Abstract
Developing chiral receptors with an endo-functionalized cavity for chiral recognition is of great significance in the field of molecular recognition. This study presents two pairs of chiral naphthotubes containing a bis-thiourea endo-functionalized cavity. Each chiral naphthotube has two homochiral centers which were fixed adjacent to the thiourea groups, causing the skeleton and thiourea groups to twist enantiomerically through chiral transfer. These chiral naphthotubes are highly effective at enantiomerically recognizing various neutral chiral molecules with an enantioselectivity up to 17.0. Furthermore, the mechanism of the chiral recognition has been revealed to be originated from differences in multiple non-covalent interactions. Various factors, such as the shape of cavities, substituents of guests, flexibility of host and binding modes are demonstrated to contribute to creating differences in the non-covalent interactions. Additionally, the driving force behind enantioselectivity is mainly attributed to enthalpic differences, and enthalpy -entropy compensation has also been observed to influence enantioselectivity.
Similar content being viewed by others
Introduction
Chiral recognition1,2,3 is important in both biological processes4,5,6 and organic synthesis7,8,9. A better comprehension of chiral recognition is essential to create more effective catalytic systems for asymmetric synthesis10,11,12,13,14, provide new materials with intriguing chiral properties15,16 and may contribute to a better understanding of the conservation of homochirality in biological molecules17,18,19,20,21, as well as guide designing of supramolecular chiral structures22,23,24,25. Over the past five decades, supramolecular chemists have endeavored to develop chiral hosts that can achieve enantioselective molecular recognition26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41. Various non-covalent interactions including hydrogen bonding, ionic, ion-dipole, dipole-dipole, van der Waals as well as π-π interaction play a critical role in differentiating chirality and achieving enantioselectivity (Se = KR(S)/KS(R), up to ~109)41. However, the chiral control of receptors and precise transmission of stereochemical information remains challenging, particularly in simplifying the process of enantioseparation42 and increasing chirality transfer efficiency43,44.
In recent years, based on the strategy of simultaneous construction we have reported a series of achiral hosts with an endo-functionalized cavity called naphthotubes45,46,47,48,49,50,51,52. These hosts share similar cavity features to bioreceptors and can selectively recognize polar groups and molecules. Therefore, we envisioned that chiral endo-functionalized naphthotubes might achieve chiral recognition for chiral guests. Indeed, chiral amide naphthotubes (Fig. 1a) can enantioselective recognize small organic molecules with decent enantioselectivities (up to 2.0)53. This is encouraging, but the enantioselectivity is far from satisfactory. The low enantioselectivity may be attributed to two aspects: (1) the chiral centers of the chiral amide naphthotubes are too distant from the hydrogen bonding sites resulting in a limited chirality transfer; (2) the smaller number of inward-directing hydrogen bonding sites cannot provide sufficient stereochemically dependent binding sites.
a Chemical structures of chiral amide naphthotubes R2,S2-1 and S2,R2-1 in previous work, the chiral centers are highlighted with cyan. b This work: biomimetic design of an endo-functionalized cavity with chiral centers located at the neighborhood of the inward-directing binding sites. The left figure is a cyclic dipeptide (CDP) cyclo-L-Arg-D-Pro complex with a cyclase (PDB: 5z53), the asterisks indicate the chiral centers.
In bioreceptors, multiple binding sites often come from sidechains or amino acid residues and are located near chiral centers54,55,56,57. For example, as shown in Fig. 1b, a chiral cyclic dipeptide cyclo-L-Arg-D-Pro can bind into the chiral cavity of a cyclase with matching binding sites58. The proximity of chiral centers and recognition sites increases the efficiency of chiral transfer between the chiral pocket and substrate, thereby contributing a high enantioselectivity in biosystem. Similarly, we wondered if migrating chiral centers closer to hydrogen bonding sites and increasing the number of binding sites could achieve better enantioselectivities. We speculate this strategy may even extend to the enantioselective recognition of bioactive chiral molecules, such as cyclic peptides with chiral centers, as depicted in Fig. 1b. For this purpose, we designed and synthesized two enantiopure bis-thiourea naphthotubes with a chiral center located near each thiourea group (CT1 and CT2, Fig. 2a, b). The chiral naphthotubes exhibit a high level of enantioselectivity (up to 17.0) in recognizing chiral cyclic dipeptides, which is better than the previous reports in terms of enantioselectivity (~9.0)59. Furthermore, the chiral naphthotubes can selectively recognize a diverse set of neutral molecules, including cyclic esters, quinuclidinol, oxazolidinones, morpholinone derivatives and drug molecules. Moreover, we have observed an interesting phenomenon in chiral recognition. In general, receptors with R (or S) homochiral centers often favor R (or S) guests, which is consistent with homochiral selection in nature60. However, this is not always the case here, we observed a few instances where R (or S) host selected an S (or R) guest for the chiral naphthotubes. These cases of heterochiral selectivity encourage further investigation into the underlying molecular mechanisms of enantioselectivity of the chiral naphthotubes.
