Abstract
The initial steps of B-cell acute lymphoblastic leukemia (B-ALL) development usually pass unnoticed in children. Several preclinical studies have shown that exposure to immune stressors triggers the transformation of preleukemic B cells to full-blown B-ALL, but how this takes place is still a longstanding and unsolved challenge. Here we show that dysregulation of innate immunity plays a driving role in the clonal evolution of pre-malignant Pax5+/− B-cell precursors toward leukemia. Transcriptional profiling reveals that Myd88 is downregulated in immune-stressed pre-malignant B-cell precursors and in leukemic cells. Genetic reduction of Myd88 expression leads to a significant increase in leukemia incidence in Pax5+/−Myd88+/− mice through an inflammation-dependent mechanism. Early induction of Myd88-independent Toll-like receptor 3 signaling results in a significant delay of leukemia development in Pax5+/− mice. Altogether, these findings identify a role for innate immunity dysregulation in leukemia, with important implications for understanding and therapeutic targeting of the preleukemic state in children.
Similar content being viewed by others
Introduction
Childhood B-ALL is considered a clonal blood disorder whose earliest stages take place during embryonic life. Hematopoietic progenitor cells carrying germline or somatic alterations affecting transcription factor genes that regulate early hematopoietic or early B cell development (such as germline ETV6, PAX5, or IKZF1 mutations, or the somatic ETV6-RUNX1 fusion) represent what are commonly defined as preleukemic cells1. These preleukemic cells are compatible with normal hematopoietic development in a large majority of cases. However, occasionally they can transform over time through the acquisition of additional somatic genetic alterations that lead to the appearance of full-blown B-ALL2,3,4,5.
The nature of the environmental or intrinsic signals that contribute to this “switch” from the preleukemic state to the leukemic state remains unclear. The majority of preleukemic genetic alterations on their own are also insufficient to cause B-ALL in mice, essentially recapitulating the scenario in humans2. Recent preclinical studies have shown that exposure to an immune stressor such as common pathogens is necessary to provide the selection advantage that allows preleukemic cells to give rise to leukemia in genetically susceptible strains of mice3,5. Epidemiologic data demonstrating that infection can act as a trigger for B-ALL development in humans further supports these findings6,7,8,9,10,11. However, the nature and order of the events by which infection triggers leukemia is still a matter of debate. The “delayed infection” hypothesis is based on the possibility that an immune system unexposed to common infections early in life may function abnormally when exposed to such infections later in life. Specifically, the abnormal immune signaling triggered by these infections is proposed to lead to a series of genetic events that culminate in the development of leukemia. Alternatively, the “population mixing” hypothesis was postulated to explain the appearance of small clusters of patients with B-ALL in a localized place and time12. It posits that childhood B-ALL arises as an uncommon result of exposure to any otherwise common infection, but this would only become noticeable as clusters of B-ALL at times of population mixing, when a relatively large number of susceptible children naïve to this infection mix with a large number of infected individuals, leading to a (largely subclinical) widespread epidemics12. In either case, a transient deficiency of the immune priming could system act together with a gut microbiome dysbiosis to drive B-ALL. However, little is known about how exposure to infection promotes such transformation of preleukemic cells.
Germline mutations affecting the B cell master regulator PAX5 or the presence of the somatic ETV6-RUNX1 fusion are associated with B-ALL development in a proportion of human carriers, and this process can be faithfully recapitulated in Pax5+/− or ETV6-RUNX1 transgenic mice3,5. Using these and other mouse models, recent studies have begun to address how preleukemic cells respond to infectious stimuli; in this way, it has been demonstrated that infection-driven B-ALL development in Pax5+/− mice is T-cell independent13. Further, the presence of the Pax5+/− or ETV6-RUNX1 mutations uniquely shapes the gut microbiota when compared to the microbiota of wild-type littermates13. Curiously, the Pax5+/−-specific gut microbiota protects these predisposed mice from leukemia development, and its alteration by antibiotic treatment early in life is sufficient to induce leukemia, even in the absence of exposure to common pathogens13. In the present study, we sought to determine how Pax5+/− and ETV6-RUNX1 preleukemic cells switch to a leukemic state using the Pax5+/− and ETV6-RUNX1 mouse models, which are uniquely suited for the study of the early stages of malignant transformation2. Herein, we describe how the temporary malfunction of innate immune signaling plays a key role in this process in Pax5+/− mice, and we identify how this is mediated by a molecular mechanism based on the partial downregulation of Myd88, finally leading to B-ALL in Pax5+/− mice. These findings have important implications for the understanding and potential therapeutic targeting of preleukemic cells in susceptible children.
Results
Microbiome disruption promotes leukemia development in Pax5 +/− mice but not other genetically prone strains
Our group recently found that both Pax5+/− and Sca1-ETV6-RUNX1 transgenic mice harbor distinct genotype-specific gut microbiomes directly related to the genetic alteration they are carrying13. This work established an experimental paradigm to evaluate and identify the factors that modulate the selective advantage of preleukemic hematopoietic precursors. Thus, we first asked whether this relationship between leukemia-predisposition genetic alteration and gut microbiome is an inherent characteristic of genetic alterations associated with infection-driven leukemias or, rather, predisposing genetic alterations associated with infection-independent leukemias. To test this possibility, we took advantage of the Sca1-Lmo2 mouse model, which develops infection-independent T-ALL14, and the Sca1-BCR-ABLp190 model, which develops infection-independent B-ALL15. We initially analyzed the impact of these genotypes on the microbiome using stool samples from Sca1-Lmo2 and Sca1-BCR-ABLp190 mice. Indeed, the effect of each genetic alteration shaped a characteristic gut microbiome that was distinct between these two mouse strains and differed from the microbiome in wild-type (WT) and Pax5+/− animals (Fig. 1a, b, Supplementary Fig. S1 and Supplementary Data 1–3). Thus, predisposing genetic alteration shapes distinct gut microbiota regardless of whether the alteration is associated with infection-dependent or infection-independent ALL development.
a Pairwise Jaccard distances (beta diversity) were computed for all samples. Distance metric was ordinated into 3D via Principal Coordinates Analysis (PCoA) and visualized via Emperor. Axes indicate percent of explained variance. b Pairwise Permanova tests with 999 permutations were applied to test for differences in beta diversity grouped by mouse genotype (WT n = 12, Sca1-BCR-ABLp190 n = 15, Sca1-Lmo2 n = 15 and Pax5+/− n = 10). Scatter dot plots visualize Jaccard distances between samples of the same genotype. Data are shown as Mean ± SD,“n = ” reports measured distances, “p“ is the p-value of the Permanova test. c Leukemia specific survival curve of Sca1-BCR-ABLp190 treated (n = 13) or untreated (n = 7) with antibiotic6s (p-value = 0.6514), Sca1-Lmo2 treated (n = 24) or untreated (n = 14) with antibiotics (p-value = 0.7935), and Sca1-ETV6-RUNX1 treated (n = 6) or untreated (n = 10) with antibiotics mice p-value > 0.9999), and Pax5+/− treated (n = 19) or untreated (n = 20) with antibiotics (p-value = 0.0002). None of them were exposed to common infections. P-values are from Log-rank (Mantel–Cox) test. Source data are provided as a Source Data file. Abx: treated with a cocktail of antibiotics for 8 weeks.
We have previously shown that altering the gut microbiome with antibiotic treatment elicits leukemia in Pax5+/− mice, even in the absence of an infectious trigger (i.e., in specific pathogen-free conditions, SPF). Therefore, we next explored whether altering the gut microbiome under SPF conditions might lead to leukemia induction in Sca1-ETV6-RUNX1, Sca1-Lmo2, and Sca1-BCR-ABLp190 mice. These mice were treated with an antibiotic cocktail (ampicillin, vancomycin, ciprofloxacin, imipenem, and metronidazole) in their drinking water for 8 weeks, which was started at around 8 weeks of age. In all three models, leukemia incidence, time to disease onset and the disease characteristics from both immunophenotypic and histological points of view were not altered by antibiotic treatment compared to non-antibiotic treated mice (Fig. 1c, Supplementary Fig. S2). Thus, modifying the gut microbiome through bacterial depletion impacts B-ALL development in Pax5+/– mice but not in these other leukemia-predisposed strains. Taken together, these results suggest that, although childhood ALL up until now have been speculated to have a common underlying association with infection, there is etiologic heterogeneity regarding the role of gut microbiome dysbiosis in the development of different genetic subtypes of childhood ALL.
