Abstract
The use of anion redox reactions is gaining interest for increasing rechargeable capacities in alkaline ion batteries. Although anion redox coupling of S2− and (S2)2− through dimerization of S–S in sulfides have been studied and reported, an anion redox process through electron hole formation has not been investigated to the best of our knowledge. Here, we report an O3-NaCr2/3Ti1/3S2 cathode that delivers a high reversible capacity of ~186 mAh g−1 (0.95 Na) based on the cation and anion redox process. Various charge compensation mechanisms of the sulfur anionic redox process in layered NaCr2/3Ti1/3S2, which occur through the formation of disulfide-like species, the precipitation of elemental sulfur, S–S dimerization, and especially through the formation of electron holes, are investigated. Direct structural evidence for formation of electron holes and (S2)n− species with shortened S–S distances is obtained. These results provide valuable information for the development of materials based on the anionic redox reaction.
Similar content being viewed by others
Introduction
The discovery of anionic redox chemistry in layered compounds was traced back to ligand–hole chemistry in chalcogenides, in which S22−, Se22−, or Te22− ions in layered structures may be involved1,2,3,4,5,6. In recent years, attention is boosted by interest in anionic redox in oxides for high-energy Li-ion batteries7,8,9,10,11,12. These anionic redox-based oxides, such as layered Li1.2Ni0.13Mn0.54Co0.13O2 (Li-rich NMC) provide nearly twice as much capacity as that of LiCoO2 and LiNi1/3Mn1/3Co1/3O2 (NMC) cathodes13,14,15. The reversible activity of lattice oxygen in Li-rich NMC should be responsible for their extraordinary capacities16,17. In these compounds, the high electrochemical activity of O 2p non-bonding states is revealed18,19,20. The reversible formation of O–O dimers, as well as electron holes on O can also be confirmed in Li2IrO3, Li2RuO3, Na3RuO418,21,22, and Li1.2Ni0.13Co0.13Mn0.54O217, respectively. Accordingly, the questions are whether these concepts could be mutually corroborated by other systems, such as layered chalcogenides, whether the reversible formation and decomposition of S–S dimers or electron holes on S could be evidenced, and what is the underlying nature of anionic redox chemistry in layered chalcogenides. The reversible anionic reduction/oxidation of (S2)2− + 2e− ↔ 2S2− was reported previously in non-layered polysulfide electrode materials23,24,25,26,27,28 and in layered materials confirmed by X-ray photoelectron spectroscopy (XPS) and X-ray absorption spectroscopy (XAS) techniques29,30,31. However, there has been a lack of core structural evidence on the formation of S–S dimers or electron holes on S in layered chalcogenides. In fact, the anionic redox activities from dimers or from electron holes is still controversial17,32,33, which is found to be dependent on the composition and structure of the electrode. Here, we report direct structural evidences for the presence of (S2)n− species with shortened S–S distances in the anionic redox processes in O3-NaCr2/3Ti1/3S2 as a model layered cathode. The highly reversible formation and decomposition of S–S dimers driven by the migration of Cr ions are directly observed. The anionic redox chemistry of sulfur involving the formation of localized electron holes, anionic dimers, disulfide-like species, as well as the precipitation of elemental sulfur are in layered NaCr2/3Ti1/3S2, in which the possible routes of anionic redox processes from the electron holes to element are clarified. A new pathway to engineer the anionic redox process for the optimal balance between the extra capacities boosted by S2−/S− redox is explored.