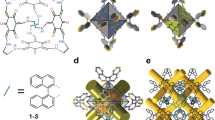
a Chemical structures of chiral bis-thiourea endo-functionalized syn-configured naphthotubes with R,R and S,S chiral centers, and b anti-configured naphthotubes with R,R and S,S chiral centers, the chiral centers are highlighted with cyan. c X-Ray single crystal structures of R,R-CT2 and S,S-CT2, the acetone molecules binding in the cavities are removed for clarity, the dihedral angles of bis-naphthalene cleft groups are marked with green lines. d Circular dichroism spectra of chiral bis-thiourea endo-functionalized naphthotubes including R,R-CT1, R,R-CT2, S,S-CT1, S,S-CT2 (50 μM in 1,2-dichloroethane, 25 °C).
Results
Synthesis and characterization
The key to synthesizing chiral naphthotubes is the production of a chiral diamine with two chiral centers (Supplementary Figs. 1–19). To avoid complicated enantioseparation, a chiral auxiliary tert-butanesulfinamide was employed to regulate the chirality during the addition of a methyl group to the chiral aldimines via Grignard reagent methylmagnesium bromide. For instance, a chiral auxiliary S-tert-butanesulfinamide created the chiral carbon center with an R configuration, and vice versa61,62. After macrocyclization between diisothiocyanate and chiral diamine (R,R) under high-dilution conditions, two configured isomers including syn-configured naphthotube R,R-CT1 (yield: 5.0%) and anti-configured naphthotube R,R-CT2 (yield: 5.1%) were separated and purified via recrystallization following column chromatography. Two configured isomers are difficult to be assigned according to their complicated NOE signals in the 2D NMR spectra (Supplementary Figs. 20–23). Fortunately, the assignment can be finally confirmed by X-ray single crystal structure of R,R-CT2 (Fig. 2c). Similarly, the other enantiomers S,S-CT1 and S,S-CT2 can be synthesized from the chiral diamine with S configuration on two chiral centers. The configured assignments were supported by X-ray single crystal structure of S,S-CT2 (Fig. 2c). In two crystal structures, the skeleton of chiral naphthotubes, especially the thiourea binding sites, undergo enantiomeric twist through intramolecular chiral transfer. Additionally, the asymmetric distribution of chiral centers in the naphthotubes causes two bis-naphthalene clefts to twist with different extents, as evidenced by the structures of X-ray single crystals and energy-minimized simulations. (Fig. 2c, Supplementary Figs. 24–26). We also attempted to introduce more chiral centers to the methylene adjacent to the thiourea groups, but to no avail, likely due to greater steric hindrance. In addition, spectral data also provide evidence of the molecule’s twisting, as depicted in Fig. 2d, the enantiomeric pairs of these chiral naphthotubes exhibit mirror-image CD spectra, and offering additional evidence for their enantiotropy of structures and enantiopurity. Chiral high performance liquid chromatography (HPLC) experiments on R,R-CT1, R,R-CT2, S,S-CT1 and S,S-CT2 showed that the enantiomeric excess (ee) values of these chiral naphthotubes are >99% (Supplementary Fig. 27), further supporting the chirality of the chiral diamines was maintained during macrocyclizations.
Chiral recognition
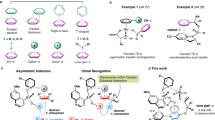
Various organic molecules, including cyclic esters (guests 1), quinuclidinol (guest 2), oxazolidinones (guests 3), morpholinones (guests 4), cyclic dipeptides (guests 5) and drug molecules (guests 6 & 7) were selected to test the recognition with chiral naphthotubes (Fig. 3a). The enantioselectivity was preliminarily evaluated by the 1H NMR spectra of 1: 1 host-guest complex (Supplementary Figs. 28–37). For example as shown in Fig. 3b, a chiral cyclic dipeptide R-5a interacts with two enantiomeric anti-configured chiral naphthotubes respectively. The differences of chemical shift change of proton a/a’ between R-5a@R,R-CT2 and R-5a@S,S-CT2 demonstrate the enantioselectivity of chiral naphthotubes. These results also provide evidence that enantioselectivity may stem from differences in non-covalent interactions between the host and guest. Moreover, the 1: 1 host-guest 1H NMR experiments in DMSO further confirm the impact of non-covalent interactions on chiral recognition (Supplementary Fig. 38). Additionally, we have attempted to conduct 1H, 1H-ROESY experiments of host and guest to obtain evidence of the differences in interactions between enantiomers. (Supplementary Figs. 39−40). However, the nuclear Overhauser effect NOE signal cannot be detected due to the rapid exchange of host-guest exchange on the NMR timescale. The Job plots of R-5a@S,S-CT2 and high-resolution mass spectra (HRMS) for host-guest complexes support a 1: 1 binding stoichiometry (Supplementary Figs. 41–43). Based on the results, the association constants between all possible combinations of hosts and guests were determined by 1H NMR titration with a 1: 1 binding mode (Supplementary Figs. 44–107), and are listed in Table 1. Typically, there are two main methods for calculating the enantioselectivities between a pair of enantiomeric guests and / or hosts. The first method involves utilizing one chiral host that bind to two enantiomeric guests, resulting in different association constants. The second method involves using two enantiomers of hosts, each with different association constants with the same chiral guest. The enantioselectivities obtained from both methods should agree with each other quantitatively and qualitatively. Since the complexes R-guest@R-host and R-guest@S-host are the enantiomers of S-guest@S-host and S-guest@R-host, respectively, they should have similar stability and thus similar association constants. Therefore, two enantioselectivities should theoretically be similar (or with their reciprocals) and this would support the reliability of the data on the association constants. As shown in Table 1, the enantioselectivities or their reciprocals are all similar for one enantiomeric pair of guests and one enantiomeric pair of hosts. This supports the reliability of our data.