Immune training by early exposure to infection does not prevent leukemia development in Pax5 +/− mice
In agreement with the delayed exposure hypothesis, epidemiologic studies suggest that exposures leading to immune stimulation (i.e., breastfeeding, daycare attendance, contact with pets, allergies) during early childhood are correlated with reduced development of the most common B-ALL subtypes (ETV6-RUNX1+ and high-hyperdiploid B-ALL)16,17,18,19,20,21. To ascertain whether the timing of infection exposure is relevant for B-ALL development in Pax5+/− mice, heterozygous mutant and control littermate WT mice were exposed to the same infectious conditions since pregnancy and birth, and then during the rest of their lifespan (we named this the “early exposure group”) (Fig. 2a). The microbiological status in the conventional facility was defined and controlled for 2 years (Supplementary Data 4). The early exposure to common pathogens induced a significant decrease in peripheral blood (PB) B-cells in both WT and Pax5+/− mice. However, this decrease does not seem to be related to leukemogenesis as WT mice never develop B-ALL. Under this scenario, specific B-ALL development was observed in 25% (7 out of 28) of Pax5+/− animals (but none of the WT mice) of the early exposure group (Fig. 2b), closely resembling the penetrance of B-ALL development in Pax5+/− mice with delayed exposure3 and in humans harboring a heterozygous PAX5 c.547G>A pathogenic variant22,23,24. The leukemias occurring in Pax5+/− early exposure mice developed between 9 and 17 months of age, similar to those developing in delayed-exposure mice3, and they exhibited the same leukemic immunophenotype (Fig. 2c and Supplementary Fig. S3–S5). Therefore, the timing of infection does not appear to impact leukemia development in Pax5+/− mice with exposure to an immune stressor (i.e., housing in a conventional, non-SPF, facility) representing the triggering event.
a Experimental setup. Female mice of the “early exposure group” got pregnant already in a conventional facility (i.e., non-SPF conditions) where they are exposed to common infections during their lifespan, and the born pups were kept in these conditions for their lifetime. Female mice from the “delayed-exposure group” got pregnant under SPF conditions, and pups were kept in SPF until one month of age, when they were moved to the conventional facility. The mice were monitored periodically. The endpoint was established at 2 years of age (22–24 months) or when the mice showed signs of disease (clinical signs or presence of blasts in peripheral blood); the animals were sacrificed and the hematopoietic and non-hematopoietic tissues were analyzed. E: embryonic period; PN: postnatal period; SPF: specific pathogen free. b B-ALL specific survival curve of Pax5+/− animals born and kept into non-SPF conditions (red, n = 28) compared to WT control mice (black, n = 21) showing a significantly (log-rank P value 0.0151) shortened life span and compared to Pax5+/− animals always kept into SPF conditions (green, n = 20) showing a significantly (log-rank P value 0.0177) shortened life span (left panel), and B-ALL specific survival curve of Pax5+/− early exposure group (red, n = 28) compared to Pax5+/− delayed-exposure group (blue, n = 41) showing similar life span (log-rank P value 0.5491) and comparing Pax5+/− animals always kept into SPF conditions (green, n = 20) to Pax5+/− delayed-exposure group (blue, n = 41) showing a significantly (log-rank P value 0.0098) shortened life span (right panel). Source data are provided as a Source Data file. c Flow cytometric analysis of hematopoietic subsets in diseased Pax5+/− early exposure mice. Representative plots of cell subsets from the bone marrow and peripheral blood show accumulation of blast B cells in Pax5+/− early exposure mice (n = 7, age: 9–17 months) compared to control age-matched littermate WT mice (n = 4, age: 8–16 months). Pax5+/− early exposure mice show a similar immune-phenotype than Pax5+/− delayed-exposure mice (n = 9, age: 6–16 months).
Nevertheless, it remained to be determined whether the mechanism driving B-ALL susceptibility in Pax5+/− mice under early exposure to infections was similar to the one driving B-ALL onset in Pax5+/− mice with delayed exposure. Examination of B cells in preleukemic Pax5+/− early exposure littermates revealed significantly reduced proportions of total B-cells in the peripheral blood (PB) when compared to WT littermates housed under similar conditions (Supplementary Fig. S6). This decrease in PB B-cell proportions was similar to what we observed in the delayed-exposure Pax5+/− group (Supplementary Fig. S6). To identify the somatically acquired second hits leading to leukemia development, we next performed whole exome sequencing of four Pax5+/− early exposure tumors and their corresponding germline. Similar to leukemias from the delayed Pax5+/− group, early exposure tumors showed recurrent mutations affecting the remaining WT Pax5 allele, with Pax5 p.P80R occurring in 2 out of 4 analyzed leukemias (Supplementary Fig. S7). Moreover, an activating Jak1 p.F837V variant was identified in one of the leukemic samples, which again highlights the importance of the JAK/STAT-signaling pathway in Pax5+/− leukemias, as shown for Jak1 and Jak3 mutations in the Pax5+/− delayed-exposure B-ALL3,25. Interestingly, 2 out of 4 mice analyzed harbored somatic mutations affecting the RAS-RAF-MEK-ERK-pathway (Nras p.G13D and Kras p.Q61H) (Supplementary Fig. S7). Somatic mutations that deregulate this signaling pathway are among the most common genetic aberrations in childhood ALL26. In summary, we obtained in vivo evidence showing that Pax5+/− mice exposed to infections early in life develop leukemia with similar incidence, latency, and molecular features as Pax5+/− mice exposed later in life. These results show that immune training by early exposure to infection does not have a protective effect against the conversion of preleukemic cells to B-ALL in mice heterozygous for a germline Pax5 mutation. These findings strongly point toward a cell-autonomous defect within Pax5+/− preleukemic cells and further reinforce the view of etiologic variation in leukemia development among different B-ALL molecular subtypes.
Reduced expression of Myd88 in immune-stressed preleukemic Pax5 +/− precursor B-cells
Innate immunity plays a critical role in shaping the evolution of cancers with a demonstrated causal association with infection27. Indeed, it has been reported that, in infection-triggered cancers, Toll-like receptors (TLRs) play a central role in inhibiting as well as in promoting cancer formation, both at the level of immune cells within the tumor microenvironment and acting directly on the cancer cells themselves27. To elucidate whether TLR signaling is involved in the transition of pre-malignant B cells to B-ALL, we next performed a Mouse Toll-Like Receptor Signaling Pathway PCR Array using precursor B cells isolated from the bone marrow (BM) of Pax5+/− and WT mice kept either under SPF (Specific Pathogen Free) conditions or exposed to natural infection (i.e., housed in the conventional facility) both for 3 or 6 months. As we previously showed, Pax5+/− mice only developed B-ALL under natural infection exposure3. Analysis of the TLR signaling pathway in preleukemic precursor B cells of Pax5+/− mice exposed to infections revealed a decrease in the expression of the adaptors Myd88 and Tirap, as well as the downstream transcription factor Nfkb1a, (Supplementary Fig. S8). Using real-time PCR quantification, we observed that low levels of Myd88 could be detected in a small percentage of Pax5+/− preleukemic mice, and that this expression was within a similar range to that observed in WT precursor B-cells (Fig. 3a). However, Myd88 expression was significantly decreased in Pax5+/− precursor B-cells from antibiotic-treated mice under SPF conditions and was equal to the level observed in Pax5+/− leukemic B-cells (Fig. 3a). The antibiotic treatment of ETV6-RUNX1 mice also induced a reduction in Myd88 expression in precursor B-cells (Fig. 3a). This finding suggests that the inability of Abx-treatment to drive leukemia progression in the Sca1-ETV6-RUNX1 model therefore, does not appear to be due to a failure to down-regulate Myd88 expression in precursor B-cells. While the Sca1-ETV6-RUNX1 model might have a different reliance on Myd88 signaling or on the cell intrinsic developmental stage where molecular alterations should take place, these results highlight the need to identify downstream mechanisms to provide possible explanations for the inability of Abx-treatment to drive leukemia progression in the Sca1-ETV6-RUNX1 model. Thus, induction of a leukemogenic state is associated with a decrease of Myd88 expression in Pax5+/− B-ALL blasts.
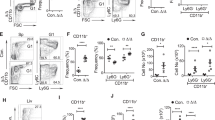
a Q-PCR of Myd88 expression levels in preleukemic Pax5+/− and Sca1-ETV6-RUNX1 and leukemic Pax5+/− proB cells. Preleukemic samples: sorted BM proB (B220low IgM-) cells from 3 months-old of wild-type (n = 5), Pax5+/− (n = 3), Sca1-ETV6-RUNX1 (n = 5) and Pax5+/− (n = 3) and Sca1-ETV6-RUNX1 (n = 5) mice treated with Abx for 8 weeks were analyzed by Q-PCR to quantified the expression of Myd88. Leukemic samples: BM leukemic cells from Pax5+/− mice treated with Abx for 8 weeks (n = 3) and Myd88+/−;Pax5+/− mice (n = 3) were analyzed by Q-PCR to quantify the expression of Myd88. All the mice analyzed were exposed to infections (in the conventional facility). Total BM from Myd88−/− mice was used as a negative control. Error bars represent the mean and SD. For the significant differences, unpaired t test p-values (two-tailed) are indicated in each case. Only relevant comparisons are shown. p = 0.0378 when comparing Pax5+/−(Abx) vs Sca1-ETV6-RUNX1(Abx); p = 0.001 when comparing Pax5+/− vs Pax5+/−(Abx); p = 0.012 when comparing Sca1-ETV6-RUNX1 vs Sca1-ETV6-RUNX1-(Abx); p = 0.004 when comparing Pax5+/− vs Sca1-ETV6-RUNX1 and *** for p-value < 0.0001. b B-ALL-specific survival of Pax5+/− and Myd88+/−; Pax5+/− mice (Pax5+/−, blue line, n = 41), (Myd88+/−;Pax5+/− dark green line, n = 30), (WT, black line, n = 23), and (Myd88+/−;Pax5+/+, brown line, n = 26) all of them exposed to common infections. Log-rank (Mantel–Cox) test p-value > 0.9999 when comparing WT vs Myd88+/−:Pax5+/+ mice, p-value = 0.0117 when comparing Pax5+/− vs Myd88+/−;Pax5+/+ mice and p-value < 0.0001 when comparing Myd88+/−;Pax5+/− vs Myd88+/−;Pax5+/+ mice. c Flow cytometric analysis of bone marrow showing the accumulation of blast B cells (B220+ IgM+/−) in the leukemic Myd88+/−;Pax5+/− mice (X012 and W182) and compared with a healthy Myd88+/− mouse (X650), all of them exposed to common infections. d Haematoxylin and eosin staining of a tumor-bearing Myd88+/−;Pax5+/− mice showing infiltrating blast cells in the spleen, Peyer’s patches, and lymph nodes, compared with an age-matched Myd88+/− mouse. Loss of normal architecture due to leukemic cell infiltration can be seen. A representative example of 16 diseased mice is shown in the figure. Magnification and the corresponding scale bar are indicated in each case. Source data are provided as a Source Data file.