Results
Enhanced capacity by anionic and cationic redox couples
The members of NaCr1−yTiyS2 series were synthesized by high-temperature solid-state reactions using the mixture of powdered Na2S, S, Ti, and Cr in various stoichiometric ratios. All the NaCr1−yTiyS2 series have O3 type structure, similar to NaTiS2 and NaCrS2 (the preliminary structural characterizations are shown in Supplementary Figs. 1, 2 and Supplementary Table 1). The electrochemical performance of NaCr1−yTiyS2 compounds was tested versus Na and cycled between 1.4 and 3.3 V (1.5–4.0 V for NaCrS2) at the current rate of C/10 and the charge/discharge curves of the initial three cycles are shown in Fig. 1a. The capacity of pure NaCrS2 comes mainly from the high voltage range above 2.55 V, corresponding to the redox between S2− and S22−31, with a characteristic of large polarization about 0.6 V. As contrast, the capacity of NaTiS2 comes mainly from low voltage range below 2.55 V, corresponding to the redox of Ti3+34, with a small polarization about 0.05 V. These features can be observed in the curves of NaCr1−yTiyS2 series, which show small polarization in low voltage range and large polarization in high voltage range. The proportion of capacity from high/low voltage range relates to the ratio of Cr/Ti doped. Among the NaCr1−yTiyS2 series, NaCr2/3Ti1/3S2 shows the highest capacity of 186 mAh g−1 in the second cycle, corresponding to the reversible deintercalation of 0.95 Na per unit. It can be found that three main stages in the charging process of NaCr2/3Ti1/3S2 consist of the first plateau between 1.4 and 2.55 V, the slope between 2.55 and 2.85 V and the second plateau at 2.90 V (Fig. 1b). The capacity retentions with 69% of the first cycle is obtained after 50 cycles in the voltage range of 1.4–3.3 V, but the capacity remains unattenuated in the stage of 1.4–2.55 and 2.55–2.85 V (Fig. 1b inset). The capacity in the range of 1.4–2.55 V is 65 mAh g−1, which conforms exactly to the Ti3+/4+ fully redox. Both the stages of 2.55–2.85 and 2.85–3.3 V correspond to the redox processes of sulfur confirmed via in situ X-ray absorption near-edge structure (XANES) spectra (see the “Methods” section for XAS details), standing for the redox of S2−/Sn− and Sn−/(S2)n−, respectively. An intuitionistic pie chart representing the increase of capacity is shown in Supplementary Fig. 3. Significantly, more sulfur can be activated by the addition of Ti (33.33%) into NaCrS2 to produce higher capacity. The cyclic voltammetry test (Fig. 1c) at a scan rate of 0.01 mV s−1 shows three single anodic peaks at 1.7, 2.7, and 2.9 V standing for the oxidation processes of Ti/S/S, respectively, as mentioned above. Their corresponding three cathodic peaks locate at 1.6, 2.3, and 2.5 V, respectively. Rate performances were also tested between 0.1–1 and 0.1–10 C. The electrode showed good rate performance and could achieve the capacity of at least 80 mAh g−1 even under such a high rate of 10 C (see Supplementary Fig. 4). After 360 cycles under 1/3 C rate, the capacity retention can reach 52.2% (see Supplementary Fig. 5). Comparing to other metal polysulfide electrodes24,25,26,27,28 for lithium or sodium batteries (Supplementary Table 2), it is clearly shown that poor cycling stability is a common problem to be solved in metal sulfide electrodes driven by anion redox reactions, to which the highly reversibility of electron holes might be a potential solution as we discuss later.
Electrochemical performance of NaCr1−yTiyS2 series. a Voltage profile for the NaCr1−yTiyS2 series versus Na+/Na at C/10 rate. The Coulombic efficiency of NaCr2/3Ti1/3S2 at the first cycle is superior to 100% for there are few Na vacancies in the pristine material without Na precursor excess. b The voltage profile over first 50 cycles for a Na/NaCr2/3Ti1/3S2 cell together with (as inset) its capacity retention in range of 1.4–2.55, 2.55–2.85 and 2.95–3.3 V at C/10 rate. c First four cyclic voltammograms for the NaCr2/3Ti1/3S2 electrode cycled between 3.3 and 1.4 V
For further insight into the structural changes of NaCr2/3Ti1/3S2 electrode in desodiation processes, in situ XRD test was performed (Fig. 2a). The sodium deintercalation/intercalation process during the first cycle is highly reversible for the whole contour is symmetrical, and the structure after discharging to 1.4 V comes back to that of the pristine. Three corresponding stages in Fig. 1 can be further analyzed in Fig. 2a. The initial structure is O3 and the first stage corresponds to O3 → P3 transition. Then the solid solution reaction is occurred in P3 phase, which corresponds to the slope part in charge curve with shortening of a/b lattice length and elongation along the c-axis. The third part is a P3-O1′ transition, which corresponds to the second plateau in Fig. 1b. A huge reduction in lattice parameter of c occurred, implying the structural contraction after complete desodiation. The calculated cell parameters during the first charging process in Fig. 2b show that the lattice parameter of c, with considering the Cr migration, is closer to the experimental value. The c length slightly decreased with the migration of Cr ions to Na vacancy sites because the electrostatic attraction between S layers becomes stronger.