For these chiral guests, two configured chiral naphthotubes both exhibit enantioselectivities, especially in the case of cyclic dipeptides, where the highest enantioselectivity reaches 17.0 for chiral naphthotubes CT1 and chiral guest S,S-5b. This is remarkable for a neutral guest molecule and a host with a neutral cavity. Compared with chiral guest S,S-5b, the chiral guest S,S-1 with similar molecular structure, however, shows a significantly decreased complexation ability and much lower enantioselective recognition. This may be caused by substituent differences in the guest. In addition, the syn-configured naphthotubes CT1 and the anti-configured naphthotubes CT2 often show different enantioselectivities to the same chiral guests. Furthermore, the chiral naphthotubes display chiral recognition of guests with bulky substituents and multiple chiral centers, including chiral drugs 6 and 7. Notably, the chiral naphthotubes exhibit different chiral preferences for selecting chiral guests with homochirality or heterochirality. The homochiral preference exists in most cases, such as 1, 2, 5b, 5c and 5d (marked with letter b in Table 1), which is consistent with the selection of homochirality in nature. However, in exceptional cases, the chiral naphthotubes exhibit a preference for selecting guests with heterochirality, such as for 3, 4a, 4b, 4c and 5a (marked with letter c in Table 1). Another noteworthy point is that the syn-configured and anti-configured host exhibit different chiral preferences towards guests 3, 4a, 4b and 4c. These findings indicate the shape of the chiral cavities and the variations of substituent groups may potentially influence the chiral recognition and preference. There are still some undiscovered molecular mechanisms behind enantioselectivity exhibited by these chiral naphthotubes, therefore it is essential to explore the underlying mechanisms.
Crystal structures and binding mode of chiral recognition
In order to gain a deeper insight of chiral recognition, we attempted to grow single crystals (Deposition Numbers 2247191 (R-5a@S,S-CT2), 2247223 (S-5a@S,S-CT2) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallo-graphic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service, the same below) of the host-guest complexes with heterochirality preliminarily. Fortunately, we were successful in obtaining a pair of single crystals of R-5a@S,S-CT2 and S-5a@S,S-CT2, which were suitable for analysis using X-ray crystallography (Supplementary Figs. 108–109). As shown in Fig. 4a, b, multiple interactions exist between the host and guest, including hydrogen bonding, N-H•••π and C-H•••π interactions. The extra C-H•••π interactions between the isopropyl and naphthyl in R-5a@S,S-CT2 may contribute to the enantioselectivity. Moreover, the different twists of bis-naphthalene clefts create slight asymmetry in the space. This may affect the number and strength of non-covalent interactions. Notably, the enantiomeric guests S-5a and R-5a adopt opposite orientations and both lying flat within the cavity of S,S-CT2. In this binding mode, both enantiomeric guests can snugly fill the twisted cavities of the hosts with structurally complementary space and binding sites.
As for the homochiral selectivity, we tried to obtain single crystals (Deposition Numbers 2247283 (S,S-5c@S,S-CT2), 2247282 (2Toluene@R,R-CT2), 2269211 (S,S-5b@R,R-CT2) of S,S-5b, S,S-5c and S,S-5d with anti-configured chiral naphthotubes. However, only the single crystals of S,S-5b@R,R-CT2 and S,S-5c@S,S-CT2 were obtained (Supplementary Figs. 110, 111). Additionally, in the growth of S,S-5c@R,R-CT2 crystal, only two toluene molecules in the cavity of R,R-CT2 were detected in the obtained crystals, despite adding excess S,S-5c to the solution of R,R-CT2 (Supplementary Fig. 112). The single crystal structures are shown in Figs. 4c, d, multiple interactions stabilize the complexation of the host and guest, and both S,S-5b and S,S-5c are observed to stand upright within the cavity, which is distinct from the case of 5a. Furthermore, considering the opposite binding modes found in case of 5a, it can be speculated the guests in S,S-5b@S,S-CT2 and S,S-5c@R,R-CT2 should adopt an opposite orientation compared with S,S-5b@R,R-CT2 and S,S-5c@S,S-CT2, respectively. Overall, the above findings in crystals provided us with evidence of chiral recognition in terms of the differences in non-covalent interactions and opposite binding mode.
Influence of flexibility on the chiral recognition
The complicated 1H NMR signals of chiral naphthotubes indicate the potential existence of rapid and asymmetric conformational variation at room temperature. The flexibility of the host may possibly influence chiral recognition. In variable temperature 1H NMR experiments, as the temperature decreased, the proton signals of S,S-CT1 and S,S-CT2 split (Fig. 5a, Supplementary Fig. 113) and the signals of protons 5 and 5’ are detected respectively at −40 °C. Additionally, in the 1H NMR spectra of complex R-5a@S,S-CT2 (Supplementary Fig. 114), the signals of S,S-CT2 split to be clear at room temperature, which likely attribute from the restriction caused by the guest R-5a in cavity, and there is almost no variation even with a temperature decrease from 20 to −40 °C. In contrast, a larger extent of conformation exchange may exist in S-5a@S,S-CT2 as the temperature decreased (Supplementary Fig. 115), which could be attributed to a potential mismatch in shape between S-5a and S,S-CT2.