Downregulation of Myd88 increases the penetrance of infection-driven B-ALL development in Pax5 +/− mice
Given the downregulation of Myd88 in preleukemic cells under antibiotic treatment as well as in Pax5+/− B-ALL blasts, we next directly tested the requirement for Myd88 in B-ALL development. First, we decreased the expression of Myd88 by generating Pax5+/−;Myd88+/− mice by interbreeding, and examined whether Pax5+/−;Myd88+/− animals were more prone to infection-induced B-ALL than Pax5+/− mice. Pax5+/−;Myd88+/− mice were exposed to natural infections, and B-ALL development was monitored. We observed that B-ALL development was significantly increased in Pax5+/−;Myd88+/− mice, where 64% (16 out of 25) developed B-ALL (Fig. 3b), closely resembling the penetrance of B-ALL development in Pax5+/− animals treated with antibiotics13. To extend these findings, SPF-housed, two months-old WT and Pax5+/− mice were intraperitoneally (i.p.) injected with PBS or 35 mg LPS (a Myd88-dependent TLR ligand) every other day for eight times over 2 weeks. This exposure of SPF-housed Pax5+/− mice to an Myd88-dependent TLR ligand generated B-ALL in 20% of the mice (Fig. 4). In contrast, the exposure of SPF-housed Pax5+/− mice to an Myd88-independent TLR ligand (mice treated with an intravenous injection of 200 μg of poly(I:C) for a total of 2 times, separated by four weeks between doses) did not generate B-ALL (Fig. 4), therefore supporting the direct participation of Myd88 modulation in B-ALL development in this model. The advent of leukemia in Pax5+/−;Myd88+/− mice under natural infection exposure occurred at between 7 and 20 months of age (Fig. 3b), similar to the Pax5+/− group. Thus, the latency in leukemogenesis was not changed. Furthermore, the critical role of Myd88 downregulation for B-ALL development in Pax5+/− mice was further confirmed by both the lack of B-ALL development in Pax5+/+;Myd88+/− mice under natural infection exposure (0 out of 26) (Fig. 3b) and the appearance of B-ALL in Pax5+/−;Myd88+/− mice even in the absence of exposure to infections (5 out 8, 62.5%) (Supplementary Fig. S9), a condition where Pax5+/− mice never develop B-ALL3. In all these cases the characterization of Pax5+/−;Myd88+/− B-ALLs showed that they are histologically and phenotypically similar to those appearing in the Pax5+/− group (Fig. 3c, d and Supplementary Figs. S10–S12).
a B-ALL-specific survival of Pax5+/− mice injected with LPS (a Myd88-depenedent TLR ligand) (orange line, n = 10), Pax5+/− mice injected with poly(I:C) (a Myd88-indepenedent TLR ligand) (light blue line, n = 10), Pax5+/− mice injected with PBS (as control) (green line, n = 10), control wild type (WT) mice injected with LPS (red line, n = 10), WT mice injected with poly(I:C) (dark blue line, n = 10), and WT mice injected with PBS (black line, n = 10), all of them housed in an SPF facility (without exposure to common infections). Log-rank (Mantel–Cox) test p-value = 0,1464 when comparing Pax5+/− mice injected with LPS and Pax5+/− mice injected with PBS. b Flow cytometry representative illustration of the percentage of leukemic B cells (B220+IgM+ subsets) in PB, BM, spleen and LN from a diseased Pax5+/− mouse injected with LPS compared to an age-matched healthy WT mouse. c Haematoxylin and eosin staining of a tumor-bearing Pax5+/− mouse injected with LPS unexposed to common infections showing infiltrating blast cells in the spleen, liver, and lymph nodes and compared with a healthy WT mouse. Loss of normal architecture can be seen due to the infiltrating cells morphologically resembling lymphoblast. Magnification and the corresponding scale bar are indicated in each case. Source data are provided as a Source Data file.
To explore the relevance of our findings for human leukemia, we compared the role of Myd88 activity on mRNA expression in from tumor cells from patients with B-ALL28,29,30,31. Using RNA microarray gene expression analyses, we identified differentially expressed genes (n = 2463) in leukemic BM from Pax5+/−;Myd88+/− mice compared with BM precursor B cells from WT mice (Supplementary Fig. S13 and Supplementary Data 5). When compared with defined signatures (genesets) previously identified as specifically up- or down-regulated in human B-ALLs28,29,31, the transcriptional profiles of mouse B-ALLs behaved similar to the ones derived from human leukemias (Fig. 5a and Supplementary Fig. S13). Similarly, the genes differentially expressed between mouse B-ALLs and normal mouse B cell progenitors showed a parallelism with genesets related to malignant transformation (Supplementary Fig. S14). Therefore, Pax5+/−;Myd88+/− B-ALLs exhibited a gene expression pattern similar to human B-ALLs, and the gene expression profiles of Pax5+/−;Myd88+/− and Pax5+/− B-ALLs did not show major differences between them or when compared with gene expression profiles derived from blast cells from patients with B-ALL (Supplementary Fig. S15 and Supplementary Data 6), indicating that Myd88 is not significantly contributing to the leukemic gene expression profile of infection-driven B-ALL.
a GSEA showing that leukemic Pax5+/−;Myd88+/− cells from diseased mice present similar profiles for genesets previously identified in human B-ALL samples28,29,31. The genesets shown are significantly enriched with a nominal p-value < 1%. Statistical analysis was done using Gene set enrichment analysis (GSEA) algorithm. b Whole Genome Sequencing in Pax5+/−;Myd88+/− B-ALLs. Oncoprint of somatic single nucleotide mutations and copy number alterations across 8 leukemia samples from Pax5+/−;Myd88+/− mice. Somatic alterations are clustered by gene. Tumor DNA was derived from whole leukemic BM or LN, while tail DNA of the respective mouse was used as reference germline material. Previously reported known human or mouse leukemia hotspot mutations are highlighted (red). Mean tumor variant allele fraction (VAF) for each single nucleotide mutation is shown on the dotplot on the right. c GSEA plots showing the 8 gene sets that are significantly enriched at nominal p-value < 1% in Pax5+/−;Myd88+/− proB cells from healthy mice. Enrichment analysis was done using the hallmarks collection from MSigDB55. Statistical analysis was done using Gene set enrichment analysis (GSEA) algorithm.
To identify somatically acquired second hits leading to B-ALL development in Pax5+/−;Myd88+/− mice, we performed whole-genome sequencing of paired tumor and germline tail DNA samples from eight Pax5+/−;Myd88+/− tumors. The percentage of leukemic cells in the sequenced samples ranged from 88.1% to 98.7%. We identified several somatically acquired recurrent mutations and copy-number variations involving B cell transcription factors in diseased Pax5+/;Myd88+/− mice (Fig. 5b, Supplementary Figs. S16, S17, Supplementary Data 7). Furthermore, recurrent mutations affecting the JAK/STAT and RAS signaling pathways were detected (Fig. 5b, Supplementary Fig. S16). The same genes are also mutated in Pax5+/− murine leukemias3,13,25 and human B-ALL samples26. Thus, the drivers of infection-associated B-ALLs are similar in both Pax5+/−;Myd88+/− and Pax5+/− leukemic cells. Collectively, these observations revealed that downregulation of Myd88 is associated with increased development of leukemia with a similar genetic profile as the leukemias observed in Pax5+/− mice and those from human patients.
