Abstract
Methane (CH4) oxidation to high value chemicals under mild conditions through photocatalysis is a sustainable and appealing pathway, nevertheless confronting the critical issues regarding both conversion and selectivity. Herein, under visible irradiation (420 nm), the synergy of palladium (Pd) atom cocatalyst and oxygen vacancies (OVs) on In2O3 nanorods enables superior photocatalytic CH4 activation by O2. The optimized catalyst reaches ca. 100 μmol h−1 of C1 oxygenates, with a selectivity of primary products (CH3OH and CH3OOH) up to 82.5%. Mechanism investigation elucidates that such superior photocatalysis is induced by the dedicated function of Pd single atoms and oxygen vacancies on boosting hole and electron transfer, respectively. O2 is proven to be the only oxygen source for CH3OH production, while H2O acts as the promoter for efficient CH4 activation through ·OH production and facilitates product desorption as indicated by DFT modeling. This work thus provides new understandings on simultaneous regulation of both activity and selectivity by the synergy of single atom cocatalysts and oxygen vacancies.
Similar content being viewed by others
Introduction
As the predominant constituent of natural gas, methane hydrate and shale gas resources, selective methane (CH4) oxidation to value-added chemicals holds considerable financial and environmental prospective1,2,3,4,5. However, the inert symmetrical tetrahedral structure of CH4 makes it rather difficult for the dissociation of the first C–H bond, which is the most important step for the activation of methane6,7,8. Industrial multistep route via steam reforming and subsequent Fischer–Tropsch synthesis could efficiently activate CH4, while it requires harsh experimental conditions (e.g., >700 °C temperature and/or high pressure), causing huge energy consumption and safety issues9,10,11,12,13. In parallel, it is relatively challenging to achieve high selectivity due to the more reactive characteristics of the desired oxygenates against both the reactant CH4 and stable product CO214,15,16,17. Therefore, selective CH4 conversion to value-added chemicals under mild conditions other than CO2 is highly attractive, while facing considerable challenges.
Photocatalysis offers an appealing alternative to drive many tough redox reactions under mild conditions including CO2 conversion18,19, N2 reduction20 and selective CH4 oxidation8. Recently, various value-added chemicals such as methanol1,21,22,23,24, formaldehyde25,26, ethanol27,28, ethane and ethylene29,30,31,32,33,34 were produced by photocatalysis. For example, we found that up to 90% selectivity with a yield of 3.5 μmol h−1 methanol could be achieved over the optimized FeOx/TiO2 photocatalyst under ambient condition using H2O2 as an oxidant22. Recently a high yield of liquid oxygenates including CH3OH, CH3OOH and HCHO were produced under full arc irradiation over Au supported ZnO, together with the good selectivity of primary products (CH3OH and CH3OOH) (<70%)1. Very recent, the yields of 18.7 μmol h−1 HCHO and 3.7 μmol h−1 CH3OH were reported on quantum BiVO4 with an excellent selectivity toward HCHO (87%) and CH3OH (99%) under 300–400 nm or 400–780 nm irradiation26. Given these significant advances in photocatalytic methane conversion, the yield and/or selectivity to high value chemicals are still quite moderate, in particular it is very challenging to achieve methane activation under visible light irradiation instead of a full arc spectrum due to a narrowed bandgap with mitigated reduction or oxidation potentials.
To realize visible-driven methane oxidation by O2 gas on narrow bandgap photocatalysts, the cocatalyst is crucial that not only promotes charge separation, but more importantly manipulates the activation energy of the methane conversion and the selectivity35,36,37,38,39,40. Furthermore rationally regulating the production of reactive oxygen species (ROS) through cocatalyst modification is necessary as ·OH radicals have been widely regarded as the main species that induced CH4 activation and overoxidation41,42. When CH3OH served as the desired products, overoxidation to HCHO or CO2 would be suppressed by lowering the oxidative potential of photogenerated hole through cocatalyst modification, thus improving the selectivity. Stimulated by molecular catalysis, single atom cocatalysts promise an extremely high efficiency, where atomic dispersed species with unsaturated coordination environment could improve the catalytic performances based on the unique electronic structure43,44,45. Meanwhile, high atom utilization efficiency could be achieved46,47. On the other hand, since CH4 exhibited low electron and proton affinity, moderate decoration of defective sites could enhance the chemical-adsorption of non-polar molecular, then promoting the activation of CH448. Therefore, the integration of both defects and single atom cocatalyst decoration could boost charge separation, weaken oxidative potential and enhance CH4 activation on a photocatalyst.
Herein, atomically dispersed palladium (Pd) supported on defective In2O3 was prepared and served as the visible-light responsive photocatalyst for CH4 conversion to high value chemicals. Under 420 nm irradiation, the production of oxygenates on the photocatalyst reached up to ca. 300 μmol in 3 h, with a very high selectivity of 82.5% over primary products. In situ XPS and EPR spectra were conducted to investigate the charge transfer dynamics. The results indicated the dedicated roles of Pd atoms and oxygen vacancies (OVs) in promoting the transfer of photo-induced holes and electrons, respectively. DFT calculation results indicated H2O could also promoted the desorption of the oxygenate products, thus suppressing overoxidation and facilitating high selectivity of primary products. The introduction of both atomic Pd and OVs further enhanced this effect on suppressing overoxidation. Isotopic labeled experiments further proved the methane conversion pathway.
Results and discussion
Visible-light photocatalytic CH4 oxidation by O2
Atomic Pd cocatalyst was prepared by the in situ photo-deposition method using K2PdCl4 and (NH4)2PdCl4 as the precursors on the visible-responsive In2O3 nanorod photocatalyst. Two types of photocatalysts were synthesized, including the defect-rich and defect-lean materials, denoted Pd-def-In2O3 and Pd-In2O3, respectively. For a comparison, other noble metals including Pt and Au modified photocatalysts were also prepared with the same dosage, denoted M-(def)-In2O3 (M = Pt and Au). With different K2PdCl4 dosage, the as-prepared samples were named as Pdx-def-In2O3, where x% represented the dosage weight percentage of Pd to In2O3. The real content of Pd in all photocatalysts was analyzed and shown in Table S1, which is very close to the nominal dosage. In the following discussion, the best sample Pd-def-In2O3 and the reference Pd-In2O3 were referred to Pd0.1-def-In2O3 and Pd0.1-In2O3 unless otherwise specified.
