical procedures
Salivary oxytocin samples
From oxytocin saliva samples containing less than 100 µl of material, 50 µl was obtained and diluted to 100 µl, sample concentrations were then multiplied by 2 (T0: 4 AM samples, 1 PM sample; T1: 3 AM samples; T2: 3 AM samples). Further, for participants displaying concentrations below the detection limit, concentrations were set to the lowest detected value across samples (T0: 14 AM samples, 12 PM samples; T1: 14 AM samples, 9 PM samples; T2: 18 AM samples, 9 PM samples). Finally, extreme outliers in oxytocin level data were identified (five inter-quartile ranges (Q3-Q1) below or above the first (Q1), respectively third (Q3) quartile) and recoded to the sample mean (across groups) (T0: 1 AM sample, 1 PM sample; T1: 3 AM samples; T2: 1 AM sample, 1 PM sample). Note that the pattern of results remained qualitatively similar also when removing these outliers.
To assess oxytocin-induced effects, salivary oxytocin levels were subjected to mixed-effects analyses of variances and Bonferroni-corrected post-hoc tests, with the factors ‘nasal spray’ (oxytocin, placebo) and ‘assessment session’ (T1, T2) as fixed effects, and the factor ‘subject’ as random effect. To correct for variance in the individuals’ baseline T0 scores, baseline values prior to nasal administration were included as a covariate in the model. Separate models were constructed for the morning and afternoon samples.
Salivary DNAm samples
Samples where OXTR DNAm could not be determined due to insufficient sample quality, were removed from analysis (T0: 2 samples, T1: 2 samples). For the DNAm level data, only one extreme outlier was identified and recoded to the sample mean (across groups), namely for CpG site -934, at T0.
Oxytocin-induced effects in OXTR DNAm were analysed using similar mixed models, i.e., with the fixed factors ‘nasal spray’ (oxytocin, placebo) and ‘assessment session’ (T1, T2), the random factor ‘subject’, and baseline (T0) scores modelled as covariate. Separate models were constructed for each CpG site (-934, −924 and -914).
Next, Spearman correlation analyses were performed to examine whether variations in treatment-induced increases in salivary oxytocin levels (averaged across morning and afternoon), were associated to variations in treatment-induced changes in OXTR DNAm, as assessed in the oxytocin group. Considering that treatment-related effects on salivary oxytocin levels were only present at assessment session T1 (not at T2, see results), association analyses were primarily focused on this assessment session. For completeness, associations are also reported for follow-up session T2, as well as for T0, allowing to examine whether baseline variations in oxytocin levels possibly relate to baseline variations in OXTR DNAm.
Further, since prior work has evidenced a link between variations in oxytocin levels and behaviour14,55, associations between salivary oxytocin levels and clinical-behavioural assessments were also performed. Specifically, exploratory examinations were performed to investigate whether individual variation in oxytocin-induced effects in endogenous oxytocinergic function relate to clinical-behavioural variations on social functioning (SRS-248), repetitive behaviours (Repetitive Behavior Scale-Revised, RBS-R56), anxiety scores (Screen for Child Anxiety Related Emotional Disorders, SCARED-NL57), and self-reported attachment (Attachment Style Classification Questionnaire, ASCQ58) (see Table S1 for a detailed outlining of the obtained questionnaires). All statistical analyses were executed with Statistica version 14 (Tibco Software Inc.).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data supporting the findings of the current report are available in Supplementary Data Source files. Source data are provided with this paper.
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed). Washington, DC (2013).
Ooi, Y. P., Weng, S. J., Kossowsky, J., Gerger, H. & Sung, M. Oxytocin and Autism Spectrum Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmacopsychiatry 50, 5–13 (2017).
Bethlehem, R. A. I., Baron-Cohen, S., van Honk, J., Auyeung, B. & Bos, P. A. The oxytocin paradox. Front Behav. Neurosci. 8, 5 (2014).
Ma, Y., Shamay-Tsoory, S., Han, S. & Zink, C. F. Oxytocin and Social Adaptation: Insights from Neuroimaging Studies of Healthy and Clinical Populations. Trends Cogn. Sci. 20, 133–145 (2016).
Quintana, D. S. et al. Low-dose oxytocin delivered intranasally with Breath Powered device affects social-cognitive behavior: a randomized four-way crossover trial with nasal cavity dimension assessment. Transl. Psychiatry 5, e602–e602 (2015).