Structural evolution of NaCr2/3Ti1/3S2 during cycling. a In situ XRD contour for NaCr2/3Ti1/3S2 cell cycling at a rate of C/5. The constant intensity peaks at 38.54°, 41.98°, and 44.82° are peaks of Al foil. b Lattice parameters calculated from the in situ XRD and from density functional theory (DFT) calculations as functions of composition during the first charge. For easy comparison, c length of full charged O1′ phase is three timed to accord with O3/P3 phase
Explicit support of redox process via in situ/operando XAS
The XAS is a powerful tool to directly provide the information of valence electrons in the vicinity of Fermi level, which is sensitive to the elements, chemical bonds, and oxidation states. Figure 3a–c show a complete in situ XAS dataset for Ti, Cr, and S during the charge and discharge process of NaCr2/3Ti1/3S2/Na battery cycled at C/10. The in situ XAS evolve contours of three elements, exhibiting overall asymmetry, which shows the similarity to their corresponding charge and discharge profiles. Several features from three elements during the charge process are listed in the selected spectra shown in Fig. 3d–f, respectively. It can be seen that during the whole charge process the Cr K-edge XAS is hardly changed, as its corresponding mapping result shows, indicating that Cr ions do not participate in the electrochemical redox reactions. Only a small rise in pre-edge, corresponding to the transition of Cr 1s electron to the 3d-t2g and 3d-eg orbitals through the hybridization of S-3p and Cr-3d states, is observed in stage 3 that might be resulting from the local distortion of Cr–S octahedron caused by movement of S. On the contrary, the Ti K-edge XAS spectra change in the first stage, the main peak located at ∼4977.8 eV shifts continuously toward high energy, finally reaches 4978.8 eV and the pre-edge slightly rises due to holes generated on Ti3dS3p partly admixed orbitals or due to the change of chemical environment between O3–P3 phase transition. The Na constant curve of Ti K-edge XAS spectra implies that there is no further oxidation reaction for Ti in subsequent stages and the oxidation reaction of Ti3+ have been finished in the first stage. The most significant spectral variations at different state of charge are found in the XAS spectra at sulfur K-edge. First of all, during the first stage, the S K-edge is hardly changed. In the second stage, the shoulder located at ~2468 eV is gradually grown. Such a shoulder is attributed to the hybridization between the Cr 3d and S 3p orbitals. According to the structural evolution of solid solution P3 phase in this charging stage, the generation of electron holes on sulfur sites should be responsible for the formation of the typical shoulder, indicating the charge compensation taking place on sulfur sites in this desodiation process. The growth of a new peak appearing at 2470.7 eV can be clearly seen on the third stage of charging process, which is consistent with the changes we have seen previously for NaCrS231 and in previous report of Li2FeS235. It corresponds to a newly formed localized electronic states on sulfur, probably stand for S–S σ*, resulting from the occurrence of 2S2−/(S2)n− (n < 4), i.e. the anionic redox process for charge compensation accompanied with the formation of S–S dimers36,37. The ex situ extended X-ray absorption fine structure (EXAFS) of sulfur was also collect after performing 2D X-ray fluorescence (XRF) and analyzed (see Fig. 3 bottom inset and Supplementary Figs. 6 and 7). An embedded peak raised at ~1.6 Å can be observed. This peak can be attributed to disulfide (S–S distance 2.05 Å) after considering phase correction, which is similar to the sulfur spectrum of FeS238. In addition, it should be noted that the line shape of S K-edge in XAS spectra varies systematically and its evolutions shows an isosbestic point upon desodiation process in the third stage, which indicates a dominating two-phase transformation39, and is in good agreement with the XRD. Finally, like the evolution behavior of Cr K-edge during the charging process, the Cr 3d-bands (see Supplementary Fig. 8) are almost inert to the discharging process. In the full discharging process, K-edge XAS spectra of Ti and S returns close to those of the pristine material in a symmetrical way, which indicates the reversibility of the charge compensation on Ti and S.