Pax5 +/− ;Myd88 +/− precursor B cells exhibit an inflammatory transcriptional profile
We next studied the function of Myd88 downregulation at the preleukemic stages by examining both the accumulation of myeloid cells and the transcriptional profiles of Pax5+/−;Myd88+/− preleukemic precursor B-cells in comparison with those from Pax5+/− preleukemic cells (Supplementary Figs. S18–21 and Supplementary Data 8–10). Myd88 downregulation was not associated with an accumulation of myeloid cells in Pax5+/−;Myd88+/− mice when compared to Pax5+/−;Myd88+/+ mice (Supplementary Fig. S18). However, Pax5+/−;Myd88+/− proB cells were characterized by an increased expression of genes involved in activation of signaling effectors (for example, Notch, apoptosis and IL2-STAT5 signaling) associated with cell proliferation and survival (Fig. 5c). Pax5+/−;Myd88+/− proB cells were also enriched in genes associated with pre-B and follicular murine B cell developmental stages (Supplementary Fig. S21A). Interestingly, the downregulation of Myd88 in Pax5+/− proB cells exacerbated the number of genes differentially expressed that are regulated by Pax5, like Cd19, Slamf6, Bst1, Foxo1, or Bcar3 (Supplementary Figure S21B). Myd88 downregulation in Pax5+/− preleukemic precursor B-cells also augmented the expression of genes involved in the interferon-α response, TNFα signaling via NFκB and interferon-γ response (Fig. 5c), in agreement with the increase in inflammatory cytokines observed in peripheral blood in Pax5+/−;Myd88+/− mice (Supplementary Fig. S22). These findings suggest that Myd88-dependent signaling was hyperactive in Pax5+/− cells, and failed to upregulate feedback inhibitors of the Myd88–NFκB signaling pathway (Fig. 5c), in agreement with previous results showing that Myd88 deficiency results in tissue-specific changes in cytokine induction and inflammation in infected mice32. These results are also in agreement with in vitro studies showing that pro‐inflammatory cytokines like IL-6/IL-1β/TNFα33 or TGF-β dependent signaling34,35 promote malignant transformation of pre‐leukemic B cells.
Engagement of Myd88-independent TLR signaling delays natural infection-driven B-ALL development
We have shown that exposure to natural infections promotes full-blown B-ALL in Pax5+/− mice and that the malignant transformation of preleukemic precursor B-cells is associated with reduced levels of Myd88 expression. Since Myd88 is a critical component in innate immunity induction following TLR ligation, we questioned whether Myd88-independent TLR ligation during the preleukemic phase might alter progression to B-ALL. Therefore, we have addressed this hypothesis by prior exposure of Pax5+/− mice to Myd88-independent TLR agonists, such as the synthetic double-stranded RNA analogue poly(I:C)36. Specifically, we established a mouse cohort consisting of 37 control littermate WT and 66 Pax5+/− mice. All mice were born in a SPF facility. Four weeks after birth, mice were transferred to a conventional facility, and 14 WT and 31 Pax5+/− mice were pre-treated with an intravenous injection of 200 μg of poly(I:C) for a total of 2 times, separated by four weeks between doses, and the remaining 23 WT and 35 Pax5+/− mice received vehicle (Fig. 6c). The microbiologic status of mice was monitored before and periodically throughout treatment, with no differences observed between treated and untreated animals (Supplementary Data 4). As a result of poly(I:C) treatment, we observed a specific increase in inflammatory cytokines and CD8+ T cells in peripheral blood in both WT and Pax5+/− mice (Fig. 6b and Supplementary Figs. S23–S25), showing that poly(I:C) administration was having a similar immune-activating effect in WT and Pax5+/− mice. We next evaluated the immediate effects of Poly(I:C) treatment on the preleukemic cell population and myeloid cell compartments. Poly(I:C) treatment does not reduce the preleukemic compartment in the bone marrow of Pax5+/− mice (Supplementary Fig. S26) but it did induce an accumulation of myeloid cells in both WT and Pax5+/− mice (Supplementary Fig. S27). However, consistent with our previous findings, 23% (8 out of 35) of Pax5+/− mice receiving vehicle developed and succumbed to leukemia (Fig. 6c). The prevalence and the phenotypic and histological characteristics of B-ALL were similar in animals treated with poly(I:C) and untreated Pax5+/− mice, while 25% (8 out of 31) of treated Pax5+/− mice developed B-ALL and died from the disease (p = 0.7930) (Fig. 6c, and Supplementary Fig. S28, S29). In contrast, the latency to leukemia in Pax5+/− mice treated with poly(I:C) was significantly longer than in the Pax5+/− control group receiving vehicle, where it was delayed until 12–21 months of age (p = 0.0019) (Fig. 6e). Thus, early Myd88-independent TLR ligation during the preleukemic phase in Pax5+/− mice resulted in significant delay of B-ALL development under oncogenic environmental conditions, supporting the view that immune modulation during the preleukemic stage can significantly alter progression to B-ALL2.
a Study design. poly(I:C) treatment fully described in methods section. SPF: specific pathogen free facility; CF: conventional facility, where the mice area exposed to common infections. b Percentages of CD8+ T cells in peripheral blood of Pax5+/− and WT mice treated with poly(I:C) compared to untreated mice. An increase in CD8+ T cells can be observed in the blood of treated mice, only being statistically significant for the Pax5+/− mice (unpaired t test (two-tailed), p-value = 0.0087) (for WT mice the unpaired t test has a nonsignificant p-value = 0.5069). Each point represents the mean of the levels of the CD8+ T cells in all mice in each group for each of the different time points (n = 31 for Pax5+/− poly(I:C)-treated mice, n = 8 for Pax5+/− untreated mice, n = 14 for WT poly(I:C) treated mice and n = 23 for WT untreated mice). c B-ALL–specific survival of mice treated with poly(I:C) (Pax5+/−, pink dashed line, n = 31; WT, blue dashed line, n = 14) and nontreated mice (Pax5+/−, pink line, n = 35; WT, blue line, n = 23) following exposure to common infections. Log-rank (Mantel–Cox) test p = 0.7930 when comparing Pax5+/− mice treated with poly(I:C) versus nontreated Pax5+/− mice, p = 0.0034 when comparing Pax5+/− versus WT poly(I:C)-treated mice and p = 0.0154 when comparing Pax5+/− versus WT mice without treatment. d Absence of changes in B-ALL incidence in Pax5+/− mice treated with poly(I:C). The treatment of Pax5+/− mice with poly(I:C) did not reduce nor increase the rate of B-ALL development. Fisher exact test (two-tailed), p = 0.9999. 8 out 35 mice in the non-treated group developed the disease (22.86%) and 8 out of 31 mice in the poly(I:C)-treated group developed B-ALL (25.81%). e poly(I:C) treatment increases the latency of B-ALL development in Pax5+/− mice. Pax5+/− untreated mice (n = 8) were diagnosed with B-ALL between 6 and 15 months of age and the Pax5+/− mice treated with poly(I:C) (n = 8) were diagnosed between 10.5 and 20.8 months of age. Error bars represent the mean and SD. Statistic test Mann–Withney (two-tailed), p-value = 0.0019. Source data are provided as a Source Data file.
Discussion
The association of B-ALL incidence with infectious stressors has been supported by epidemiological data for several decades now; however, until recently, there were no experimental models available capable of providing a suitable biological mechanistic explanation for these findings3,4,5,13,25. However, leukemogenesis in these animal models occurs relatively late related to its age peak in humans, and we do not yet have an explanation for this finding2. We believe that the time to leukemogenesis is, to a large extent, related to the species-specific differences in the dependence of early B cell progenitors from IL7. Murine early B cell progenitors are strongly dependent on IL7 signaling, and when IL7 is removed from cell cultures, these cells die. IL7, when bound to its receptor, activates Jak3 and phosphorylates STAT5. We speculate that this may be the cause explaining why, under the selection pressure of exposure to a common infectious environment, murine B cell progenitors need more time to acquire Jak3 mutations in comparison with their human counterparts, probably because human B cell progenitors are to a large extent independent of IL737.
Using these mouse models, here we have provided evidence showing that gut microbiome dysbiosis is a universal characteristic of all genetic preleukemic conditions tested. However, altering the gut microbiome through bacterial depletion exclusively has a specific impact on B-ALL development in genetically predisposed Pax5+/– mice, therefore highlighting the complex interaction between genetic subtypes and environmental factors that drive leukemogenesis in childhood B-ALL. Indeed, our study of variation in susceptibility to B-ALL by genetic subtypes has allowed us to identify for the first time an etiologic heterogeneity in the progression of the disease among subtypes of childhood B-ALL, and our results suggest that the use of gut microbiota as a modifiable therapeutic target to reduce or abolish the impact of immune stress in predisposed carriers may only apply to specific genetic subtypes of childhood B-ALL. In agreement with this finding, our studies show that, in Pax5+/− mice, immune training by early exposure to infection does not have a protective effect against the conversion of the preleukemic clone into B-ALL, therefore conflicting with the “delayed infection” hypothesis for this specific molecular subtype of B-ALL, and instead supporting Kinlen’s “population mixing” explanation for the epidemiological findings. In this way, our results reinforce the notion of etiologic variation in childhood B-ALL development and the existence of a specific “gene/environment” interaction (i.e., infection) triggering B-ALL development.
Unexpectedly, our data also show that B-ALL development falls into the category of cancers associated with a dysregulation of the molecular mechanisms of innate immunity, which plays a central role in the clonal evolution of pre-malignant B-cell precursors toward B-ALL as a result of immune stress. The molecular landscape characterization of innate immune regulators that promote the initiation of B-ALL has allowed us the identification of Myd88 as one key player in this process. Indeed, a preleukemic B-cell-dependent role was evidenced by an increase in B-ALL incidence upon Myd88 downregulation in the leukemia-prone Pax5+/− model (Fig. 7). The downregulation of Myd88 was shown to function in preleukemic B-cells by upregulating the expression of genes involved in interferon-α response, TNFα signaling via NFκB and interferon-γ response and activation of signaling effectors (for example, Notch, apoptosis and IL2-STAT5 signaling) associated with cell proliferation and survival. This inflammation-dependent role for Myd88 in triggering B-ALL within preleukemic B-cells has also been shown for other TLRs in cancer27. The characterization of the innate immunity signaling within preleukemic precursor B cells showed that all Pax5+/− preleukemic cells are not identical (Fig. 6). As proposed, this variability in the expression of Myd88 under immune stress could provide a means for preleukemic cells to adapt their behavior to the environmental conditions and take advantage of potential fluctuations in the immune proficiency of the organism under stress conditions2. We speculate that such adaptation to new conditions might in turn lead to a rewiring of preleukemic cells themselves, which might affect immune molecules (such as TLRs and other immunomodulatory molecules), favouring the acquisition of second hits. Indeed, this preleukemic heterogeneity could contribute to the variable disease risks across susceptible children.