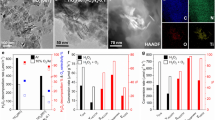
Typical noble metal cocatalysts (Pt, Pd, Au) loaded on In2O3 nanorods were first tested via photocatalytic CH4 conversion with O2 as the oxidant (Fig. 1a and Table S2). Under 420 nm irradiation, the products including CH3OH, CH3OOH and HCHO over Pd-In2O3 reaches 13.4, 32.3 and 27.5 μmol in 3 h reaction, respectively. The selectivity of the primary products (CH3OH and CH3OOH) was 62.1% and the selectivity to the overoxidation products (HCHO and CO2) was 37.9%. In a comparison, Au-In2O3 and Pt-In2O3 performed almost 100% over-oxidized products (HCHO), while with the trace yields of 1.4 and 0.9 μmol HCHO, respectively. Such difference was probably caused by the intrinsic characteristics of different noble metals, such as higher dehydrogenation capability of Pd than Au49,50. Meanwhile, Pt and Au-loaded catalysts seemed to over-oxidize of the major product CH3OH to HCHO as 100% selectivity of HCHO over the Pt and Au-loaded catalysts was observed. One possible reason is due to the weak ability of Pt or Au to trap photoholes as Pt and Au were mainly reported as electrons acceptors51,52,53, as such photoholes left on the VB of In2O3 with a strong oxidation ability could over-oxidize the products to HCHO. Further introduction of defective sites to form Pd-def-In2O3 led to an improved oxygenates yield to 179.7 μmol, 2.5 times higher than that of Pd-In2O3 (73.2 μmol). In parallel, the selectivity of the primary products was improved from 62.1 to 80.4%, suggesting that deep-oxidation to HCHO and CO2 was greatly suppressed under the synergy of Pd single atoms and OVs. In the case of Au-def-In2O3 and Pt-def-In2O3, defect modification exhibited the similar phenomenon on promoting CH4 conversion although the yield was much lower than that achieved on the Pd modified photocatalyst. Apparent quantum efficiency (AQE) of the photocatalytic reaction was determined to be 2.89% at 420 nm for Pd-def-In2O3, further indicating the efficient visible-light-driven activity.
Investigations on a diverse noble metal species, b K2PdCl4 dosage during synthesis, c molar ratio of CH4/O2, d H2O dosage, e total pressure and f cycling tests over the best sample Pd-def-In2O3. Standard reaction conditions: 20 mg photocatalyst, 50 ml distilled H2O, 19 bar CH4, 1 bar O2, 3 h. For reaction condition investigation, only the specified parameter was changed.
The effect of Pd single atoms was explored over Pdx-def-In2O3. As shown in Fig. 1b, Pdx-def-In2O3 photocatalysts exhibited much higher oxygenates production than that of the pristine In2O3. With the raising of K2PdCl4 dosage, the production of the liquid oxygenates exhibited the volcanic trend, increasing from 48.7 μmol on Pd0.01-def-In2O3 to 179.7 μmol on Pd0.1-def-In2O3. Furthermore, the increasing K2PdCl4 dosage resulted in the decreased photocatalytic performance. Notably, the selectivity exhibited slight improvement from 76.5 to 82.8%. With a close look at the above results, OVs and Pd cocatalyst played the synergistic role in optimizing the activity and selectivity for photocatalytic CH4 conversion.
Molar ratio of CH4 to O2 was tuned over Pd-def-In2O3 (Fig. 1c). The production of oxygenates demonstrated the volcanic trend again, with the highest oxygenate production and selectivity achieved at 1 bar O2 pressure. Reducing molar ratio of CH4 to O2 caused the decreased production to 116.0 μmol of primary products when CH4/O2 = 10/10, mainly ascribed to the decrease of CH4 concentration. In parallel, the increased concentration of O2 induced overoxidation and decreased selectivity of the primary products from 80.4 to 67.6 %. With the increase of H2O dosage (Fig. 1d), the production of oxygenates gradually increased, reaching the highest value with 100 ml H2O dosage. The highest oxygenates achieved 299.0 μmol, 2.3 times improvement than that of 25 ml dosage (128.0 μmol) over Pd-def-In2O3. Moreover, the selectivity of the primary products improved from 74.2 to 82.5% with H2O dosage increasing from 25 to 100 ml, which could be attributed to the enhanced desorption of the products from the surface of the photocatalyst when more water was used as discussed later. Notably, in the absence of H2O dosage, CO2 (8.5 μmol) was produced as the only product, suggesting the critical role of H2O in promoting CH4 activation as well suppressing overoxidation, probably ascribed to the production of ·OH radical and promotion desorption of oxygenates by H2O54. While increasing the total pressure of the gaseous reactants, CH4 dissolved increased and the oxygenate production gradually increased (Fig. 1e), e.g., only trace amount of HCHO (4.1 μmol) produced at 1 bar and reaching the highest yield of 179.7 μmol when the pressure was 20 bar. To investigate the stability of the optimized photocatalyst, we carried out the cycling test experiment over Pd-def-In2O3 photocatalyst. No obvious decrease of oxygenates was observed under 15 h reaction (Fig. 1f), demonstrating the good stability of Pd-def-In2O3.
Structural identification
X-ray diffraction (XRD) patterns were recorded to probe the crystalline structure of the representative photocatalysts (In2O3, Pd-In2O3 and Pd-def-In2O3) (Fig. S1). The diffraction peaks on all three samples at 30.7°, 35.5°, 51.0° and 60.7° were well matched with the standard phase of In2O3 (PDF#71-2194). While no Pd and PdOx diffraction peaks were observed on Pd-In2O3 and Pd-def-In2O3, indicating the high dispersion of Pd species. The slightly weakened relative intensity from 100% of In2O3 to 97% and 93% of Pd-In2O3 and Pd-def-In2O3 could be probably ascribed to the introduction of defects. Raman spectra (Fig. S2) further supported the well-established In2O3 phase. The typical Raman peaks for In2O3 were clearly observed at 130.6, 305.1 and 494.8 cm−1 55. For Pd-In2O3 and Pd-def-In2O3, the dominant peak exhibited a slight left-shift from 130.6 to 129.9 cm−1, attributed to the surface stain effect induced by the Pd cocatalyst deposition56.
Electron paramagnetic resonance (EPR) spectra were conducted to evaluate the spin electrons including OVs (Fig. 2a). For the pristine In2O3 and Pd-In2O3, a single Lorentz peak at g = 1.882 was observed, ascribed to the electrons on the conduction band (CB)57,58. In the case of Pd-def-In2O3, the signal of this peak exhibited much stronger intensity than the others, suggesting the higher electron density on CB. Meanwhile, an additional Lorentz peak was observed at g = 2.001, which could be attributed to the free-electrons trapped by the OVs58, thus suggesting the existence of OVs in Pd-def-In2O3. High-resolution O1s XPS measurement of Pd-def-In2O3 photocatalyst was then conducted and shown in Fig. S3a. The binding peaks centered at 529.72 eV, 531.59 eV and 533.02 eV were assigned to the lattice oxygen, OVs and adsorbed moisture, respectively, which directly supported the existence of OVs, consistent with the EPR results and amorphous layer discussed below59. In comparison, Pd-In2O3 (Fig. S3b) exhibited a relatively low content of 14% at 531.59 eV, much lower than that of Pd-def-In2O3 (30%).
a EPR spectra of In2O3, Pd-In2O3 and Pd-def-In2O3. b, c HRTEM and d HAADF and EDS-mapping images of Pd-def-In2O3. Blue, red and green colors represent indium, oxygen and palladium elements, respectively. e Aberration corrected HAADF-STEM image of Pd-def-In2O3, where Pd single atoms with a weak intensity are indicated by yellow circles. f Line scan measured along the x-y rectangle region marked in e. g Fourier transforms of EXAFS of the Pd K-edge spectra of Pd-def-In2O3, Pd foil and PdO.