In situ K-edge X-ray absorption spectroscopy mapping. Mapping of a Cr, b Ti, and c S valance state, the corresponding voltage profiles for the first cycle is on the right side. d–f The divided in situ XAS K-edge XAS spectra of three stages as marked in the bottom inset during the first charge process. The inset in the bottom part is converted R space of ex situ sulfur K-edge EXAFS for pristine and full charged samples, showing an embedded peak of disulfide S–S distance
Following the trail of S–S dimer formation in microstructure
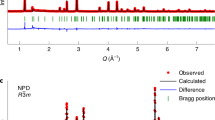
To further assess the reason behind the charge compensation during the first cycle, we tested the NaCr2/3Ti1/3S2 electrode at three stages in charging process by XRD, selected-area electron diffraction (SAED), and high-angle annular dark field-scanning transmission electron microscopy (HAADF-STEM). The Rietveld refined XRD schemes are presented in Fig. 4a, b. In the refined results, no crystalline impurity was observed. The structure parameters refined are listed in Supplementary Tables 3 and 4.
Structure characterization. a XRD refinement patterns of powder NaCr2/3Ti1/3S2 (red circles), fitted profile (black solid line), and difference (purple solid line) using synchrotron XRD (wavelength 0.6884 Å). Bragg positions are indicated as green vertical tick marks. The inset is structure schematic of NaCr2/3Ti1/3S2 observed along a-axis, legend: orange (Na), blue (Cr/Ti), and yellow balls (S). Impurity peaks at 11° and 14° can be attributed to Na2SO3 as a common impurity in commercial Na2S. b XRD refinement patterns of powder Na0.66Cr2/3Ti1/3S2 and structure schematic of Na0.66Cr2/3Ti1/3S2 as inset. c Selected-area electron diffraction (SAED) pattern of NaCr2/3Ti1/3S2 taken from [010] zone axis. d SAED pattern of Na0.66Cr2/3Ti1/3S2 taken from [010] zone axis. e HAADF-STEM image of the pristine NaCr2/3Ti1/3S2 particle along a/b-axis, scale bar 1 nm. f HAADF-STEM image of the Na0.66Cr2/3Ti1/3S2 particle along a/b-axis, scale bar 1 nm
The microstructures are confirmed by SAED patterns and atomic scale HADDF-STEM images (Fig. 4c–f). The SAED pattern for pristine NaCr2/3Ti1/3S2 and one-third charged Na0.66Cr2/3Ti1/3S2 (Fig. 4c, d) presents numerous diffraction points, and can be well indexed in the expected O3—the R-3m space group and P3—the P3m1 space group, respectively, which are in good agreement with the XRD results. In addition, we noticed that the shapes of diffraction points are regular, indicating good order of atoms without stacking faults or twin lamella. Figure 4e, f exhibit the images of HAADF-STEM for NaCr2/3Ti1/3S2 and Na0.66Cr2/3Ti1/3S2 projected both along [100] direction at atomic-level, showing the typical layered structures. From the STEM images it is clear that the atoms are in good order in long range and can fit the predicted structures well, no disorder area or polymorphism was found. It can be certified from the images that during the early stages of first charging process there are no obvious signals in darker layers of Na, indicating that Cr3+ ions were not migrated. Some stacking faults are captured in the following phase P3 Na0.5Cr2/3Ti1/3S2 (see Supplementary Fig. 9), the corresponding SAED result showed a multiphase diffraction pattern. The slabs of S–Cr/Ti–S are obviously slid with Na ion kept in prismatic sites. Neither formation of S–S nor migration of Cr is observed in this phase, probably because the remaining Na can form Na–S ionic bond with S to stabilize the hole in S ion, similar to the reason that no O–O species formed in Nax[Mg0.28Mn0.72]O232, which has good cyclic performance as well. This could reveal the reason for great cyclic performance in stage 2.