Immune stress (e.g. infection exposure, antibiotic treatment, etc.) can affect both healthy (a) and Pax5+/− preleukemic carriers (b) alike, and in both of them it can elicit a partial downregulation of Myd88 expression in individual B cell progenitors. However, only in Pax5+/− preleukemic progenitors will this downregulation interfere with innate immune signaling in a way that allows the appearance of secondary mutations leading to the development of a full-blown leukemia. The incidence of B-ALL is directly proportional to the percentage of Pax5+/− preleukemic cells having a downregulation of Myd88 expression.
The initial steps of B-ALL development in children pass usually undetected and, when the disease becomes evident at diagnosis, the delineation of the consecutive genetic events progressively leading to the leukemia is complicated by the accumulation of many oncogenic driver and passenger mutations. Therefore, key to the identification of the driver role of innate immunity dysregulation is the study of leukemia cells while they are appearing, in order to understand the sequential events initiating infection-driven B-ALL. This approach has allowed us to identify previously unknown molecular mechanisms taking place in the still unnoticed stages of human B-cell leukemogenesis, thus opening new opportunities for the development of effective strategies for the early detection and prevention of childhood B-ALL. Collectively, these data raise the possibility that innate immunity boosting might represent a viable strategy for risk reduction or prevention in childhood-related B-ALL; early innate immune response induction by Myd88-independent Toll-like receptor ligation during the preleukemic phase in Pax5+/− mice resulted in significant delay of B-ALL development under oncogenic environmental conditions, supporting the view that immune modulation during the preleukemic stage can significantly alter progression to B-ALL. TLR signaling induction produces disparate effects depending on the genetic subtype of childhood B-ALL and the experimental approach used. While early innate immune response induction by TLR ligation could reduce leukemia penetrance in the Eμ-RET and E2A–PBX1 transgenic mouse models38, ex vivo Toll-like receptor signaling induction by exposure to lipopolysaccharide triggers transformation of ETV6–RUNX1+ precursor B cells39. Our results also suggest that the elimination of these rewired preleukemic cells, or protection against this environmental adaption, might help curtail or prevent leukemic development in genetically predisposed carriers more effectively than strategies based on general immune training. Likewise, immune training by early exposure to infection does not prevent Pax5+/− preleukemic carriers against B-ALL. Overall, these findings have important implications for the understanding and potential therapeutic targeting of the preleukemic state in children.
Methods
Data reporting
Sample sizes were determined on the basis of the literature describing mouse modeling of natural infection-driven leukemia3,4,5,13,25 and were justified by power calculations estimating 90% power to detect differential leukemia incidence. The experiments were not randomized and the investigators were not blinded to allocation during experiments and outcome assessment.
Mouse model for natural immune stress-driven leukemia
Pax5+/−, Sca1-ETV6-RUNX1, Sca1-Lmo2 and Sca1-BCR-ABLp190 mice have been previously described4,14,15,40. The Sca1-ETV6-RUNX1 construct was generated by placing the human ETV6-RUNX1 cDNA under the control of the stem‐cell‐specific Sca1 promoter, as has been previously described4. Pax5+/− mice were crossed with Myd88+/− mice (B6.129P2(SJL)-Myd88<tm1.1Defr>/J)41 to generate Pax5+/−;Myd88+/− mice. These Pax5+/−;Myd88+/− mice were bred and maintained in the SPF area of the animal house until the moment when they were exposed to conventional pathogens present in non-SPF animal facilities, as previously described3. All mouse experiments were performed following the applicable Spanish and European legal regulations, and had been previously authorized by the pertinent institutional committees of both University of Salamanca and Spanish Research Council (CSIC). Pax5+/−(age of these mice range from 1 month-old until disease development), Sca1-ETV6-RUNX1 (2 month-old mice were used in the study), Sca1-Lmo2 (2 month-old mice were used in the study), Sca1-BCR-ABLp190 (2 month-old mice were used in the study), and Pax5+/−;Myd88+/− mice (age of these mice range from 1 month-old until disease development) of a mixed C57BL/6×CBA background were used in this study. For the experiments, Pax5+/−, Sca1-ETV6-RUNX1, Sca1-Lmo2 and Sca1-BCR-ABLp190, and Pax5+/−;Myd88+/− of the same litter were used. Both male and female mice were used in all the studies with the exception of the microbiome analysis in which only female mice were included in the study in order to avoid gender biases in microbiome results. Housing environmental conditions included a temperature of 21 °C ± 2°, humidity of 55% ±10%, and a 12 h∶12 h light: dark cycle. Mice had access to food and water ad libitum. During housing, animals were monitored daily for health status. When required, SPF-housed two months old WT or Pax5+/− mice were i.p. injected with PBS or 35 mg LPS corresponding to 35 EU (Escherichia coli 0111:B4, LPS-EB Ultrapure, Invivogen). When the animals showed evidence of illness, they were humanely killed, and the main organs were extracted by standard dissection. The maximal tumor size/burden approved by the institutional committees has not been exceeded in any mice included in the experiments. All major organs were macroscopically inspected under the stereo microscope, and then representative samples of tissue were cut from the freshly dissected organs, and were immediately fixed. Differences in Kaplan–Meier survival plots of transgenic and WT mice were analyzed using the log-rank (Mantel-Cox) test. Statistical analyses were performed by using GraphPad Prism v5.01 (GraphPad Software).
Antibiotic treatment
Mice were treated with an antibiotic regimen as described42. Groups of mice were given a cocktail of antibiotics (ampicillin, 1 g/L, Ratiopharm; vancomycin, 500 mg/L, Cell Pharm; ciprofloxacin, 200 mg/L, Bayer Vital; imipenem, 250 mg/L, MSD; metronidazole, 1 g/L, Fresenius) added to their drinking water ad libitum for a period of eight weeks.
poly(I:C) treatment
Pax5+/− and WT mice were born in SPF conditions and were maintained there until they reached 1 month of age. At that time, 31 Pax5+/− and 14 WT mice were transferred to a conventional facility (CF) and administered the first dose of poly(I:C) (intravenous injection of 200 μg/mouse). As a control group, 35 Pax5+/− and 24 WT mice were transferred to CF and maintained under the same conditions without poly(I:C) administration. 4 weeks later, the second dose of poly(I:C) was administered to the previously treated animals. Mice were monitored periodically. The endpoint was established at 2 years of age (22–24 months) or when the mice showed signs of disease (clinical signs or presence of blasts in peripheral blood). SPF: specific pathogen-free facility; CF: conventional facility, where the mice area exposed to common infections.
FACS analysis
Nucleated cells were obtained from total mouse bone marrow (flushing from the long bones), peripheral blood, thymus, or spleen. Contaminating red blood cells were lysed with red cell lysis buffer (RCLB) and the remaining cells were washed in PBS with 1% FCS. After staining, all cells were washed once in PBS and were resuspended in PBS with 1% FCS containing 10 µg/mL propidium iodide (PI) to excluded dead cells from both analyses and sorting procedures. The samples and the data were acquired in an AccuriC6 Flow Cytometer and analyzed using Flow Jo software. Specific fluorescence of FITC, PE, PI, and APC excited at 488 nm (0.4 W) and 633 nm (30 mW), respectively, as well as known forward and orthogonal light scattering properties of mouse cells were used to establish gates. Nonspecific antibody binding was suppressed by preincubation of cells with CD16/CD32 Fc-block solution (BD Biosciences). For each analysis, a total of at least 50,000 viable (PI-) cells were assessed. The gating strategy is exemplified in Supplementary Fig. S30.
The following antibodies were used for flow cytometry: anti-B220 (RA3-6B2, cat. 103212), CD4 (RM4-5, 1:250, cat. 100516), CD8a (53-6.7, 1:250, cat. 100708), CD11b/Mac1 (M1/70, 1:200, cat. 553310), CD19 (1D3, cat. 152404), CD117/c-Kit (2B8, 1:200, cat. 105807), Ly-6G/Gr1 (RB6-8C5; cat. 108412), IgM (R6-60.2, cat. 406509) and CD25 (PC61, cat. 553866) antibodies. All antibodies were purchased from BioLegend and used at a 1:100 dilutions unless otherwise indicated.
Histology
Animals were humanely euthanized by cervical dislocation; tissue samples were formalin-fixed and embedded in paraffin. Pathology assessment was performed on hematoxylin-eosin stained sections under the supervision of Dr. Oscar Blanco, an expert pathologist at the Salamanca University Hospital.