High-resolution transmission electron microscope (HRTEM) images further proved the defective structure of Pd-def-In2O3 (Fig. 2b, c). Pd-def-In2O3 reserved the nanorod morphology with the average dimension of 240 nm in diameter and 1449 nm in length (insert of Figs. 2b and S4), the same as that of the In2O3 substrate and Pd-In2O3. In addition, a thin amorphous/defective layer of ca. 4 nm was observed on the edge (Fig. 2c). The bulk crystal plane distance of 0.415 nm was indexed to the (211) facet of In2O3. On the contrary, there is not such defective layer as indicated in Fig. S5. Elemental distribution of the corresponding area was analyzed by the EDS-mapping images (Fig. 2d). Obviously, Pd-def-In2O3 exhibited uniform palladium distribution with indium and oxygen elements. No Pd nanoparticles or clusters were found from the HAADF-STEM images at different scales apart from the 42 nm In2O3-assembled nanorods (Fig. S4). The aberration corrected HAADF-STEM image in Fig. 2e clearly indicated the atomic distribution of Pd, where the weak intensity spots cycled by the yellow corresponded to Pd atoms. The x-y line scan along the yellow rectangle of Fig. 2e clearly presents the atomic dispersion of Pd as shown in Fig. 2f. To further investigate the coordination environment of Pd species, Pd K-edge X-ray absorption near edge structure spectra of Pd-def-In2O3 with Pd foil and PdO references were measured as shown in Fig. S6. The absorption edge position of Pd-def-In2O3 was observed between Pd foil and PdO, suggesting the valence state of Pd in Pd-def-In2O3 was between 0 and +260. Fourier transforms of EXAFS of the Pd K-edge (Fig. 2g) exhibited only a predominant peak at ca. 1.5 Å for Pd-def-In2O3, which was ascribed to the first-shell of the Pd-O bond with reference to the PdO sample. In parallel, no peaks corresponding to Pd-O-Pd and Pd-Pd at 3.1 Å and 2.5 Å were detected, indicating the atomically dispersed Pd sites in Pd-def-In2O3, with the absence of metallic Pd and PdO clusters. Therefore the best sample was composed of single atom Pd and OVs on In2O3 nanorods.
Mechanism investigation
UV-vis diffuse reflection spectra (UV-DRS) were conducted to evaluate the photoabsorption ability of the representative photocatalysts (In2O3, Pd-In2O3 and Pd-def-In2O3) (Fig. 3a). All three photocatalysts exhibited the similar band photoabsorption onset at ca. 450 nm, indicating that the modification of single atom Pd and OVs had little influence on the bandgap energy, which would not be the decisive reason for the induced improvement of photocatalysis. Meanwhile, the intrinsic defective structure in the nanocrystallines led to the tail adsorption to 560 nm, and the introduction of OVs resulted in a further red-shift of this tail to 600 nm, similar to the reported on TiO261. Meanwhile, since Pd exhibited the atomic dispersion but not metal nanoparticles, it thus could not cause a photoabsorption peak via the localized surface plasmon resonance effect62.
a UV-DRS spectra of In2O3, Pd-In2O3 and Pd-def-In2O3. Insert shows the optical images of In2O3, Pd-In2O3 and Pd-def-In2O3. b In situ Pd3d XPS spectra of Pd0.3-def-In2O3 in dark and under extended light irradiation. Solid-state in situ EPR spectra of c In2O3, d Pd-In2O3 and e, f Pd-def-In2O3 in dark and under light irradiation for different reaction time.
In situ high-resolution Pd3d X-ray photoelectric spectra (XPS) in dark and under light irradiation were then conducted to investigate the charge transfer direction (Figs. 3b and S7). Notably, high-resolution Pd3d XPS spectra of Pd0.1-def-In2O3 showed a very weak Pd signal (Fig. S8), centered at ca. 336.0 eV between the binding energy of Pd0 (335.38 eV) and Pd2+ (336.55 eV)63. The de-convoluted peaks of Pd0 and Pd2+ at 335.38 and 336.55 eV showed the relative content of 58% and 42%, respectively, indicating that the Pd valance states were equivalent to a mixture of Pd0 and Pd2+. However, due to the limit detection sensitivity of the in situ XPS spectra (Thermo ESCALAB 250Xi instrument), the in situ XPS under light irradiation for Pd0.1-def-In2O3 was featureless as shown in Fig. S9. Therefore, Pd0.3-def-In2O3 that prepared under the identical conditions but with a higher content of Pd (0.284 wt%) was employed to study the function of Pd through the in situ XPS measurement (Fig. 3b). The oxidation state for Pd0.3-def-In2O3 in dark (Fig. 3b) was mainly close to Pd0, suggesting that Pd aggregation happened with a high dosage of K2PdCl4 precursor. It could be seen that Pd3d XPS spectra exhibited an attractive phenomenon that a left-shift of the strongest peaks was observed from 335.77 eV in dark to 336.03 eV for 20 min irradiation and to 336.29 eV for 40 min irradiation. Further extending irradiation time could not result into a continuous left-shift when comparing the XPS peaks under light irradiation for 40 and 60 min (Fig. S7). Such left shift to higher binding energy indicated a more positive valence of Pd under light irradiation, thus suggesting the role of Pd as the hole acceptor under light irradiation. The solid-state in situ EPR spectra under light irradiation provided further experimental evidence as shown in Fig. 3c–f. For pristine In2O3, EPR signal at g = 1.882 (Fig. 3c) was attributed to the spin electrons on the CB of In2O3 substrates57,58. Under light irradiation, this signal for In2O3 showed an increased intensity by 1.4 times due to the photo-excitation process. Similarly, Pd-In2O3 also exhibited such EPR signal that associated with the CB electrons at g = 1.882, and an enhanced intensity under light irradiation. However, the enhancement of this EPR signal for Pd-In2O3 was about 1.7 times, much larger than that of In2O3 (1.4 times) (Fig. 3d). Such greatly enhanced intensity thus suggested that Pd could accept photo-induced holes, then leading to the suppressed charge recombination and an improved concentration of spin electrons that were photo-excited to the CB.
In situ EPR spectra under light irradiation also gave information on the role of OVs. Under light irradiation for 30 s, the EPR signal intensity for Pd-def-In2O3 at g = 1.882 (Fig. 3e) that associated with the spin electrons on the CB increased by 1.2 times. Further prolonging irradiation time did not result in continuous increased EPR intensity at g = 1.882, suggesting that dynamic equilibrium between photo-excitation and charge transfer quickly reached. In parallel, the related signal at g = 2.001 that assigned to the OVs performed gradually increasing intensity from 100 to 226% (Fig. 3f) with the prolonged irradiation for 360 s. Such enhanced intensity indicated the role of OVs as electron acceptors and continuous electron transfer from the CB to the OVs. Therefore, single atom Pd and OVs separately acted as the hole and electron acceptors under light irradiation, which would greatly contribute to the enhanced charge separation.