To track the formation of S–S dimers and the migration of Cr ions in the full charged material, STEM images were used to analyze the microstructure. When observed along c-axis, apparent differences can be found between pristine O3 and full charged O1′ structures (Fig. 5a, b). In Fig. 5a, one spot represents an atom column consisting of Na, Cr, Ti, and S, showing a typical structure built by anionic close packing, with interior angles of polygons in regular 60° and 120°. In Fig. 5b, full charged O1′ structure shows a twisted polygon with interior angles in 67° and 113°. The brighter spots represent Cr/Ti cation columns and the darker spots represent S columns. We marked a hexagon built by S atoms and it can be seen that side lengths differ and there are two opposite sides, which are quite short. Each dotted triangle in the hexagon marked three S atoms in the same layer, the average length for sides of triangle are 0.293, 0.338, and 0.347 nm, respectively. In contrast, the S–S distance in the same layer in O3–NaCr2/3Ti1/3S2 is 0.355 nm. That is a direct evidence for the formation of S–S dimers in the full charged structure, but that is not saying the S–S distance is exactly 0.293 nm, the formation of S–S dimer is caused by S atom moving around the equilibrium position, and the STEM image is statistical average result. The S–S dimer could be formed between neighboring layers according to our calculation results discussed later, similar to Li’s conclusion in Li-rich Li1.2Ni0.2Mn0.6O240. In Fig. 5c and Supplementary Fig. 10 we observe the full charged sample along a/b-axis, from the layered structure the Cr migration could clearly be seen. There are brighter points representing Cr atoms in the Na vacancies. From the intensity profiles between cation layers (on the right side) it can be seen that the blue colored peaks are much higher than the red colored peaks, so it can be concluded that blue peaks represents Cr/Ti atoms and red peak represent small amount of migrated Cr (the reason why Cr migrated instead of Ti will be discussed in calculation part by comparing the total energy and diffusion barrier), we analyzed the statistical average of peak areas and found the result between red/blue is ~0.24, that means ~29% of Cr migrated. The full deintercalation of Na can also be deduced from this figure for the uniform spacing between S layers accords with the typical spacing of S–Cr/Ti–S (0.27–0.29 nm for NaCr2/3Ti1/3S2 and TiS2, respectively), much smaller than the S–Na–S spacing (0.37–0.44 nm for NaCr2/3Ti1/3S2 and Na0.5Cr2/3Ti1/3S2). In addition, we selected three layers of S–Cr/Ti–S to show how the migration of Cr influences internal layer S–S distance and formation of S–S dimers (on the bottom side). Since the STEM image is average result of atom column, the peak intensity for Cr/Ti layer is much smoother than S layers, indicating irregular distribution of S around the equilibrium position. In situ EXAFS of Cr and ex situ EXAFS of Cr, Ti, and S were also performed to certify the migration as shown in Supplementary Figs. 11–13, respectively. In Supplementary Fig. 11a, the position of the first peak representing Cr–S distance decreases in the charging process and increases in the discharging process, which is in good agreement with DFT calculation results (see Supplementary Fig. 14). The intensity of the second peak decreases in the charging process, indicating the generation of Cr vacancy and migration of Cr atoms. The second shell peaks do not completely disappear at charged state in in situ EXAFS data shown in Supplementary Fig. 10e, excluding the possibility of complete amorphization.
Characterization of full charged O1′ NaCr2/3Ti1/3S2 and after cycling. STEM image of a, the pristine NaCr2/3Ti1/3S2 particle along c-axis, scale bar 1 nm. b the full charged Na0Cr2/3Ti1/3S2 particle along c-axis, scale bar 1 nm. c The full charged Na0Cr2/3Ti1/3S2 particle along a/b-axis, scale bar 1 nm. d SAED pattern of full charged Na0Cr2/3Ti1/3S2 particle along [010]-axis. e SAED pattern of discharged sample after 10 cycles. f First one-third charged Na0.66Cr2/3Ti1/3S2 along a/b-axis, scale bar 10 nm, inset scale bar 5 nm. g Discharged sample after 10 cycles along a/b-axis, scale bar 100 nm
To confirm the existence of S–S bond, ex situ Raman test was performed. In Supplementary Fig. 15, the evolution of Raman peak can be observed at different charging states, a new peak at about 487 cm−1 arose in the charging process and disappeared in discharging process. According to previous reports on Li2Sx (x ≥ 2) sulfides, the S–S dimers show a Raman shift in the range of 400–600 cm−141. And comparing to the samples like FeS2, CH–S–S–S–CH3 which are certified to have S–S bond, this Raman peak could be assigned for S–S (Δν). XPS measurements were also performed to confirm the valence of sulfur in the electrochemical process. In Supplementary Fig. 16 it can be seen that in pristine sample of NaCr2/3Ti1/3S2 there are only peaks at 160.6 eV standing for S2− 2p342 and after full charging the peaks of S22−43 and higher valence like elemental sulfur and S2O32− appear. After discharging process, XPS peaks from elemental sulfur and S2O32 are observed. These results verified the formation of S22−, and that the elemental sulfur and its oxidized products are irreversible formed on the surface of electrodes.