V(D)J recombination
V(D)J recombination analysis was carried out as previously described43. Immunoglobulin rearrangements were amplified by PCR using the primers below. Cycling conditions consisted of an initial heat-activation at 95 °C followed by 31–37 cycles of denaturation for 1 min at 95 °C, annealing for 1 min at 65 °C, and elongation for 1 min 45 s at 72 °C. This was followed by a final elongation for 10 min at 72 °C. To determine the DNA sequences of individual V(D)J rearrangements, the PCR fragments were isolated from the agarose gel and cloned into the pGEM-Teasy vector (Promega); the DNA inserts of at least ten clones corresponding to the same PCR fragment were then sequenced. The primer pairsused are listed in Table 1.
16S rRNA sequencing and analysis
DNA extraction
Fresh fecal samples were collected from each mouse (WT or Pax5+/− mice) at defined time points. Fecal DNA was isolated using QIAamp DNA Stool Mini Kit (Qiagen_Cat. No. 51604), following the stool pathogen detection protocol. DNA was extracted following the manufacturer’s instructions with a modification: heating step in ASL buffer at 95 °C and eluting the DNA in 100 μl ATE buffer. Quantity of DNA was done by Picogreen (Invitrogen, cat.#P7589) method using Victor 3 fluorometry. After performing quality control, qualified samples proceed to library construction.
Targeted 16S V4 region sequencing
We targeted and amplified the V3-V4 region of the 16S rRNA gene by PCR using barcoded primers. Library construction was done by Macrogen (https://www.macrogen-europe.com/) using the Library Kit Herculase II Fusion DNA Polymerase Nextera XT Index Kit V2. To verify the size of PCR-enriched fragments, we checked the template size distribution by running on an Agilent Technologies 2100 Bioanalyzer using a DNA 1000 chip. V3-V4 paired-end sequencing was performed using an Illumina MiSeq (La Jolla, CA) according to the manufacturer’s protocols. The base calls (BCL) binary generated by the Illumina sequencer was converted into FASTQ files utilizing the Illumina package cl2fastq.
Sequencing data processing and statistical analysis
Microbiome bioinformatics were performed with QIIME 2 2019.1044. Raw sequence data were demultiplexed and quality filtered using the q2‐demux plugin followed by denoising with DADA245 (via q2‐dada2). All amplicon sequence variants (ASVs) were aligned with mafft46 (via q2‐alignment) and used to construct a phylogeny with fasttree247 (via q2‐phylogeny). Alpha‐diversity metrics (observed features and Faith’s Phylogenetic Diversity48), beta diversity metrics (weighted UniFrac49, unweighted UniFrac50, Jaccard distance, and Bray‐Curtis dissimilarity51), and Principle Coordinate Analysis (PCoA) were estimated using q2‐diversity after samples were rarefied (subsampled without replacement) to 80992 sequences per sample. Taxonomy was assigned to ASVs using the q2‐feature‐classifier52 classify‐sklearn naı̈ve Bayes taxonomy classifier against the SILVA 132 99% OTUs reference sequences53. For the comparative analysis of the taxonomy of the different groups, the feature table was filtered to keep features with a minimum frequency of 10 and present in at least 10 samples. The comparative analysis was determined using Linear Discriminant Analysis (LDA) Effect Size (LEfSe)54.
Mouse toll-like receptor signaling pathway PCR array
RNA from sorted BM precursor B (B220low IgM−) cells of wild type, and Pax5+/− was extracted with the TRIzol reagent (Invitrogen), and the cDNAs prepared with the RT2 First Strand kit (Qiagen) were preamplified using the RT² HT First Strand Kit cDNA cell cycle synthesis kit (Qiagen). The expression of TLR genes and their signaling pathways was analyzed in samples using the mouse RT² Profiler PCR Array (Qiagen). The PCR array DataanalysisV4web (Qiagen) source was used to calculate the threshold cycle (ΔCt) values and to obtain ΔCt values normalized to those of housekeeping genes. To determine the differences in relative expression between B cell populations, comparative analyses were performed (2–ΔΔCt) using the PCR Array Data Analysis Software (Qiagen).
Real-time PCR quantification (Q-PCR) of Myd88
Expression of Myd88 was analyzed by Q-PCR in preleukemic samples (sorted BM precursor B (B220low IgM-) cells of wild-type (n = 3), Pax5+/−(n = 3) and Sca1-ETV6-RUNX1 (n = 5) and Pax5+/− (n = 3) and Sca1-ETV6-RUNX1 (n = 5) mice treated with Abx for 8 weeks as well as in leukemic samples (leukemic cells from Pax5+/− mice treated with Abx for 8 weeks (n = 3) and leukemic cells from Myd88+/−;Pax5+/− mice (n = 3)). All of them exposed to infections (in mice housed in conventional facility). cDNA for use in quantitative PCR studies was synthesized using reverse transcriptase (SuperScript VILOTM cDNA Synthesis Kit; Invitrogen). Real-time PCR reactions were performed in an Eppendorf MasterCycler Realplex machine. Assays used for quantitative PCR are commercially available from IDT (Integrated DNA Technologies): Myd88 (Assay ID: Mm.PT.58.8716051.gs) and GAPDH (Assay ID: Mm.PT.39a.1). In addition, the probes were designed so that genomic DNA would not be detected during the PCR. Measurement of GAPDH gene product expression was used as an endogenous control. Total bone marrow from Myd88−/− mice were used as negative control. All samples were run in triplicate. The comparative CT Method (ΔΔCt) was used to calculate relative expression of the transcript of interest and a positive control. The change in threshold cycle (ΔCt) of each sample was calculated as the Ct value of the tested gene (target) minus the Ct value of GAPDH (endogenous control). The ΔΔCt of each sample was obtained by subtracting the ΔCt value of the reference from the ΔCt value of the sample. The ΔCt reference value used was the ΔCt obtained from the total spleen of wild-type immunized mice. The fold change in each group, calculated as 2-ΔΔCt sample, was compared.
Quantification of serum cytokine levels
Serum cytokine levels were analyzed using the Cytometric Bead Array (CBA) immunoassay systems (BD Biosciences), which assesses simultaneously IL6, IL10, MCP1, IFNg, TNFa e IL12p70 (CBA Inflammation Kit) and IL-2, IL-4, IL-6, IL-10, IL-17A, TNF alpha and IFN gamma in serum from the mice (Mouse Th1 Th2 Th17 Cytokine Kit #560,485; BDB). Briefly, 50 μL of the serum was incubated for 2 h at room temperature with 50 μL of anticytokine MAb-coated beads and 50 μL of the appropriate PE-conjugated anticytokine antibody detector. After this incubation period, samples were washed once (5 min at 200 g) to remove the excess of detector antibodies. Immediately afterward, data acquisition was performed on a FACSCanto II flow cytometer (BDB) using the FACSDiva software program (BDB). During acquisition, information was stored for 3,000 events corresponding to each bead population analyzed per sample (total number of beads >9000). For data analysis, FCAP Array Software v3.0 program (BDB) was used.
Preleukemic pro-B cell culture
Pro-B cells were obtained from the BM of 2–3 weeks old WT, Pax5+/−, and Pax5+/−;Myd88+/− mice, respectively. B cells were isolated from the BM via positive MACS separation for B220. Cells were cultivated in special pro-B-cell medium (IMDM medium + l-glutamine + HEPES, 2% FCS, 1 mM penicillin–streptomycin, 0.03% Primaton, 50 µM beta-mercaptoethanol, 2 µg/ml cyproxin) supplemented with 5 ng/ml murine recombinant IL7 on ST2-feeder cells.
Microarray data analysis
Total RNA was isolated in two steps using TRIzol (Life Technologies) followed by RNeasy Mini-Kit (Qiagen) purification, following the manufacturer’s RNA Clean-up Protocol with the optional On-Column DNase treatment. The integrity and the quality of the RNA were verified by electrophoresis, and its concentration was measured. Samples were analyzed using Applied Biosystems Transcriptome Analysis Console (TAC) software v4.0.1 with the following parameters: (i) Analysis Version: version 1, (ii) Summarization Method: Gene Level—RMA, (iii) Genome Version: mm10 (Mus musculus) and (iv) Annotation: MoGene-1_0-st-v1.na36.mm10.transcript.csv. Differentially expressed genes were evaluated by specific statistical criteria (p-value < 0.05 and absolute fold change >2). Gene Set Enrichment Analyses were performed using GSEAv4.3.255,56,57, hallmark collection of gene sets55, murine B cell developmental stages gene sets58, and Pax5-regulated gene sets59,60. Gene expression signatures that are specifically upregulated or downregulated in human B-ALL28,29,30,31 were also tested for enrichment within tumor specimens using GSEA, the list of gene sets is included in Supplementary Data 11.
Whole-genome sequencing
Tumor DNA was derived from the BM where the percentage of blast cells was higher than 80% in each B-ALL and germline control DNA was obtained from the tail when the mice were 4-week old. Genomic DNA libraries were prepared from sheared DNA with the HyperPrep Library Preparation Kit (Roche PN 07962363001). Paired-end 150 cycle sequencing was performed on a NovaSeq 6000 (Illumina). Illumina paired-end reads were preprocessed and were mapped to the mouse reference genome (mm10) with BWA61. We used an ensemble approach to call somatic mutations (SNV/indels) with multiple published tools, including Mutect262, SomaticSniper63, VarScan264, MuSE65, and Strelka266. The consensus calls by at least two callers were considered as confident mutations. The consensus call sets were further manually reviewed for the read depth, mapping quality, and strand bias to remove additional artifacts. Somatic copy-number alternations were determined by CNVkit67. For somatic structural variants (SV), four SV callers were implemented in the workflow for SV calling, including Delly68, Lumpy69, Manta70, and Gridss71. The SV calls passing the default quality filters of each caller were merged using SURVIVOR72 and genotyped by SVtyper73. The intersected call sets were manually reviewed for the supporting soft-clipped and discordant read counts at both ends of a putative SV site using IGV.