Stoop, R. Neuromodulation by oxytocin and vasopressin in the central nervous system as a basis for their rapid behavioral effects. Curr. Opin. Neurobiol. 29, 187–193 (2014).
Shamay-Tsoory, S. G. & Abu-Akel, A. The Social Salience Hypothesis of Oxytocin. Biol. Psychiatry 79, 194–202 (2016).
Anagnostou, E. et al. Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: A randomized controlled trial. Mol. Autism 3, 1–9 (2012).
Bernaerts, S., Boets, B., Bosmans, G., Steyaert, J. & Alaerts, K. Behavioral effects of multiple-dose oxytocin treatment in autism: A randomized, placebo-controlled trial with long-term follow-up. Mol. Autism 11, 1–14 (2020).
Le, J. et al. Infrequent Intranasal Oxytocin Followed by Positive Social Interaction Improves Symptoms in Autistic Children: A Pilot Randomized Clinical Trial. Psychother Psychosom 1–13 (2022).
Sikich, L. et al. Intranasal Oxytocin in Children and Adolescents with Autism Spectrum Disorder. N. Engl. J. Med. 385, 1462–1473 (2021).
Daniels, N. et al. Effects of multiple-dose intranasal oxytocin administration on social responsiveness in children with autism: a randomized, placebo-controlled trial. Mol. Autism 14, 16 (2023).
Horta, M., Kaylor, K., Feifel, D. & Ebner, N. C. Chronic oxytocin administration as a tool for investigation and treatment: A cross-disciplinary systematic review. Neurosci. Biobehav. Rev. 108, 1–23 (2020).
Moerkerke, M. et al. Endogenous Oxytocin Levels in Autism—A Meta-Analysis. Brain Sci. 2021 11, 1545 (2021).
Evenepoel, M. et al. Endogenous oxytocin levels in children with autism: Associations with cortisol levels and oxytocin receptor gene methylation. Transl. Psychiatry 13, 1–9 (2023). 2023 13:1.
John, S. & Jaeggi, A. V. Oxytocin levels tend to be lower in autistic children: A meta-analysis of 31 studies. Autism 25, 2152–2161 (2021).
Parker, K. J. et al. Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc. Natl. Acad. Sci. USA 114, 8119–8124 (2017).
Daughters, K. et al. Salivary Oxytocin Concentrations in Males following Intranasal Administration of Oxytocin: A Double-Blind, Cross-Over Study. (2015).
Huffmeijer, R. et al. Salivary levels of oxytocin remain elevated for more than two hours after intranasal oxytocin administration. Neuroendocrinol. Lett. 33, 22467107–330112 (2012).
Quintana, D. S. et al. Saliva oxytocin measures do not reflect peripheral plasma concentrations after intranasal oxytocin administration in men. Horm. Behav. 102, 85–92 (2018).
Riem, M. M. E., van IJzendoorn, M. H. & Bakermans-Kranenburg, M. J. Hippocampal volume modulates salivary oxytocin level increases after intranasal oxytocin administration. Psychoneuroendocrinology 101, 182–185 (2019).
Weisman, O., Zagoory-Sharon, O. & Feldman, R. Intranasal oxytocin administration is reflected in human saliva. Psychoneuroendocrinology 37, 1582–1586 (2012).
Procyshyn, T. L. et al. Effects of oxytocin administration on salivary sex hormone levels in autistic and neurotypical women. Mol. Autism 11, 1–11 (2020).
van IJzendoorn, M. H., Bhandari, R., van der Veen, R., Grewen, K. M. & Bakermans-Kranenburg, M. J. Elevated Salivary Levels of Oxytocin Persist More than 7 h after Intranasal Administration. Front Neurosci. 6, 174 (2012).
Jurek, B. & Neumann, I. D. The oxytocin receptor: From intracellular signaling to behavior. Physiol. Rev. 98, 1805–1908 (2018).
Alaerts, K., Steyaert, J., Vanaudenaerde, B., Wenderoth, N. & Bernaerts, S. Changes in endogenous oxytocin levels after intranasal oxytocin treatment in adult men with autism: An exploratory study with long-term follow-up. Eur. Neuropsychopharmacol. 43, 147–152 (2021).