Photocurrent responses (Fig. S10) were tested to evaluate the charge separation efficiency. Pristine In2O3 exhibited a relatively low photocurrent density of 61.8 μA cm−2. After photo-depositing high dispersed Pd cocatalyst, Pd-In2O3 nearly doubled photocurrent density to 120.6 μA cm−2. The photocurrent density was further improved to 168.1 μA cm−2 on Pd-def-In2O3, almost 2.7 and 1.4 times enhancement than that of In2O3 and Pd-In2O3, respectively. Such highest photocurrent density on Pd-def-In2O3 attributed to the most efficient charge separation, indicating the defects and single atom Pd could greatly enhance charge transfer, which is consistent with the analysis mentioned above. Steady-state fluorescence (PL) spectra further evidenced the enhanced charge separation efficiency. As shown in Fig. S11, a relatively strong PL emission peak was observed for the pristine In2O3, attributed to the severe charge recombination. In comparison, the PL intensity for Pd-In2O3 was greatly weakened, indicating the suppressed charge recombination. For Pd-def-In2O3 photocatalyst, the most weakened PL peak were observed, ascribed to the most enhanced charge separation efficiency, which was corresponding with the photocurrent analysis. Time-decay PL spectra were conducted to evaluate the PL lifetime. As shown in Fig. S12, Pd-def-In2O3 photocatalyst exhibited the slowest PL decay kinetics. The fitting results (Table S3) showed that Pd-def-In2O3 exhibited the average PL lifetime at 4.99 ns, longer than that of In2O3 (3.60 ns) and Pd-In2O3 (4.28 ns), which would be beneficial to the efficient utilization of separated charge carriers.
ROS including ·OOH and ·OH radicals were widely regarded as the main active species for CH4 activation64 and monitored by in situ EPR spectra with 5, 5-dimethyl-1-pyrroline N-oxide (DMPO) as the spin-electron trapping agents. As shown in Fig. 4a, six prominent characteristic signals (4 strong and 2 relatively weak peaks) with the hyperfine splitting constants measured as AN = 15.4 G and AH = 10.6 G were clearly observed and attributed to the DMPO-OOH adduct65. Such detected ·OOH radicals over different photocatalysts came from the reduction of O2 molecule with photo-induced electrons and H+. A stronger intensity of DMPO-OOH was observed for Pd-def-In2O3, suggesting the production of ·OOH radical was enhanced by the integration of single atom Pd and OVs. On the other hand, in situ EPR spectra under light irradiation was used to monitor the generation of ·OH radical with DMPO as the trapping agent in H2O. The 1:2:2:1 quartet signal was observed and assigned to the DMPO-OH adduct, suggesting the generation of ·OH radical (Fig. 4b). It was obvious that Pd-def-In2O3 produced much more ·OH under identical conditions than Pd-In2O3 and In2O3 was the worse. It is believed that ·OH initially activates CH4 to methyl radical (·CH3), thus Pd-def-In2O3 performed CH4 activation best followed by Pd-In2O3, which is consistent with the step by step enhanced photocatalytic performances by Pd and then both Pd and OVs, indicating that OVs could promote charge separation and also facilitate water oxidation reaction on Pd. Coumarin was used as the probe for ·OH radical detection due to the easy reaction between coumarin and ·OH to produce 7-hydroxycoumain (7-HC) that could be detected by UV-vis spectra at 412 nm (Fig. 4c). The results further supported that Pd-def-In2O3 held the strongest ability for ·OH production, which facilitated CH4 activation. Therefore, single atom Pd worked as the hole acceptor, which then catalyzed ·OH radical production from water oxidation. Simultaneously, OVs acted as the electron acceptor, which then catalyzed O2 reduction to generate ·OOH radical.
In situ EPR spectra of a DMPO-OOH and b DMPO-OH for the observation of reactive ·OOH and ·OH radicals over different photocatalysts. AH and AN were hyperfine coupling constants of hydrogen and nitrogen elements, respectively. c PL intensity of 7-HC vs. time over different photocatalysts for the quantification of ·OH. Isotopic labeled experiments d oxygen source and e carbon source for methanol production in the presence of isotopic labeled H218O, 18O2 or 13CH4.
The reaction pathway was investigated by isotopic labeled experiments, including using H218O and 18O2. In the presence of 3 ml H218O, 1 bar 16O2 and 19 bar CH4, no isotopic labeled CH318OH (m/z = 33 and 34) was detected by GCMS (Fig. 4d), suggesting H2O was not the oxygen source that directly participated the formation of oxygenates. In parallel, when using 3 ml H216O, 1 bar 18O2 and 19 bar CH4, the signals at m/z = 34 and 33 were attributed to the isotopic labeled CH318OH and its fragment (Fig. 4d), suggesting O2 was the only oxygen source for CH3OH formation. Carbon source for methanol production were also studied in the presence of 5 bar isotopic labeled 13CH4 (Fig. 4e), where the signal of mass spectra (MS) at m/z = 33 was ascribed to 13CH3OH, demonstrating that CH4 was the carbon source for oxygenates production.
DFT calculations (Fig. 5) were conducted to explain the improved selectivity of primary products. It should be noted that timely desorption of the primary products on the active sites could efficiently avoid its deep-oxidation to HCHO and CO2. As ·OH radical was regarded as the main species that induced oxidation on single atom Pd cocatalyst, it was accordingly considered that the efficient desorption of primary products like CH3OH on Pd is critical to suppress further oxidation. Four models including In2O3 with (111) facet, Pd-In2O3, Pd-def-In2O3(close) with one OV close to single atom Pd and Pd-def-In2O3(far) with one OV far away from the single atom Pd modification were built. Then the adsorption energies of H2O and CH3OH on these models were calculated by the density functional theory (DFT) since the strong adsorption of H2O might promote the desorption of CH3OH. As shown in Figs. 5a, b and S13, the adsorption energies of H2O on In2O3, Pd-In2O3, Pd-def-In2O3(far) and Pd-def-In2O3(close) were −0.76, −1.57, −1.76 and −2.14 eV, respectively, much larger than the CH3OH adsorption energy of −0.47, −1.38, −1.40 and −1.50 eV on the specified model. Such large adsorption energies indicate CH3OH could be easily replaced by H2O on In2O3 or Pd atoms, promoting the desorption of CH3OH. Moreover, adsorption energies of water further increased with the introduction of both Pd atom and OV, demonstrating such co-modification of Pd atoms and OVs could promote the adsorption of H2O most efficiently, which was consistent with the increased production of ·OH radicals as analyzed by the in situ EPR and coumarin experiments. Though the adsorption of CH3OH was also enhanced due to the introduction of Pd atoms and OVs, H2O adsorption energy was enhanced much more, thus water could facilitate the desorption of primary products and avoid overoxidation as indicated in Fig. 1d. It also suggested that a close distance of Pd with OVs was preferable for the competitive adsorption of H2O, thus facilitating the desorption of CH3OH.