In addition, we noted that the diffraction points (Fig. 5d) in O1′ image are more blurred if comparing to O3/P3 image in Fig. 4c, d, and there are diffraction lines along the c-axis, indicating anion defects cause by irregular distribution of S. The in situ XRD pattern of Na0Cr2/3Ti1/3S2 shows the same conclusion (Supplementary Fig. 17). (1 0–2) peak and (1 0 4) peak are broadened and their relative intensities to the (003) peak are lower than those values without any defects, indicating through-plane anion defects, similar to P2-type Nax[Fe1/2Mn1/2]O244. These defects should be accompanied by the formation of S–S dimers.
In order to figure out the reason of capacity attenuation in Fig. 1b, the SAED pattern (Fig. 5e) of full discharged sample of NaCr2/3Ti1/3S2 after 10 cycles was investigated. These diffraction lines along c-axis indicating S defect as well, and there is a set of impurity spots, stands for heterogeneous or amorphous phases, which can be directly seen in STEM image of the sample (Supplementary Fig. 18), these phases are likely to appear after several cycles and believed to be electrochemical inactive18. There is already a crack tip (Fig . 5f) in the first charging process. This kind of crack tip evolves into a long crack penetrating the whole crystal (Fig . 5g) after 10 cycles. Wang’s group has systematically studied the reason for intragranular crack generation of LiNi1/3Mn1/3Co1/3O2 under high-voltage cycling, and found the deeper Li-ion extraction under high voltage and too large changes in lattice are the primary causes45. In our study, there are indeed large changes in lattice during phase transition between Na0.5Cr2/3Ti1/3S2 and full charged Na0Cr2/3Ti1/3S2. The crack is parallel to the c-axis, corresponding to the change in c-axis length from 20.66 to 16.79 Å, which could be a main factor for crack generation. Near the edge of crack in the sample after 10 cycles, the intensity decay of sulfur layer is faster than that of Cr/Ti layer (Supplementary Fig. 19). The difference in intensity increased from 270 on 1.2 nm from the edge to 349 on the site of the edge, which means that the crack might appear accompanied by sulfur loss. The sulfur near the crack surface is tending to be oxidized by electrolyte or disproportionate into S2− and element sulfur, and the high valence oxidation products could be dissolved in electrolyte, which can further be confirmed by XPS measurements (Supplementary Fig. 20). We compared XPS spectra of the glass fiber separator before and after cycles, and found that the relative amount of S on glass fiber separator is increased by cycling. The peak area ratio between S 2p and C 1s is 0.04% for clean separator, 1.389% after 10 cycles and 3.445% after 50 cycles. It is clear that there is almost no sulfur in clean separator, and sulfur is precipitated in the form of elemental sulfur in the first 10 cycles, for its peak position is at 163.8 eV, accords with elemental sulfur46. But sulfur exists as higher valence on separator after 50 cycles, with peak position at 169.3 eV, we suppose this could be S2O32− or polythionate because of the “tip effect”47 on the surface of materials. Apparently, the formation of elemental sulfur should be responsible for the capacity attenuation and destruction of regular layer structure, resulting in the formation of localized heterogeneous or amorphous phases.