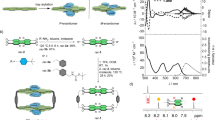
a Variable-temperature 1H NMR spectra of S,S-CT2 (500 MHz, CDCl3, 0.5 mM, 25 °C), the temperature decrease from 20 °C to -40 °C. b Circular dichroism spectra of anti-configured chiral naphthotubes with R-5a and S-5a under saturated binding (25 μM in 1,2-dichloroethane, 25 °C). c Energy-minimized structures of R-5a@S,S-CT2, S-5a@S,S-CT2 and d S,S-5c@S,S-CT2, S,S-5c@R,R-CT2, which were obtained by DFT (M06-2x/def2-svp) calculations with the PCM solution model in chloroform at 298 K, the relative energies are shown (M06-2x/ma-def2-tzvpp), the dihedral angles of bis-naphthalene clefts in host-guest complexes are marked with red lines, the butoxy are replaced with methoxy to simplify the calculation.
The circular dichroism (CD) spectra provided additional insights into the conformational changes that occur in chiral naphthotubes upon binding with chiral guests. As depicted in Fig. 5b and Supplementary Figs. 116–117, the mirror-imaged spectra of the enantiomeric pairs suggest that the host-guest complexes undergo a similar twist upon binding. Additionally, the extent of the conformational change is more predominant in host-guest complexes with higher affinity. For example, the CD intensity undergoes a larger extent of enhancement when S,S-CT2 binding with heterochiral guest R-5a than homochiral guest S-5a. The results indicate S,S-CT2 may undergo a larger extent of twist when binding with R-5a than S-5a, just like wringing a towel with different levels of force. Furthermore, the observed variations in the UV-Vis and fluorescent spectra of the binding process, specifically in relation to the naphthyl groups, provide evidence supporting the involvement of naphthyl groups. On the contrary, when S,S-5b and S,S-5c are capsulated into the cavity of anti-configured naphthotubes, respectively. The Cotton signals of S,S-5b@S,S-CT2 and S,S-5c@S,S-CT2 with homochirality show a significant enhancement than those with heterochirality (Supplementary Figs. 118–119). Besides, the syn-configured chiral naphthotubes also demonstrated a similar result when capturing S,S-5b into the cavity (Supplementary Fig. 120). The exception is S-5d was incorporated into R,R-CT2 and S,S-CT2, respectively, S-5d@R,R-CT2 exhibited a slightly stronger Cotton signal than S-5d@S,S-CT2, possibly due to the additional interactions between the phenyl groups and the host (Supplementary Fig. 121). Above results indicate a conformational variation exists in the binding process, especially for the host-guest enantiomer with higher affinity.
Theoretical calculations
Based on the binding mode of chiral recognition obtained by single crystals, theoretical calculations were performed using Density Functional Theory (DFT) and considering the solvent effect at 298 K. As shown in Fig. 5c, the stability of R-5a@S,S-CT2 is higher than that of S-5a@S,S-CT2, with a lower free energy of −1.59 kJ/mol, which is generally in qualitative agreement with the 1H NMR titration result (ΔΔG = −2.18 kJ/mol). And multiple non-covalent interactions, including hydrogen bonding, N-H•••π and C-H•••π interactions, are observed by non-covalent interaction analysis (using independent gradient model based on Hirshfeld partition, IGMH) for both complexes (Supplementary Fig. 122). The results show that the chiral naphthotube S,S-CT2 have stronger non-covalent interactions with R-5a than S-5a. These findings demonstrate that the differences of multiple non-covalent interactions should be the basis of the enantioselectivity. Besides, in the complexes of S,S-5c@S,S-CT2 and S,S-5c@R,R-CT2. Stronger non-covalent interactions are also be observed to lead S,S-5c to prefer to stay in the cavity of S,S-CT2 (Fig. 5d, Supplementary Fig. 123). In like manner, it is similar in the case of S,S-5b within anti-configured cavities (Supplementary Fig. 124). Through further analysis of the thermodynamic parameters, we found that the driving force behind enantioselectivity is mainly attributed to enthalpic differences, and enthalpy -entropy compensation has also been observed to influence enantioselectivity (Supplementary Tab. 2). And the entropy change may be caused by the different conformational twists in chiral recognition, which are indicated by the dihedral angles of bis-naphthalene clefts (Fig. 5c, d).
The calculations further confirmed the opposite binding mode in the chiral recognition. Therefore, we also carried out the related calculation of S,S-5b with an opposite orientation binding mode in syn-configured chiral naphthotube. The non-covalent interaction analysis and thermodynamic parameters reveal that the enantioselectivity is mainly attributed to enthalpic differences (Supplementary Fig. 125, Supplementary Tab. 2). From the exploration of the chiral recognition mechanism of cyclic dipeptides mentioned above, it can be seen the enantioselectivity whether homochiral or heterochiral selectivity originate from the differences of multiple non-covalent interactions. And the shape of cavities, substituents of guests, flexibility of host and binding modes, are demonstrated to contribute to creating differences in the non-covalent interactions.