Based on the above results, aerobic photocatalytic CH4 conversion mechanism over Pd-def-In2O3 was proposed (Fig. 6). The bandgap structure of Pd-def-In2O3 was established in terms of UV-DRS spectra (Fig. 3a) and Mott-Schottky plots (Fig. S14), where the calculated bandgap energy, the CB position and the valence band position were determined to be 2.71 eV, −0.16 V and 2.55 V (vs. RHE), respectively, which is sufficient to drive O2 reduction to produce ·OOH radicals and H2O oxidation to produce ·OH radicals. Compared with the widely reported large bandgap semiconductor photocatalysts, e.g., TiO2, this relatively weak oxidation potential mitigated the potential to overoxidation. The exact energy level of OVs in the optimized catalyst was hard to be established probably due to its low content. Generally, under 420 nm irradiation, electrons (e−) were excited to the CB of In2O3 nanorod photocatalyst, while leaving holes (h+) on the valence band. Then the photo-induced electrons were trapped by the OVs, activating O2 with H+ to produced ·OOH radicals as detected by the in situ EPR spectra. In parallel, Pd atoms served as the hole acceptors (Pd + h+ → Pdδ+), and then reacted with the adsorbed H2O to produced ·OH (Pdδ+ + H2O → Pd0 + ·OH + H+). CH4 molecules were next activated by the as-produced ·OH to ·CH3. The coupling reaction between ·CH3 and ·OOH then generated the primary products (CH3OOH), and subsequently transferred to CH3OH as indicated in Fig. 6b. Compared with the pristine In2O3, the incorporation of Pd single atoms significantly promoted charge separation and facilitated the generation of reactive species, thus promoting CH4 conversion to oxygenates. Pd atoms loading also moderated the oxidation ability of photoinduced holes on In2O3 as indicated in Fig. 6a, reducing the overoxidation of the primary products. Further decoration of OVs could strengthen the promoted charge separation efficiency, which eventually resulted in the superior photocatalytic CH4 conversion activity and selectivity. In order to suppress overoxidation, it was also critical to enhance the desorption of primary oxygenate products by H2O, as supported by the DFT calculation.
In summary, visible-light-driven CH4 conversion at ambient temperature was reported over the In2O3 nanorod photocatalyst loaded by Pd single atoms cocatalysts and OVs. Under 420 nm irradiation, superior yield (99.7 μmol h−1) and selectivity (82.5%) of the primary products were achieved on the Pd-def-In2O3 photocatalyst under optimized reaction conditions. In situ XPS and EPR spectra under visible light irradiation indicated that Pd and OVs acted as the hole and electron acceptors, respectively, thus synergistically boosting charge separation and transfer. Isotopic labeled experiments proved that O2 was the only oxygen source for oxygenates production, while H2O was the promoter of CH4 activation through the production of ·OH radical as monitored by the in situ EPR spectra with DMPO as the spin-trapping agent. DFT calculation results suggested that H2O performed much larger adsorption energies than CH3OH on either In2O3, def-In2O3 or Pd-def-In2O3, suggesting the stronger adsorption of H2O than CH3OH, which was beneficial to the timely desorption of CH3OH produced, thus avoiding further overoxidation. The introduction of Pd and OVs could further improve the selectivity of primary oxygenates mainly through the greatly enhanced adsorption of H2O and the reduced oxidation potential of photoinduced holes. This work thus provided a useful avenue on co-modification of photocatalysts by oxidative single atom cocatalyst and OVs for simultaneous regulation of both activity and selectivity through enhancing charge separation, moderating photohole oxidation ability and timely promoting desorption of primary products by a solvent.
Methods
Synthesis of In2O3 nanorods
In2O3 nanorods were prepared according to the previous study66. Typically, 12.0 g urea and 1.5 g InCl3 were dissolved in 135 g H2O, followed by stirring at 80 °C for 14 h. After naturally cooling down, the reactant was centrifuged and washed with H2O for several times. The white powder was then dried in vacuum at 60 °C overnight. After thermal-treating in air at 700 °C for 5 h at a ramping rate of 5 °C min−1, yellow powder was obtained and denoted as In2O3.
Synthesis of Pdx-In2O3 and Pdx-def-In2O3 nanorods
Pdx-In2O3 and Pdx-def-In2O3 nanorods were prepared through photo-deposition with ammonium tetrachloropalladate(II) ((NH4)2PdCl4) and potassium tetrachloropalladate(II) (K2PdCl4) as the precursors, respectively. The synthesis was conducted in the multichannel reactor (Beijing Perfectlight Technology Co., Ltd). For Pdx-def-In2O3 preparation, 200 mg In2O3 was first dispersed through sonication with the aqueous solution containing 10 vol.% methanol. Then certain amount of K2PdCl4 solution was added. After sealing and purging with ultrapure argon (99.999 vol.%) for 30 min, the reactor was bottom-irradiated for 3 h to facilitate Pd photo-deposition. The suspension was then centrifuged, washed with H2O for several times and dried under vacuum at 60 °C overnight. The as-prepared samples were denoted as Pdx-def-In2O3, where x% represented the mass percentage of palladium to In2O3 substrates. OVs in Pdx-def-In2O3 were probably generated due to the quite acidic condition of K2PdCl4 solution with a pH value of 3.84, lower than that of (NH4)2PdCl4 solution with pH = 4.20. Pdx-In2O3 with no OVs was prepared under identical conditions except the usage of (NH4)2PdCl4 instead of K2PdCl4.
Characterizations
XRD were measured to obtain the crystalline structure on the D8 ADVANCE diffractometer (Bruker Co., Ltd). Palladium and potassium contents were measured through inductively coupled plasma optical emission spectrometer (ICP-OES) (Agilent 5110 ICP-OES/MS). Chlorine contents in the photocatalyst were analyzed by the ion chromatography (ICS-5000). Both K+ and Cl- ions did not exist in the as-prepared photocatalyst according to the measurements (Table S1). Raman spectra were collected on the DXR 2DXR2 instrument (Thermo Fisher Scientific, Co., Ltd). HRTEM images were captured on the Talos F200X instrument (FEI Co., Ltd). UV-DRS spectra were measured on the UV-3600 plus spectrophotometer (Shimadzu Co., Ltd). The extend X-ray absorption fine structure (EXAFS) spectra were collected at BL14W1 station in Shanghai Synchrotron Radiation Facility. Photocurrent test was conducted on the electronic workstation (CHI660E) on the three-electrode system. Ag/AgCl electrode, platinum sheet electrode and Na2SO4 solution (0.1 M) were used as the reference electrode, counter electrode and electrolyte, respectively. The mixtures of photocatalyst, ethanol and Nifion solution (Shanghai Adamas Reagent Co., Ltd) were suspended and sonicated to prepare the working electrode. In situ XPS in dark and under light irradiation for different irradiation time were measured on the Thermo ESCALAB 250Xi instrument with an Al Kα radiation source. In situ solid-state EPR spectra in dark and under light irradiation were measured at room temperature with 20 mg photocatalyst on the ELEXSYS II EPR instrument.