Sanger sequencing
Mutations were validated using Sanger sequencing on a 3130 Genetic Analyzer (Applied Biosystems). The following primer pairs were used: mJak3, forward: CGGGATGTGGGGCTTTAACT, reverse: GCAGACACGGGGTATAGTGG; mJak1 forward: CCAGACAGCCAGGAGAACAG, reverse: CGTCTGCATAGTACCCACCC; mPax5 forward: CTCGTACATGCACGGAGACA, reverse: GGACCCTTCAGTACACCAGC.
Statistical analysis
Statistical analyses were performed using GraphPad Prism v5.01 (GraphPad Software). Statistical significance was calculated by two-tailed unpaired t test, paired t test, Mann–Whitney test or Kruskal–Wallis test. Data normality was tested using the D’Agostino and Pearson test (alpha level = 0.05). Survival analyses were performed using the log-rank/Mantel–Cox test. Two-side Fisher exact test was used to compare leukemia incidence. P values are depicted on the figures only for significant differences. According to power analysis calculations, assuming the development of leukemia as the experimental endpoint, and an expected proportion of 0.23 B-ALL-positive untreated animals at 20 months of age, a sample size of 34 in each group has a 73% power to detect a decrease in the incidence of the disease to a proportion of 0.03 B-ALL-positive mice in polyI:C-treated animals, with a significance level (alpha) of 0.05.
Data availability
Authors can confirm that all relevant data are included in the paper and/or its supplementary information files. All data reported in this article are deposited in the NCBI’s Gene Expression Omnibus (GEO)74, available under the GEO Series accession number GSE221597 and to NCBI’s Sequence Read Archive (SRA) under the BioProject accession number PRJNA912234. The remaining data are available within the Article, Supplementary Information or Source Data file. Source data are provided with this paper.
References
Cobaleda, C., Vicente-Duenas, C., Ramirez-Orellana, M. & Sanchez-Garcia, I. Revisiting the concept of childhood preleukemia. Trends Cancer 8, 887–889 (2022).
Cobaleda, C., Vicente-Duenas, C. & Sanchez-Garcia, I. Infectious triggers and novel therapeutic opportunities in childhood B cell leukaemia. Nat. Rev. Immunol. 21, 570–581 (2021).
Martin-Lorenzo, A. et al. Infection exposure is a causal factor in b-cell precursor acute lymphoblastic leukemia as a result of Pax5-inherited susceptibility. Cancer Discov. 5, 1328–1343 (2015).
Rodriguez-Hernandez, G. et al. Infection exposure promotes ETV6-RUNX1 precursor B-cell leukemia via impaired H3K4 demethylases. Cancer Res. 77, 4365–4377 (2017).
Rodriguez-Hernandez, G. et al. Infectious stimuli promote malignant B-cell acute lymphoblastic leukemia in the absence of AID. Nat. Commun. 10, 5563 (2019).
Cazzaniga, G. et al. Possible role of pandemic AH1N1 swine flu virus in a childhood leukemia cluster. Leukemia 31, 1819–1821 (2017).
Francis, S. S., Selvin, S., Yang, W., Buffler, P. A. & Wiemels, J. L. Unusual space-time patterning of the Fallon, Nevada leukemia cluster: evidence of an infectious etiology. Chem. Biol. Interact. 196, 102–109 (2012).
Hagan, T. et al. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell 178, 1313–1328 e1313 (2019).
Kroll, M. E., Stiller, C. A., Murphy, M. F. & Carpenter, L. M. Childhood leukaemia and socioeconomic status in England and Wales 1976-2005: evidence of higher incidence in relatively affluent communities persists over time. Br. J. Cancer 105, 1783–1787 (2011).
Greaves, M. F. Speculations on the cause of childhood acute lymphoblastic leukemia. Leukemia 2, 120–125 (1988).
Greaves, M., Cazzaniga, V. & Ford, A. Can we prevent childhood Leukaemia? Leukemia 35, 1258–1264 (2021).
Kinlen, L. Evidence for an infective cause of childhood leukaemia: comparison of a Scottish new town with nuclear reprocessing sites in Britain. Lancet 2, 1323–1327 (1988).
Vicente-Duenas, C. et al. An intact gut microbiome protects genetically predisposed mice against leukemia. Blood 136, 2003–2017 (2020).
Garcia-Ramirez, I. et al. Lmo2 expression defines tumor cell identity during T-cell leukemogenesis EMBO J 37 (2018).
Martin-Lorenzo, A. et al. Loss of Pax5 exploits Sca1-BCR-ABL(p190) susceptibility to confer the metabolic shift essential for pB-ALL. Cancer Res 78, 2669–2679 (2018).
Rudant, J. et al. Childhood acute lymphoblastic leukemia and indicators of early immune stimulation: a Childhood Leukemia International Consortium study. Am. J. Epidemiol. 181, 549–562 (2015).
Kamper-Jorgensen, M. et al. Childcare in the first 2 years of life reduces the risk of childhood acute lymphoblastic leukemia. Leukemia 22, 189–193 (2008).
Ajrouche, R. et al. Childhood acute lymphoblastic leukaemia and indicators of early immune stimulation: the Estelle study (SFCE). Br. J. Cancer 112, 1017–1026 (2015).
Amitay, E. L. & Keinan-Boker, L. Breastfeeding and childhood leukemia incidence: a meta-analysis and systematic review. JAMA Pediatr. 169, e151025 (2015).
Urayama, K. Y., Buffler, P. A., Gallagher, E. R., Ayoob, J. M. & Ma, X. A meta-analysis of the association between day-care attendance and childhood acute lymphoblastic leukaemia. Int J. Epidemiol. 39, 718–732 (2010).
Greaves, M. A causal mechanism for childhood acute lymphoblastic leukaemia. Nat. Rev. Cancer 18, 471–484 (2018).
Auer, F. et al. Inherited susceptibility to pre B-ALL caused by germline transmission of PAX5 c.547G>A. Leukemia 28, 1136–1138 (2014).
Shah, S. et al. A recurrent germline PAX5 mutation confers susceptibility to pre-B cell acute lymphoblastic leukemia. Nat. Genet. 45, 1226–1231 (2013).
Duployez, N. et al. Germline PAX5 mutation predisposes to familial B-cell precursor acute lymphoblastic leukemia. Blood 137, 1424–1428 (2021).
Casado-Garcia, A. et al. Transient inhibition of the JAK/STAT pathway prevents B-ALL development in genetically predisposed mice. Cancer Res. 82, 1098–1109 (2022).
Case, M. et al. Mutation of genes affecting the RAS pathway is common in childhood acute lymphoblastic leukemia. Cancer Res. 68, 6803–6809 (2008).
Man, S. M. & Jenkins, B. J. Context-dependent functions of pattern recognition receptors in cancer. Nat. Rev. Cancer 22, 397–413 (2022).
Linka, Y. et al. The impact of TEL-AML1 (ETV6-RUNX1) expression in precursor B cells and implications for leukaemia using three different genome-wide screening methods. Blood Cancer J. 3, e151 (2013).
Juric, D. et al. Differential gene expression patterns and interaction networks in BCR-ABL-positive and -negative adult acute lymphoblastic leukemias. J. Clin. Oncol. 25, 1341–1349 (2007).
Chiaretti, S. et al. Gene expression profiles of B-lineage adult acute lymphocytic leukemia reveal genetic patterns that identify lineage derivation and distinct mechanisms of transformation. Clin. Cancer Res. 11, 7209–7219 (2005).
Kohlmann, A. et al. Pediatric acute lymphoblastic leukemia (ALL) gene expression signatures classify an independent cohort of adult ALL patients. Leukemia 18, 63–71 (2004).
Behera, A. K. et al. MyD88 deficiency results in tissue-specific changes in cytokine induction and inflammation in interleukin-18-independent mice infected with Borrelia burgdorferi. Infect. Immun. 74, 1462–1470 (2006).
Beneforti, L. et al. Pro-inflammatory cytokines favor the emergence of ETV6-RUNX1-positive pre-leukemic cells in a model of mesenchymal niche. Br. J. Haematol. 190, 262–273 (2020).
Ford, A. M. et al. The TEL-AML1 leukemia fusion gene dysregulates the TGF-beta pathway in early B lineage progenitor cells. J. Clin. Invest. 119, 826–836 (2009).
Portale, F. et al. Activin A contributes to the definition of a pro-oncogenic bone marrow microenvironment in t(12;21) preleukemia. Exp. Hematol. 73, 7–12.e14 (2019).
Alexopoulou, L., Holt, A. C., Medzhitov, R. & Flavell, R. A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413, 732–738 (2001).
Ghia, P., ten Boekel, E., Rolink, A. G. & Melchers, F. B-cell development: a comparison between mouse and man. Immunol. Today 19, 480–485 (1998).