Based on these findings regarding chiral recognition, we proceeded to investigate the chiral recognition of chiral guests 4 within the chiral naphthotubes. Specifically, we focused on three guests, S-4a, S-4b and S-4c, which all share morpholinone groups. These groups contain two different hybridized oxygen atoms that can act as hydrogen-bonding receptors. Thus, the chiral guests 4 can adopt four possible binding modes in an asymmetrical cavity. The results of theoretical calculations indicate the chiral guests 4 adopt a similar opposite binding mode within chiral cavities of naphthotubes (Supplementary Figs. 126–129). Further analysis of spectral and thermodynamic data indicated that the enantioselectivity was mainly driven by enthalpy, resembling the driven force observed in chiral guests 5 (Supplementary Figs. 130–131, Supplementary Tab. 2). These findings are generally in qualitative agreement with the 1H NMR and spectral results. Moreover, the results again highlight the importance of multiple non-covalent interactions in chiral recognition.
Discussion
Reviewing the mechanisms of chiral recognition in a previous study, this mechanism is similar to the four-location model used to explain protein’s ability to discriminate between L- and D-isomers in biosystem63. In four-location model, a minimum of four designated locations may include a direction needed for chiral discrimination. In the case here, the enantioselectivity was found to originate from variations in multiple non-covalent interactions also include an orientation, thus, which should be referred to as the multipoint location model. The above investigations have provided us with a better understanding of the molecular basis of chiral selection, and additionally to comprehend the conservation of homochirality in biosystems.
In summary, we have presented the synthesis, characterization, chiral recognition, and mechanism of chiral recognition of two pairs of chiral naphthotubes. The thiourea groups with fixed chiral centers are responsible for the molecular skeleton, which includes a twist in an enantiomeric manner. The validity of the chiral introduction method was confirmed by single crystal structures and CD spectra. The presence of multiple endo-functionalized hydrogen bonding sites enhances the ability of the naphthotubes to selectively capture various chiral guests, including cyclic esters, quinuclidinol, oxazolidinones, morpholinone derivatives, cyclic peptides and drug molecules. Furthermore, we used various techniques, including 1H NMR titrations, circular dichroism spectroscopy, X-ray single crystallography and DFT calculations reveal the mechanism and the driving force of enantioselectivity. This study provides a comprehensive understanding of the molecular basis of enantioselectivity within the chiral naphthotubes. Moreover, it is helpful to comprehend the conservation of homochirality in nature and guide the designing of chiral supramolecular receptors.
Methods
General
All the reagents involved in this research were commercially available and used without further purification unless otherwise noted. Solvents were either employed as purchased or dried before use by standard laboratory procedures. Thin-layer chromatography (TLC) was carried out on 0.25 mm Leyan silica gel plates (60F-254). Column chromatography was performed on silica gel (200-300 mesh) as the stationary phase. 1H, 13C NMR, 2D NMR spectra were performed on Bruker Avance-500 NMR spectrometers. Chemical shifts are reported in ppm with residual solvents or tetramethylsilane (TMS) as the internal standards. The following abbreviations were used for signal multiplicities: s, singlet; d, doublet; dd, doublet of doublet; m, multiplet. Host-guest complexes were prepared by simply mixing the guests and hosts in 1: 1 stoichiometry in the corresponding solvent. Electrospray-ionization high-resolution mass spectrometry (ESI-HRMS) experiments were conducted on an applied Q-EXACTIVE mass spectrometry system. Circular Dichroism (CD) and UV-Vis spectra were recorded on an Applied Photo Physics Chirascan CD spectropolarimeter, using a 1 cm quartz cuvette. Fluorescent spectra were recorded on a spectrofluorometer (Edinburgh FS5), using a 1 cm quartz cuvette. Specific rotations were measured on Rudolph Research Analytical Autopol I Polarimeter (589 nm) in a 1 dm length cell under 25 °C.
Synthesis and characterization of chiral naphthotubes
The diisothiocyanate (561 mg, 0.92 mmol; in 60 mL CH2Cl2) and chiral diamine hydrochloride (Optical pure compounds with R,R / S,S configuration, 577 mg, 0.92 mmol; in 60 mL CH2Cl2) in two separate syringes were added dropwise via a double-channel syringe pump to the solution of N,N-Diisopropylethylamine (DIEA, 646 mg, 871 μL, 5.0 mmol) in CH2Cl2 (400 mL) during the course of 10 h. The resulting mixture was stirred at room temperature for 24 h. After the solvent was removed in vacuum, the residue was purified by column chromatography (SiO2, CH2Cl2: MeOH = 1000/5) to give the compound CT1 and CT2 as a white solid. The enantiomers (R,R / S,S) are synthesized by similar methods. Specific rotation (R,R-CT1: [α]25D = +135.0 (c, 0.002, Dichloroethane), R,R-CT2: [α]25D = +10.0 (c, 0.002, Dichloroethane); S,S-CT1: [α]25D = −136.7 (c, 0.002, Dichloroethane), S,S-CT2: [α]25D = −10.0 (c, 0.002, Dichloroethane)). ESI-HRMS: m/z calculated for R,R-CT1 [M + H]+ C70H77N4O8S2: 1165.5178, found 1165.5173 (error = −0.4 ppm); m/z calculated for R,R-CT2 [M + H]+ C70H77N4O8S2: 1165.5178, found 1165.5178 (no error); m/z calculated for S,S-CT1 [M + H]+ C70H77N4O8S2: 1165.5178, found 1165.5175 (error = −0.3 ppm); m/z calculated for S,S-CT2 [M + H]+ C70H77N4O8S2: 1165.5178, found 1165.5173 (error = −0.4 ppm). The NMR and HPLC characterization data of the naphthotubes are provided in the Supplementary Information Figs. 10–13, 15–16 and Fig. 9.