De Dreu, C. K. W. Oxytocin modulates cooperation within and competition between groups: An integrative review and research agenda. Horm. Behav. 61, 419–428 (2012).
Kusui, C. et al. DNA methylation of the human oxytocin receptor gene promoter regulates tissue-specific gene suppression. Biochem Biophys. Res Commun. 289, 681–686 (2001).
Moerkerke, M. et al. Oxytocin receptor gene (OXTR) DNA methylation is associated with autism and related social traits – A systematic review. Res Autism Spectr. Disord. 85, 101785 (2021).
Gregory, S. G. et al. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med 7, 1–13 (2009).
Gulliver, D. et al. Targeting the Oxytocin System: New Pharmacotherapeutic Approaches. Trends Pharm. Sci. 40, 22–37 (2019).
Neumann, I., Douglas, A. J., Pittman, Q. J., Russell, J. A. & Landgraf, R. Oxytocin Released within the Supraoptic Nucleus of the Rat Brain by Positive Feedback Action is Involved in Parturition-Related Events. J. Neuroendocrinol. 8, 227–233 (1996).
Ludwig, M. et al. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature 418, 85–89 (2002).
Huang, H. et al. Chronic and Acute Intranasal Oxytocin Produce Divergent Social Effects in Mice. Neuropsychopharmacology 39, 1102–1114 (2013).
Peters, S., Slattery, D. A., Uschold-Schmidt, N., Reber, S. O. & Neumann, I. D. Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology 42, 225–236 (2014).
Zimmermann-Peruzatto, J. M. et al. The Impact of Oxytocin Gene Knockout on Sexual Behavior and Gene Expression Related to Neuroendocrine Systems in the Brain of Female Mice. Cell Mol. Neurobiol. 37, 803–815 (2017).
Ford, C. L. & Young, L. J. Refining oxytocin therapy for autism: context is key. Nat. Rev. Neurol. 18, 67–68 (2021). 2021 18:2.
Itskovich, E., Bowling, D. L., Garner, J. P. & Parker, K. J. Oxytocin and the social facilitation of placebo effects. Molecular Psychiatry 2022 1–10 (2022).
Valstad, M. et al. The correlation between central and peripheral oxytocin concentrations: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 78, 117–124 (2017).
Marazziti, D. et al. A relationship between oxytocin and anxiety of romantic attachment. Clin. Pract. Epidemiol. Ment. Health 2, 1–6 (2006).
Ferreira, A. C., Osório, F. & de, L. Peripheral oxytocin concentrations in psychiatric disorders – A systematic review and methanalysis: Further evidence. Prog. Neuropsychopharmacol. Biol. Psychiatry 117, 110561 (2022).
Carter, C. S. Oxytocin pathways and the evolution of human behavior. Annu Rev. Psychol. 65, 17–39 (2014).
Porges, S. W. The Polyvagal Theory: Neurophysiological Foundations of Emotions, Attachment, Communication, Self-Regulation (Norton Series on Interpersonal Neurobiology). (WW Norton & Company., 2011).
Martin, J. et al. Oxytocin levels in saliva correlate better than plasma levels with concentrations in the cerebrospinal fluid of patients in neurocritical care. J. Neuroendocrinol. 30, e12596 (2018).
Moerkerke, M. et al. Can repeated intranasal oxytocin administration affect reduced neural sensitivity towards expressive faces in autism? A randomized controlled trial. Journal of Child Psychology and Psychiatry (2023).
Alaerts, K. et al. At the Head and Heart of Oxytocin’s Stress-Regulatory Neural and Cardiac Effects: A Chronic Administration RCT in Children with Autism. Psychother Psychosom 1–14 (2023).
Lord, C. et al. ADOS-Autisme diagnostisch observatieschema Handleiding. (2012).
Constantino, J. & Gruber, C. Social responsiveness scale 2nd. ed: SRS-2. Manual. Western Psychological Services. (2012).
Wechsler, D. WISC-V-NL. Wechsler Intelligence Scale for Children, Fifth Edition, Dutch version. Preprint at (2018).
Yatawara, C. J., Einfeld, S. L., Hickie, I. B., Davenport, T. A. & Guastella, A. J. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: A randomized clinical crossover trial. Mol. Psychiatry 21, 1225–1231 (2016).