Photocatalytic CH4 conversion
Photocatalytic CH4 conversion was conducted in a top-irradiated high-pressure reactor with 200 ml volume. LED lamp (420 nm, PLS-LED100C, Beijing Perfectlight Technology Co., Ltd) was used as the light source. Radiation spectrum of the LED lamp contained only visible light centering at 421 ± 7 nm (Fig. S15). Typically, 20 mg photocatalyst was dispersed in 50 ml distilled water. After sealing and purging with ultrapure O2 (99.999 vol.%) for 20 min, 1 bar O2 and 19 bar CH4 (99.999 vol.%) were flowed into the reactor. The temperature of the reactor was maintained at 25 °C by the cold-water bath. After reacting for 3 h, the gaseous and liquid products were measured by the gas chromatography (GC2014, Shimadzu Co., Ltd) equipped with thermal conductivity detector and flame ionization detector, which showed that no CO product was detected for all of the photocatalytic reactions under the present experimental conditions. CH3OOH was measured through 1H nuclear magnetic resonance (NMR) (AVANCE III, JEOL Ltd). As CH3OOH and CH3OH have the same number of methyl, the area ratio of CH3OOH to CH3OH in 1H NMR should be the molar ratio of CH3OOH to CH3OH. Thus, CH3OOH was quantified with 1H NMR. The representative GC and NMR curves were shown in Figs. S16 and S17. HCHO was measured through the colorimetric method on the UV-3600 Plus spectrometer (Shimadzu Co., Ltd)67.
AQE was then measured. In the experiment, 100 mg Pd-def-In2O3 photocatalyst was first dispersed in 100 ml distilled water. Then the suspension was stirred and purged with ultrapure O2 (99.999 vol.%) for 20 min. After flowing 1 bar O2 and 19 bar CH4 into the reactor, the reaction was conducted for 2 h with a Xe lamp as the light source equipped with a 420 nm bandpass filter. Light intensity was measured as 36.6 mW cm−2 by the light intensity meter (PL-MW2000, Beijing Perfectlight Technology Co., Ltd). The as-produced CH3OOH, CH3OH and HCHO were 19.1, 7.6 and 8.4 μmol, respectively. Since the formations of CH3OOH, CH3OH and HCHO needed 1, 3 and 5 photogenerated charges1, respectively, AQE was calculated based on the following equation:
n(CH3OOH), n(CH3OH) and n(HCHO) represent the number of CH3OOH, CH3OH and HCHO, respectively, and n(photons) represents the number of the irradiated photons during methane conversion.
Isotope labeling experiments
For carbon source investigation: 20 mg Pd-def-In2O3 photocatalyst was dispersed in 3 ml H2O. After the reactor being degassed for 30 min, 1 bar O2 and 5 bar 13CH4 were injected into the reactor. After reacting for 6 h, the suspension was filtered and then the solvent was analyzed by GC-MS (QP2020, Shimadzu Co., Ltd) equipped with the Cap WAX column.
For oxygen source investigation: 20 mg Pd-def-In2O3 photocatalyst was dispersed in 3 ml H216O or H218O. After the reactor being degassed for 30 min, 1 bar 18O2 or 16O2 and 5 bar CH4 were injected into the reactor. After reacting for 6 h, the suspension was filtered and then the solvent was analyzed by GC-MS (QP2020, Shimadzu Co., Ltd).
Monitor of the reactive species
DMPO was used as the spin-trapping agent to monitor the reactive species including ·OOH and ·OH radicals. For ·OOH radical detection, 10 mg Pd-def-In2O3 photocatalyst was dispersed into 5 ml DMPO/methanol solution. After purging with ultrapure O2 (99.999 vol.%) for 20 min, in situ EPR spectra in dark and under light irradiation were collected. For ·OH radical detection, 10 mg Pd-def-In2O3 photocatalyst was dispersed in 5 ml aqueous DMPO solution. After purging with ultrapure O2 (99.999 vol.%) for 20 min, in situ EPR spectra in dark and under light irradiation were collected.
Analysis of hydroxyl radical (·OH)
Coumarin was used as the probe for the quantification of ·OH via the production of 7-HC41. Typically, 20 mg photocatalyst was dispersed in 100 ml aqueous coumarin solution (5 × 10−4 M). After stirring for 30 min in dark, the suspension was irradiated with the LED light source (420 nm, PLS-LED100C, Beijing Perfectlight Technology Co., Ltd). Certain amount of suspension was sampled and filtered in the 10 min intervals. PL intensity of the produced 7-HC in the solution was then measured on the F4500 spectrofluorometer.
DFT calculation of adsorption energies
The first-principles were employed to perform all the DFT calculations within the generalized gradient approximation using the PBE formulation. The projected augmented wave potentials have been chosen to describe the ionic cores and take valence electrons into account using a plane wave basis set with a kinetic energy cutoff of 400 eV. Partial occupancies of the Kohn–Sham orbitals were allowed using the Gaussian smearing method and a width of 0.05 eV. The electronic energy was considered self-consistent when the energy change was smaller than 10−4 eV. A geometry optimization was considered convergent when the force change was smaller than 0.05 eV/Å. Grimme’s DFT-D3 methodology was applied to describe the dispersion interactions. During structural optimizations, the 2 × 2 × 1 Monkhorst-Pack k-point grid for Brillouin zone was used for k-point sampling for structures. Finally, the adsorption energies (Eads) were calculated as Eads = Ead/sub − Ead − Esub, where Ead/sub, Ead, and Esub were the total energies of the optimized adsorbate/substrate system, the adsorbate in the structure, and the clean substrate, respectively.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Li, X., Wang, C. & Tang, J. Progress and Challenges of Methane Transformation by Photocatalysis, Nat. Rev. Mater. https://doi.org/10.1038/s41578-022-00422-3 (2022).
Sushkevich, V. L., Palagin, D., Ranocchiari, M. & Bokhoven, J. A. V. Selective anaerobic oxidation of methane enables direct synthesis of methanol. Science 356, 523–527 (2017).
Sher Shah, M. S. A. et al. Catalytic oxidation of methane to oxygenated products: recent advancements and prospects for electrocatalytic and photocatalytic conversion at low temperatures. Adv. Sci. 7, 2001946 (2020).
Hu, D., Ordomsky, V. V. & Khodakov, A. Y. Major routes in the photocatalytic methane conversion into chemicals and fuels under mild conditions. Appl. Catal. B-Environ. 286, 119913 (2021).
Meyet, J. et al. Monomeric copper(II) sites supported on alumina selectively convert methane to methanol. Angew. Chem. Int. Ed. 58, 9841–9845 (2019).