Determination of the association constants
The association constants for the complexes of CTs with most guests (except S,S-CT2 with S-5d) are small (<105 M−1) and the chemical exchange is fast at the NMR timescale. Thus, we used direct NMR titrations to determine their association constants. The data were analyzed using the instrumental internal software package and fitted by “one set of binding sites” model to give the association constants (Ka). Non-linear fitting data are shown in Supplementary Figs. 44−107. For the cases (S,S-CT2 complex with S-5d) with large association constants (>105 M−1) and fast exchange kinetics, the binding affinities were determined by competitive NMR titrations and using guest S-5a (binding constant with S,S-CT2 is 1.2 × 104 M−1) as the outgoing guest. The data from competitive titrations was nonlinearly fitted64 according to the equations developed by Prof. Werner Nau (available from their website, http://www.jacobs-university.de/ses/wnau). All 1H NMR titration experiments were repeated 3 times, and the averaged values and standard deviations are given.
Data availability
The X-ray crystallographic coordinates for structures generated in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2268760 (for R,R-CT2·2Acetone), 2269592 (for S,S-CT2·2Acetone), 2247191 (for R-5a@S,S-CT2), 2247223 (for S-5a@S,S-CT2), 2247283 (for S,S-5c@S,S-CT2), 2247282 (for 2Toluene@R,R-CT2), 2269211 (for S,S-5b@R,R-CT2). These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information Files. The atomic coordinates of structures for DFT calculation are provided as a Source Data file. Source data are provided with this paper.
References
Huang, W.-H., Zavalij, P. Y. & Isaacs, L. Chiral recognition inside a chiral cucurbituril. Angew. Chem. Int. Ed. 46, 7425–7427 (2007).
Zehnacker, A. & Suhm, M. A. Chirality recognition between neutral molecules in the gas phase. Angew. Chem. Int. Ed. 47, 6970–6992 (2008).
Hu, M. et al. Chiral recognition and enantiomer excess determination based on emission wavelength change of AIEgen rotor. Nat. Commun. 11, 161 (2020).
Reetz, M. T. Controlling the enantioselectivity of enzymes by directed evolution: practical and theoretical ramifications. Proc. Natl Acad. Sci. USA. 101, 5716–5722 (2004).
Thomas, C. M. & Ward, T. R. Artificial metalloenzymes: proteins as hosts for enantioselective catalysis. Chem. Soc. Rev. 34, 337–346 (2005).
Bautista-Barrufet, A. et al. Optical control of enzyme enantioselectivity in solid phase. ACS Catal. 4, 1004–1009 (2014).
List, B. & Yang, J. W. The organic approach to asymmetric catalysis. Science 313, 1584–1586 (2006).
Lacour, J. & Linder, D. A counterion strategy. Science 317, 462–463 (2007).
Mohr, J. T., Krout, M. R. & Stoltz, B. M. Natural products as inspiration for the development of asymmetric catalysis. Nature 455, 323–332 (2008).
Safont-Sempere, M. M., Fernández, G. & Würthner, F. Self-sorting phenomena in complex supramolecular systems. Chem. Rev. 111, 5784–5814 (2011).
Chen, L.-J., Yang, H.-B. & Shionoya, M. Chiral metallosupramolecular architectures. Chem. Soc. Rev. 46, 2555–2576 (2017).
Hong, C. M., Bergman, R. G., Raymond, K. N. & Toste, F. D. Self-assembled tetrahedral hosts as supramolecular catalysts. Acc. Chem. Res. 51, 2447–2455 (2018).
Wenz, K. M., Leonhardt-Lutterbeck, G. & Breit, B. Inducing axial chirality in a supramolecular catalyst. Angew. Chem. Int. Ed. 57, 5100–5104 (2018).
Chu, D. et al. Boosting enantioselectivity of chiral molecular catalysts with supramolecular metal-organic cages. CCS Chem. 4, 1180–1189 (2022).
Yashima, E. et al. Supramolecular helical systems: helical assemblies of small molecules, foldamers, and polymers with chiral amplification and their functions. Chem. Rev. 116, 13752–13990 (2016).
Duan, Y., & Che, S. Chiral mesostructured inorganic materials with optical chiral response. Adv. Mater. https://doi.org/10.1002/adma.202205088 (2023).
Hein, J. E. & Blackmond, D. G. On the origin of single chirality of amino acids and sugars in biogenesis. Acc. Chem. Res. 45, 2045–2054 (2012).