Dadds, M. R. et al. Nasal oxytocin for social deficits in childhood autism: A randomized controlled trial. J. Autism Dev. Disord. 44, 521–531 (2014).
Guastella, A. J. et al. Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology 38, 612–625 (2013).
Luis Royo, J., Hidalgo, M. & Ruiz, A. Pyrosequencing protocol using a universal biotinylated primer for mutation detection and SNP genotyping. Nat. Protoc. 2, 1734–1739 (2007).
Krol, K. M., Puglia, M. H., Morris, J. P., Connelly, J. J. & Grossmann, T. Epigenetic modification of the oxytocin receptor gene is associated with emotion processing in the infant brain. Dev. Cogn. Neurosci. 37, 1–8 (2019).
Alaerts, K., Bernaerts, S., Vanaudenaerde, B., Daniels, N. & Wenderoth, N. Amygdala–Hippocampal Connectivity Is Associated With Endogenous Levels of Oxytocin and Can Be Altered by Exogenously Administered Oxytocin in Adults With Autism. Biol. Psychiatry Cogn. Neurosci. Neuroimag 4, 655–663 (2019).
Bodfish, J. W., Symons, F. J., Parker, D. E. & Lewis, M. H. Varieties of repetitive behaviour in autism: comparison to mental retardation. J. Autism Dev. Disord. 30, 237–243 (2000).
Muris, P., Bodden, D., Hale, W., Birmaher, B. & Mayer, B. SCARED-NL. Vragenlijst over angst en bang-zijn bij kinderen en adolescenten. Handleiding bij de gereviseerde Nederlandse versie van de Screen for Child Anxiety Related Emotional Disorders. Preprint at (2007).
Finzi, R., Cohen, O., Sapir, Y. & Weizman, A. Attachment Styles in Maltreated Children: A Comparative Study. Child Psychiatry Hum. Dev. 31, 113–128 (2000).
Acknowledgements
This work was supported by a KU Leuven grant (C14/17/102), a Doctor Gustave Delport fund of the King Baudouin Foundation (2019-J1811190-212989) and a TBM grant of the Flanders Fund for Scientific Research (FWO-TBM T001821N) granted to K.A. and B.B., as well as by the Branco Weiss fellowship of the Society in Science – ETH Zurich granted to K.A. and the Excellence of Science grant (EOS; G0E8718N; HUMVISCAT) and Flanders Fund for Scientific Research grant (FWO; G023923N) granted to B.B.. M.M. is supported by a KU Leuven Postdoctoral Mandate. J.P. is supported by the Marguerite-Marie Delacroix foundation and a postdoctoral fellowship of the Flanders Fund for Scientific Research (FWO; 1257621 N). T.T. is supported by the Fund Child Hospital UZ Leuven. S.VdD. is supported by a KU Leuven Postdoctoral Mandate and a postdoctoral fellowship of the Flanders Fund for Scientific Research (FWO; 12C9723N). M.E. is supported by an aspirant fellowship of the Flanders Fund for Scientific Research (FWO; 11N1222N).
Author information
Authors and Affiliations
Contributions
M.M. conceptualization, methodology, investigation, data curation, validation, writing—original draft, writing—review and editing, visualization, project administration. N.D. conceptualization, investigation, data curation, validation, writing—review and editing, project administration. L.T. methodology, investigation, data curation, validation, writing—review and editing. T.T. methodology, investigation, data curation, validation, writing—review and editing. M.E. methodology, writing—review and editing. S.VdD. data curation, validation, writing—review and editing. E.D.: data curation, validation, writing—review and editing. J.P. data curation, validation, writing—review and editing. V.C. methodology, writing—review and editing. S.C. methodology, writing—review and editing. B.V. methodology, writing—review and editing. L.W. methodology, writing—review and editing. J.S. supervision, validation, writing—review and editing, funding acquisition. B.B. supervision, conceptualization, validation, writing—review and editing, funding acquisition. K.A. supervision, conceptualization, methodology, validation, writing—original draft,writing—review and editing, visualization, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interest.
Peer review
Peer review information
Nature Communications thanks Hidenori Yamasue, Simone Shamay-Tsoory and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moerkerke, M., Daniels, N., Tibermont, L. et al. Chronic oxytocin administration stimulates the oxytocinergic system in children with autism. Nat Commun 15, 58 (2024). https://doi.org/10.1038/s41467-023-44334-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-44334-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.