Ravi, M., Ranocchiari, M. & van Bokhoven, J. A. The direct catalytic oxidation of methane to methanol—a critical assessment. Angew. Chem. Int. Ed. 56, 16464–16483 (2017).
Zakaria, Z. & Kamarudin, S. K. Direct conversion technologies of methane to methanol: an overview. Renew. Sust. Energ. Rev. 65, 250–261 (2016).
Song, H., Meng, X., Wang, Z.-J., Liu, H. & Ye, J. Solar-energy-mediated methane conversion. Joule 3, 1606–1636 (2019).
Agarwal, N. et al. Aqueous Au-Pd colloids catalyze selective CH4 oxidation to CH3OH with O2 under mild conditions. Science 358, 223–227 (2017).
Hammond, C. et al. Direct catalytic conversion of methane to methanol in an aqueous medium by using copper-promoted Fe-ZSM-5. Angew. Chem. Int. Ed. 51, 5129–5133 (2012).
Okolie, C. et al. Conversion of methane into methanol and ethanol over nickel oxide on ceria-zirconia catalysts in a single reactor. Angew. Chem. Int. Ed. 56, 13876–13881 (2017).
Ma, J. et al. Efficient photoelectrochemical conversion of methane into ethylene glycol by WO3 nanobar arrays. Angew. Chem. Int. Ed. 60, 9357–9361 (2021).
Zhou, Y. et al. Direct piezocatalytic conversion of methane into alcohols over hydroxyapatite. Nano Energy 79, 105449 (2021).
Schwach, P., Pan, X. & Bao, X. Direct conversion of methane to value-added chemicals over heterogeneous catalysts: challenges and prospects. Chem. Rev. 117, 8497–8520 (2017).
Tian, J. et al. Direct conversion of methane to formaldehyde and CO on B2O3 catalysts. Nat. Commun. 11, 5693 (2020).
Yu, T. et al. Highly selective oxidation of methane into methanol over Cu-promoted monomeric Fe/ZSM-5. ACS Catal. 11, 6684–6691 (2021).
Jin, Z. et al. Hydrophobic zeolite modification for in situ peroxide formation in methane oxidation to methanol. Science 367, 193–197 (2020).
Wang, Y. et al. Unique hole-accepting carbon-dots promoting selective carbon dioxide reduction nearly 100% to methanol by pure water. Nat. Commun. 11, 2531 (2020).
Wang, Y., Godin, R., Durrant, J. R. & Tang, J. Efficient hole trapping in carbon dot/oxygen-modified carbon nitride heterojunction photocatalysts for enhanced methanol production from CO2 under neutral conditions. Angew. Chem. Int. Ed. 60, 20811–20816 (2021).
Han, Q. et al. Rational design of high-concentration Ti3+ in porous carbon-doped TiO2 nanosheets for efficient photocatalytic ammonia synthesis. Adv. Mater. 33, e2008180 (2021).
Zhou, W. et al. Highly selective aerobic oxidation of methane to methanol over gold decorated zinc oxide via photocatalysis. J. Mater. Chem. A 8, 13277–13284 (2020).
Xie, J. et al. Highly selective oxidation of methane to methanol at ambient conditions by titanium dioxide-supported iron species. Nat. Catal. 1, 889–896 (2018).
Feng, N. et al. Efficient and selective photocatalytic CH4 conversion to CH3OH with O2 by controlling overoxidation on TiO2. Nat. Commun. 12, 4652 (2021).
Luo, L. et al. Binary AuCu reaction sites decorated ZnO for selective methane oxidation to C1 oxygenates with nearly 100% selectivity at room temperature. J. Am. Chem. Soc. 144, 740–750 (2022).
Wei, S. et al. Aerobic oxidation of methane to formaldehyde mediated by crystal-O over gold modified tungsten trioxide via photocatalysis. Appl. Catal. B-Environ. 283, 119661 (2021).
Fan, Y. et al. Selective photocatalytic oxidation of methane by quantum-sized bismuth vanadate. Nat. Sustain. 4, 509–515 (2021).
Zhou, Y., Zhang, L. & Wang, W. Direct functionalization of methane into ethanol over copper modified polymeric carbon nitride via photocatalysis. Nat. Commun. 10, 506 (2019).
Yang, Z. et al. Efficient photocatalytic conversion of CH4 into ethanol with O2 over nitrogen vacancy-rich carbon nitride at room temperature. Chem. Commun. 57, 871–874 (2021).
Yu, X. et al. Stoichiometric methane conversion to ethane using photochemical looping at ambient temperature. Nat. Energy 5, 511–519 (2020).
Li, X., Xie, J., Rao, H., Wang, C. & Tang, J. Pt and CuOx decorated TiO2 photocatalyst for oxidative coupling of methane to C2 hydrocarbons in a flow reactor. Angew. Chem. Int. Ed. 59, 19702–19707 (2020).
Ishimaru, M., Amano, F., Akamoto, C. & Yamazoe, S. Methane coupling and hydrogen evolution induced by palladium-loaded gallium oxide photocatalysts in the presence of water vapor. J. Catal. 397, 192–200 (2021).
Yu, L., Shao, Y. & Li, D. Direct combination of hydrogen evolution from water and methane conversion in a photocatalytic system over Pt/TiO2. Appl. Catal. B-Environ. 204, 216–223 (2017).
Chen, Z. et al. Non‐oxidative coupling of methane: n-type doping of niobium single atoms in TiO2-SiO2 induces electron localization. Angew. Chem. Int. Ed. 60, 11901–11909 (2021).
Li, N. et al. Plasma-assisted photocatalysis of CH4 and CO2 into ethylene. ACS Sustain. Chem. Eng. 7, 11455–11463 (2019).
Cho, Y. et al. Disordered-layer-mediated reverse metal–oxide interactions for enhanced photocatalytic water splitting. Nano Lett. 21, 5247–5253 (2021).
Ma, J. et al. Exploring the size effect of Pt nanoparticles on the photocatalytic nonoxidative coupling of methane. ACS Catal. 11, 3352–3360 (2021).
Song, H. et al. Selective photo-oxidation of methane to methanol with oxygen over dual-cocatalyst-modified titanium dioxide. ACS Catal. 10, 14318–14326 (2020).
Chen, F., Ma, T., Zhang, T., Zhang, Y. & Huang, H. Atomic-level charge separation strategies in semiconductor-based photocatalysts. Adv. Mater. 33, 2005256 (2021).
Ling, T., Jaroniec, M. & Qiao, S.-Z. Recent progress in engineering the atomic and electronic structure of electrocatalysts via cation exchange reactions. Adv. Mater. 32, 2001866 (2020).
Lee, B.-H. et al. Reversible and cooperative photoactivation of single-atom Cu/TiO2 photocatalysts. Nat. Mater. 18, 620–626 (2019).
Nosaka, Y. & Nosaka, A. Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 117, 11302–11336 (2017).