Soai, K., Kawasaki, T. & Matsumoto, A. Asymmetric autocatalysis of pyrimidyl alkanol and its application to the study on the origin of homochirality. Acc. Chem. Res. 47, 3643–3654 (2014).
Brewer, A. & Davis, A. P. Chiral encoding may provide a simple solution to the origin of life. Nat. Chem. 6, 569–574 (2014).
Frenkel-Pinter, M., Samanta, M., Ashkenasy, G. & Leman, L. J. Prebiotic peptides: molecular hubs in the origin of life. Chem. Rev. 120, 4707–4765 (2020).
Sallembien, Q., Bouteiller, L., Crassous, J. & Raynal, M. Possible chemical and physical scenarios towards biological homochirality. Chem. Soc. Rev. 51, 3436–3476 (2022).
Xing, P. & Zhao, Y. Controlling supramolecular chirality in multicomponent self-assembled systems. Chem. Soc. Rev. 51, 2324–2334 (2018).
Zhang, L., Wang, H.-X., Li, S. & Liu, M. Supramolecular chiroptical switches. Chem. Soc. Rev. 49, 9095–9120 (2020).
Huang, S., Yu, H. & Li, Q. Supramolecular chirality transfer toward chiral aggregation: asymmetric hierarchical self-assembly. Adv. Sci. 8, 2002132 (2021).
Weyandt, E. et al. Controlling the length of porphyrin supramolecular polymers via coupled equilibria and dilution-induced supramolecular polymerization. Nat. Commun. 13, 248 (2022).
Kyba, E. B., Koga, K., Sousa, L. R., Siegel, M. G. & Cram, D. J. Chiral recognition in molecular complexing. J. Am. Chem. Soc. 95, 2692–2693 (1973).
Kubo, Y., Maeda, S., Tokita, S. & Kubo, M. Colorimetric chiral recognition by a molecular sensor. Nature 382, 522–524 (1996).
Zhang, X. X., Bradshaw, J. S. & Izatt, R. M. Enantiomeric recognition of amine compounds by chiral macrocyclic receptors. Chem. Rev. 97, 3313–3362 (1997).
Mutihac, L., Lee, J. H., Kim, J. S. & Vicens, J. Recognition of amino acids by functionalized calixarenes. Chem. Soc. Rev. 40, 2777–2796 (2011).
Zhang, G.-W. et al. Triptycene-based chiral macrocyclic hosts for highly enantioselective recognition of chiral guests containing a trimethylamino group. Angew. Chem. Int. Ed. 55, 5304–5308 (2016).
Niedbała, P., Dąbrowa, K., Wasiłek, S. & Jurczak, J. Recognition of chiral carboxylates by synthetic receptors. Molecules 26, 6417 (2021).
Petti, M. A., Shepodd, T. J., Barrans, R. E. & Dougherty, D. A. “Hydrophobic” binding of water-soluble guests by high-symmetry, chiral hosts. an electron-rich receptor site with a general affinity for quaternary ammonium compounds and electron-deficient systems. J. Am. Chem. Soc. 110, 6825–6840 (1988).
Webb, T. H., Suh, H. & Wilcox, C. S. Enantioselective and diastereoselective molecular recognition of alicyclic substrates in aqueous media by a chiral, resolved synthetic receptor. J. Am. Chem. Soc. 113, 8554–8555 (1991).
Coterón, J. M., Vicent, C., Bosso, C. & Penadés, S. Glycophanes, Cyclodextrin-cyclophane hybrid receptors for apolar binding in aqueous solutions. a stereoselective carbohydrate-carbohydrate interaction in water. J. Am. Chem. Soc. 115, 10066–10076 (1993).
Kano, K. Mechanisms for chiral recognition by cyclodextrins. J. Phys. Org. Chem. 10, 286–291 (1997).
Sansone, F., Barboso, S., Casnati, A., Sciotto, D. & Ungaro, R. A new chiral rigid cone water soluble peptidocalix[4]arene and its inclusion complexes with α-amino acids and aromatic ammonium cations. Tetrahedron Lett. 40, 4741–4744 (1999).
Singh, H. & Warmuth, R. Chiral recognition by hemicarcerand-like host in aqueous solution. Tetrahedron 58, 1257–1264 (2002).
Bouchet, A. et al. Enantioselective complexation of chiral propylene oxide by an enantiopure water-soluble cryptophane. J. Org. Chem. 76, 4178–4181 (2011).
Ríos, P. et al. Enantioselective carbohydrate recognition by synthetic lectins in water. Chem. Sci. 8, 4056–4061 (2017).
Han, X.-N., Li, P.-F., Han, Y. & Chen, C.-F. Enantiomeric water-soluble octopus[3]arenes for highly enantioselective recognition of chiral ammonium salts in water. Angew. Chem. Int. Ed. 61, e202202527 (2022).
Kim, K. M. et al. Template‐directed quantitative one‐pot synthesis of homochiral helical receptors enabling enantioselective binding. Angew. Chem. Int. Ed. 59, 22475–22479 (2020).
Scriba, G. K. E. Chiral recognition in separation sciences. part i: polysaccharide and cyclodextrin selectors. TrAC-Trends Anal. Chem. 120, 115639 (2019).
Dong, J., Liu, Y. & Cui, Y. Supramolecular chirality in metal-organic complexes. Acc. Chem. Res. 54, 194–206 (2021).