Fidanza, M. et al. Inhibition of precursor B-cell malignancy progression by toll-like receptor ligand-induced immune responses. Leukemia 30, 2116–2119 (2016).
Swaminathan, S. et al. Mechanisms of clonal evolution in childhood acute lymphoblastic leukemia. Nat. Immunol. 16, 766–774 (2015).
Urbanek, P., Wang, Z. Q., Fetka, I., Wagner, E. F. & Busslinger, M. Complete block of early B cell differentiation and altered patterning of the posterior midbrain in mice lacking Pax5/BSAP. Cell 79, 901–912 (1994).
Adachi, O. et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9, 143–150 (1998).
Cording, S. et al. Commensal microbiota drive proliferation of conventional and Foxp3(+) regulatory CD4(+) T cells in mesenteric lymph nodes and Peyer’s patches Eur. J. Microbiol. Immunol. (Bp) 3, 1–10 (2013).
Cobaleda, C., Jochum, W. & Busslinger, M. Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors. Nature 449, 473–477 (2007).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019).
Callahan, B. J. et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Katoh, K., Misawa, K., Kuma, K. & Miyata, T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059–3066 (2002).
Price, M. N., Dehal, P. S. & Arkin, A. P. FastTree 2-approximately maximum-likelihood trees for large alignments. PLoS ONE 5, e9490 (2010).
Faith, D. P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 61, 1–10 (1992).
Lozupone, C. A., Hamady, M., Kelley, S. T. & Knight, R. Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl Environ. Microbiol 73, 1576–1585 (2007).
Lozupone, C. & Knight, R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ. Microbiol 71, 8228–8235 (2005).
Bray, J. R. & Curtis, J. T. An ordination of upland forest communities of southern Wisconsin. Ecol. Monogr. 27, 325–349 (1957).
Bokulich, N. A. et al. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 6, 90 (2018).
McDonald, D. et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 6, 610–618 (2012).
Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60 (2011).
Liberzon, A. et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 1, 417–425 (2015).
Mootha, V. K. et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273 (2003).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Green, M. R. et al. Signatures of murine B-cell development implicate Yy1 as a regulator of the germinal center-specific program. Proc. Natl Acad. Sci. USA 108, 2873–2878 (2011).
Revilla, I. D. R. et al. The B-cell identity factor Pax5 regulates distinct transcriptional programmes in early and late B lymphopoiesis. EMBO J. 31, 3130–3146 (2012).
Schebesta, A. et al. Transcription factor Pax5 activates the chromatin of key genes involved in B cell signaling, adhesion, migration, and immune function. Immunity 27, 49–63 (2007).
Li, H. & Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26, 589–595 (2010).
Cibulskis, K. et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat. Biotechnol. 31, 213–219 (2013).
Larson, D. E. et al. SomaticSniper: identification of somatic point mutations in whole genome sequencing data. Bioinformatics 28, 311–317 (2012).
Koboldt, D. C. et al. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 22, 568–576 (2012).
Fan, Y. et al. MuSE: accounting for tumor heterogeneity using a sample-specific error model improves sensitivity and specificity in mutation calling from sequencing data. Genome Biol. 17, 178 (2016).
Kim, S. et al. Strelka2: fast and accurate calling of germline and somatic variants. Nat. Methods 15, 591–594 (2018).
Talevich, E., Shain, A. H., Botton, T. & Bastian, B. C. CNVkit: genome-wide copy number detection and visualization from targeted DNA sequencing. PLoS Comput. Biol. 12, e1004873 (2016).
Rausch, T. et al. DELLY: structural variant discovery by integrated paired-end and split-read analysis. Bioinformatics 28, i333–i339 (2012).
Layer, R. M., Chiang, C., Quinlan, A. R. & Hall, I. M. LUMPY: a probabilistic framework for structural variant discovery. Genome Biol. 15, R84 (2014).
Chen, X. et al. Manta: rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics 32, 1220–1222 (2016).
Cameron, D. L. et al. GRIDSS: sensitive and specific genomic rearrangement detection using positional de Bruijn graph assembly. Genome Res. 27, 2050–2060 (2017).
Jeffares, D. C. et al. Transient structural variations have strong effects on quantitative traits and reproductive isolation in fission yeast. Nat. Commun. 8, 14061 (2017).
Chiang, C. et al. SpeedSeq: ultra-fast personal genome analysis and interpretation. Nat. Methods 12, 966–968 (2015).
Edgar, R., Domrachev, M. & Lash, A. E. Gene expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210 (2002).
Acknowledgements
The authors thank all the members of their laboratories for discussion. Research at C. Cobaleda’s laboratory was partially supported by Ministerio de Ciencia e Innovación/AEI/FEDER (PID2021-122787OB-I00), and a Research Contract with the “Fundación Síndrome de Wolf-Hirschhorn o 4p-”. Institutional grants from the “Fundación Ramón Areces” and “Banco de Santander” to the CBMSO are also acknowledged. Research in C. Vicente-Dueñas group has been funded by Instituto de Salud Carlos III through the project “ PI22/00379 and by a “Miguel Servet Grant” [CPII19/00024-AES 2017-2020; co-funded by European Regional Development Fund (ERDF)/European Social Fund (ESF) “A way to make Europe”/“Investing in your future”]. Kim Nichols receives funding from the American Lebanese Syrian Associated Charities (ALSAC) and R01CA241452 from the National Cancer Institute Research in ISG group is partially supported by FEDER and by SAF2015-64420-R MINECO/FEDER, UE; by RTI2018-093314-B-I00 MCIU/AEI/FEDER, UE; by PID2021-122185OB-I00 MCIU/AEI/FEDER, UE, and by Junta de Castilla y León (UIC-017, CSI001U16, CSI234P18, and CSI144P20). Research in BdA group is partially supported by RTI2019-09114-B-I00 MCIU/AEI/FEDER, UE. M. Ramírez-Orellana and I. Sánchez-García have been supported by the Fundacion Unoentrecienmil (CUNINA project). C. Cobaleda, M. Ramírez-Orellana, and I. Sánchez-García have been supported by the Fundación Científica de la Asociación Española contra el Cáncer (PRYCO211305SANC). A. Casado-García (CSI067-18) and M. Isidro-Hernández (CSI021-19) are supported by FSE-Conserjería de Educación de la Junta de Castilla y León 2019 and 2020 (ESF, European Social Fund) fellowship, respectively. S. Alemán-Arteaga is supported by an Ayuda para Contratos predoctorales para la formación de doctores (PRE2019-088887). J. Martínez-Cano is supported by a predoctoral fellowship FPI-UAM 2019. We also thank the Hartwell Center and Dr. Ti-Cheng Chang from the Center for Bioinformatics at SJCRH for their support of the whole genome sequencing and bioinformatic pipelines.
Author information
Authors and Affiliations
Contributions
Initial conception of the project was designed by M.R.-O., C.V.-D., C.C., K.E.N., and I.S.-G. Development of methodology was performed by M.I.-H., A.C.-G., N.O., S.A.-A., B.R.-C., J.M.-C., A.M., E.G.S., O.B., M.L.G., A.O., D.A.-L., J.D.L.R., S.R., P.P.-M., A.G.-M., F.J.G.C., M.B.G.C., M.R.-O., B.d.A., C.V.-D., C.C., K.E.N. and I.S.-G. O.B., M.B.G.C., F.J.G.C., and C.V.-D. performed pathology review. Management of patient information was performed by O.B., M.B.G.C., F.J.G.C., S.R., P.P.-M., M.R.-O., C.V.-D., K.E.N., and I.S.-G. M.I.-H., A.C.-G., N.O., S.A.-A., B.R.-C., J.M.-C., A.M., A.O., D.A.-L., J.D.L.R, S.R., P.P.-M., A.G.-M., F.J.G.C., M.B.G.C., M.R.-O., B.d.A., C.V.-D., C.C., K.E.N. and I.S.-G. were responsible for analysis and interpretation of the data (e.g., statistical analysis, biostatistics, computational analysis). Paper preparation was performed by M.I.-H., A.C.-G., N.O., S.A.-A., B.R.-C., J.M.-C., A.M., E.G.S., O.B., M.L.G., A.O., D.A.-L., J.D.L.R, S.R., P.P.-M., A.G.-M., F.J.G.C., M.B.G.C., M.R.-O., B.d.A., C.V.-D., C.C., K.E.N. and I.S.-G. Administrative, technical, or material support (i.e., reporting or organizing the data, constructing databases) was compiled by M.I.-H., A.C.-G., N.O., S.A.-A., B.R.-C., D.A.-L., C.V.-D., C.C., K.E.N., and I.S.-G. The study was supervised by M.R.-O., C.V.-D., C.C., K.E.N., and I.S.-G.
Corresponding authors
Ethics declarations
Competing interests
O.B. reports personal fees from Takeda and Clinigen outside the submitted work. K.N. reports grants from Incyte outside the submitted work. No disclosures were reported by the other authors.
Peer review
Peer review information
Nature Communications thanks Hiroyuki Oshiumi, Jayanth Kumar Palanichamy and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Isidro-Hernández, M., Casado-García, A., Oak, N. et al. Immune stress suppresses innate immune signaling in preleukemic precursor B-cells to provoke leukemia in predisposed mice. Nat Commun 14, 5159 (2023). https://doi.org/10.1038/s41467-023-40961-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-40961-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.