Xiong, L. & Tang, J. Strategies and challenges on selectivity of photocatalytic oxidation of organic substances. Adv. Energy Mater. 11, 2003216 (2021).
Lin, L. et al. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts. Nature 544, 80–83 (2017).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Wang, C. et al. Co and Pt dual‐single‐atoms with oxygen‐coordinated Co-O-Pt dimer sites for ultrahigh photocatalytic hydrogen evolution efficiency. Adv. Mater. 33, 2003327 (2021).
Guo, W., Wang, Z., Wang, X. & Wu, Y. General design concept for single-atom catalysts toward heterogeneous catalysis. Adv. Mater. 33, e2004287 (2021).
Zhang, W. et al. Emerging dual‐atomic‐site catalysts for efficient energy catalysis. Adv. Mater. 33, 2102576 (2021).
Li, Q., Ouyang, Y., Li, H., Wang, L. & Zeng, J. Photocatalytic conversion of methane: recent advancements and prospects. Angew. Chem. Int. Ed. 61, e202108069 (2021).
Jiang, W. et al. Pd-modified ZnO-Au enabling alkoxy intermediates formation and dehydrogenation for photocatalytic conversion of methane to ethylene. J. Am. Chem. Soc. 143, 269–278 (2020).
Lv, D. et al. Effect of Pd and Au on hydrogen abstraction and C–C cleavage in photoconversion of glycerol: beyond charge separation. J. Phys. Chem. C. 124, 20320–20327 (2020).
Wolff, C. M. et al. All-in-one visible-light-driven water splitting by combining nanoparticulate and molecular co-catalysts on CdS nanorods. Nat. Energy 3, 862–869 (2018).
Wang, Q. et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat. Mater. 15, 611–615 (2016).
Qi, Y. et al. Redox-based visible-light-driven Z-scheme overall water splitting with apparent quantum efficiency exceeding 10%. Joule 2, 2393–2402 (2018).
Latimer, A. A., Kakekhani, A., Kulkarni, A. R. & Nørskov, J. K. Direct methane to methanol: the selectivity–conversion limit and design strategies. ACS Catal. 8, 6894–6907 (2018).
Shanmuganathan, V., Kumar, J. S., Pachaiappan, R. & Thangadurai, P. Transition metal ion-doped In2O3 nanocubes: investigation of their photocatalytic degradation activity under sunlight. Nanoscale Adv. 3, 471–485 (2021).
Xu, C. Y., Zhang, P. X. & Yan, L. Blue shift of Raman peak from coated TiO2 nanoparticles. J. Raman Spectrosc. 32, 862–865 (2001).
Siedl, N., Gügel, P. & Diwald, O. First combined electron paramagnetic resonance and FT-IR spectroscopic evidence for reversible O2 adsorption on In2O3–x nanoparticles. J. Phys. Chem. C. 117, 20722–20799 (2013).
Reyes-Gil, K. R., Sun, Y., Reyes-García, E. & Raftery, D. Characterization of photoactive centers in N-Doped In2O3 visible photocatalysts for water oxidation. J. Phys. Chem. C. 113, 12558–12570 (2009).
Loh, J. Y. Y., Mohan, A., Flood, A. G., Ozin, G. A. & Kherani, N. P. Waveguide photoreactor enhances solar fuels photon utilization towards maximal optoelectronic—photocatalytic synergy. Nat. Commun. 12, 402 (2021).
Wang, G. et al. Synergistic modulation of the separation of photo-generated carries via engineering of dual atomic sites for promoting photocatalytic performance. Adv. Mater. 33, 2105904 (2021).
Li, L. et al. Sub-10 nm rutile titanium dioxide nanoparticles for efficient visible-light-driven photocatalytic hydrogen production. Nat. Commun. 6, 5881 (2015).
Zorić, I., Zäch, M., Kasemo, B. & Langhammer, C. Gold, platinum, and aluminum nanodisk plasmons: material independence, subradiance, and damping mechanisms. ACS Nano 5, 2535–2546 (2011).
García-Trenco, A. et al. PdIn intermetallic nanoparticles for the hydrogenation of CO2 to methanol. Appl. Catal. B-Environ. 220, 9–18 (2018).
Zhu, W. et al. Facet-dependent enhancement in the activity of bismuth vanadate microcrystals for the photocatalytic conversion of methane to methanol. ACS Appl. Nano Mater. 1, 6683–6691 (2018).
Clément, J.-L. et al. Assignment of the EPR spectrum of 5,5-Dimethyl-1-pyrroline N-Oxide (DMPO) superoxide spin adduct. J. Org. Chem. 70, 1198–1203 (2005).
Wang, L. et al. Photocatalytic hydrogenation of carbon dioxide with high selectivity to methanol at atmospheric pressure. Joule 2, 1369–1381 (2018).
Chen, J., Stepanovic, S., Draksharapu, A., Gruden, M. & Browne, W. R. A non-heme iron photocatalyst for light-driven aerobic oxidation of methanol. Angew. Chem. Int. Ed. 57, 3207–3211 (2018).
Acknowledgements
L.L., L.F., H.L. and J.X. are grateful for the China Postdoctoral Science Foundation (No. 2019M663802) and the Shannxi Key Research Grant (No. 2020GY−244). Y.X. and J.T. are thankful for financial support from the UK EPSRC (EP/S018204/2), Leverhulme Trust (RPG−2017-122), Royal Society Newton Advanced Fellowship grant (NAF/R1/191163) and Royal Society Leverhulme Trust Senior Research Fellowship (SRF/R1/21000153).
Author information
Authors and Affiliations
Contributions
J.T. conceived and supervised the entire project. L.L., L.F. and H.L. conducted the material synthesis, characterizations and photocatalytic methane conversion tests. L.L. drafted the manuscript under the guidance of J.T., Y.X. and J.X. helped to discuss the catalytic results and improve the manuscript. C.-R.C. and D.-Y.Y. contributed to DFT calculations. All authors discussed the results, commented on the manuscript and contributed to the revision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Fumiaki Amano and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luo, L., Fu, L., Liu, H. et al. Synergy of Pd atoms and oxygen vacancies on In2O3 for methane conversion under visible light. Nat Commun 13, 2930 (2022). https://doi.org/10.1038/s41467-022-30434-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-30434-0
This article is cited by
-
Insight into selectivity of photocatalytic methane oxidation to formaldehyde on tungsten trioxide
Nature Communications (2024)
-
In-situ transformed Mott-Schottky heterointerface in silver/manganese oxide nanorods boosting oxygen reduction, oxygen evolution, and hydrogen evolution reactions
Nano Research (2024)
-
Selective photocatalytic oxidation of methane to C1 oxygenates by regulating sizes and facets over Au/ZnO
Nano Research (2024)
-
Internal quantum efficiency higher than 100% achieved by combining doping and quantum effects for photocatalytic overall water splitting
Nature Energy (2023)
-
Sustainable methane utilization technology via photocatalytic halogenation with alkali halides
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.