Wang, Y., Wu, H. & Stoddart, J. F. Molecular triangles: a new class of macrocycles. Acc. Chem. Res. 54, 2027–2039 (2021).
Yang, L.-P., Wang, X., Yao, H. & Jiang, W. Naphthotubes: macrocyclic hosts with a biomimetic cavity feature. Acc. Chem. Res. 53, 198–208 (2020).
Yao, H. et al. Molecular recognition of hydrophilic molecules in water by combining the hydrophobic effect with hydrogen bonding. J. Am. Chem. Soc. 140, 13466–13477 (2018).
Ma, Y.-L. et al. Biomimetic recognition of organic drug molecules in water by amide naphthotubes. CCS Chem. 2, 1078–1092 (2020).
Huang, X. et al. Biomimetic recognition and optical sensing of carboxylic acids in water by using a buried salt bridge and the hydrophobic effect. Angew. Chem. Int. Ed. 60, 1929–1935 (2021).
Ma, Y.-L. et al. Biomimetic recognition-based bioorthogonal host-guest pairs for cell targeting and tissue imaging in living animals. CCS Chem. 4, 1977–1989 (2022).
Wang, X. et al. Switchable bifunctional molecular recognition in water using a ph-responsive endo-functionalized cavity. Nat. Commun. 13, 2291 (2022).
Li, M.-S., Dong, Y.-W., Quan, M. & Jiang, W. Stabilization of imines and hemiaminals in water by an endo-functionalized container molecule. Angew. Chem. Int. Ed. 61, e202208508 (2022).
Li, M.-S. et al. The influence of small biomolecules, salts and buffers on the molecular recognition of amide naphthotube in aqueous solutions. Chem. Eur. J. 29, e202202972 (2023).
Chai, H. et al. Enantioselective recognition of neutral molecules in water by a pair of chiral biomimetic macrocyclic receptors. CCS Chem. 2, 440–452 (2020).
Manglik, A. et al. Crystal structure of the μ-opioid receptor bound to a morphinan antagonist. Nature 485, 321–326 (2012).
Zhang, C. et al. High-resolution crystal structure of human protease-activated receptor 1 bound to the antagonist vorapaxar. Nature 492, 387–392 (2012).
Wang, C. et al. Structural basis for molecular recognition at serotonin receptors. Science 340, 610–614 (2013).
Xu, P. et al. Structural insights into the lipid and ligand regulation of serotonin receptors. Nature 592, 469–473 (2021).
Chen, C.-C. et al. The crystal structure of a class of cyclases that catalyze the cope rearrangement. Angew. Chem. Int. Ed. 57, 15060–15064 (2018).
Cristofaro, M. F. & Chamberlin, A. R. Enantioselective and diastereoselective molecular recognition of cyclic dipeptides by a C2 macrolactam host. J. Am. Chem. Soc. 116, 5089–5098 (1994).
Hawbaker, N. A. & Blackmond, D. G. Energy threshold for chiral symmetry breaking in molecular self-replication. Nat. Chem. 11, 957–962 (2019).
Liu, G., Cogan, D. A. & Ellman, J. A. Catalytic asymmetric synthesis of tert-butanesulfinamide. application to the asymmetric synthesis of amines. J. Am. Chem. Soc. 119, 9913–9914 (1997).
Liu, G., Cogan, D. A., Owens, T. D., Tang, T. P. & Ellman, J. A. Synthesis of Enantiomerically pure N-tert-butanesulfinyl imines (tert-butanesulfinimines) by the direct condensation of tert-butanesulfinamide with aldehydes and ketones. J. Org. Chem. 64, 1278–1284 (1999).
Mesecar, A. D. & Koshland, D. E. A new model for protein stereospecificity. Nature 403, 614–615 (2000).
Guo, D.-S., Uzunova, V. D., Su, X., Liu, Y. & Nau, W. M. Operational calixarene-based fluorescent sensing systems for choline and acetylcholine and their application to enzymatic reactions. Chem. Sci. 2, 1722–1734 (2011).
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (22125105, W.J., 21801125, L.P.Y., 22301127, L.H.), Department of Education of Guangdong Province (2020ZDZX2060, W.J.) and Guangdong Basic and Applied Basic Research Foundation (2022A1515110991, L.H.). The calculations were supported by Center for Computational Science and Engineering at Southern University of Science and Technology, and the CHEM high-performance supercomputer cluster (CHEM-HPC) located at department of chemistry, SUSTech.
Author information
Authors and Affiliations
Contributions
W.J. conceived and designed the experiments. S.-M.W. carried out the experiments and performed the DFT calculations. Y.-F.W., H.Y solved crystal structures. L.-P.Y., X.W., S.-M.W., Y.-F.W., L.H., L.S.Z., H.N., and Y.-T.Z. analyzed the data. L.-P.Y., X.W., S.-M.W., Y.-F.W. wrote the manuscript with contributions from all the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Chun-Yi Sun and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, SM., Wang, YF., Huang, L. et al. Chiral recognition of neutral guests by chiral naphthotubes with a bis-thiourea endo-functionalized cavity. Nat Commun 14, 5645 (2023). https://doi.org/10.1038/s41467-023-41390-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-41390-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.