Verifying the redox process theoretically
To gain further insight into microstructure and electronic structure evolution of the charging process, density functional theory (DFT) calculations were performed to reveal the changes in crystal structure, total energy, bonding, density of state (DOS), and diffusion barriers. Figure 6a–c showed the DOSs of Cr, S, and Ti in NaCr2/3Ti1/3S2, Na0.58Cr2/3Ti1/3S2, and Na0Cr2/3Ti1/3S2, respectively. In the pristine structure Cr/Ti are all about trivalent, Ti has electronic structure of 3d1, Cr has the electronic structure of 3d3 of high spin, with three electrons spinning up in t2g orbital. In previous reports48 of NaxTiS2 and our experimental results of NaTiS2 cathode (Supplementary Fig. 21), Ti3+ cannot be totally oxidized to Ti4+, leading to a relatively low capacity of ~160 mAh g−1 comparing to theoretical capacity of 196 mAh g−1, indicating that if charge transfer all comes from Ti, Ti3+ will be oxidized only to Ti3.8+ eventually. According to the electrochemical curves in Fig. 1b, oxidation to Ti3.8+ corresponds to a capacity of 52 mAh g−1, which is exactly corresponding to the inflection point of the curve at 2.1 V. It accords with the typical voltage range for the redox of Ti3+/Ti3.8+. And oxidation to Ti4+ corresponds to a capacity of 64 mAh g−1, which corresponds to the inflection point at 2.55 V. From the analysis above, we have figured out that the capacity comes from the oxidation of Ti and S. To figure out the degree of sulfur redox, the exact valence of Ti oxidation should be clear, we also analyzed the DOS of Na0.75Cr2/3Ti1/3S2 (Supplementary Fig. 22), when Ti is oxidized to Ti3.75+, Ti3.75+ still has occupied orbital at Ef, making it possible to be further oxidized to Ti4+. The same conclusion of full oxidation of Ti3+ to Ti4+ can be drawn according to the research of Anton Van der Ven49 in NaTiS2 system, they found O1–P3 hybrid structure is stable at low Na concentration, which consists with our result. After the full oxidation of Ti, the orbital coupling of Cr 3d and S 3p can be seen from DOS image of Na0.58Cr2/3Ti1/3S2 in Fig. 6b, the DOS intensity of S 3p near Ef is higher than Cr 3d, indicating S is easier to lose electron and be oxidized. These orbitals spread across Ef, which means the orbitals are underfilled, corresponding to the conclusion that sulfur provides electron and forms underfilled 3p bands. In the full charged Na0Cr2/3Ti1/3S2, the DOS of Cr near Ef is the highest and the energy band discontinuity from the splitting of t2g and eg is weaker, implying that the octahedral field effect is weakened due to the oxidation and deviation of sulfur. In Fig. 6d we show the calculated voltage platform comparing to experimental electrochemical curve. The calculated voltage matches with the experimental value, and we calculated the phase diagram (see Supplementary Fig. 23), it can be drawn that the structure evolution process calculated is in accord with the experimental results.
Density of states and computed voltages. The DOS of a NaCr2/3Ti1/3S2, b Na0.58Cr2/3Ti1/3S2, and c Na0Cr2/3Ti1/3S2 with the Fermi level marked using vertical dashed lines. d Calculated voltage platform comparing to experimental electrochemical curve. The columns above the platform are contribution of elements in the redox process, coming from integration of DOS in the range of −0.1 to 0 eV
From the STEM images in Fig. 5c we confirmed the migration phenomenon of Cr, the migration of Cr makes it possible to form S–S bond in energy and orbital perspective. We calculated the total energy after different ratio of Cr migrated to Na vacancies. In Supplementary Fig. 24 it can be drawn that ~25% of Cr migration grants the lowest total energy, close to the 29% intensity ratio we drawn in STEM. From the optimized structure (Supplementary Fig. 25), we found the S–S bond or so-called S–S dimer formed between layers, and the S atom which formed S–S bond was once bonding with migrated Cr. From the perspective of orbital, the migration of Cr with Cr–S bonds broken will leave sulfur with isolated non-bonding p orbital, which are capable of forming S–S bonds.
To calculate the diffusion barrier for Cr migration in Na0Cr2/3Ti1/3S2, climbing-image nudged elastic band (CINEB) method is used. As shown in Supplementary Fig. 26, Cr3+ has lower diffusion barrier than Ti4+ in the first step, 0.56 eV for Cr and 1.69 eV for Ti, respectively, mainly for Ti4+ has higher electrostatic force towards S anion. The diffusion path is shown in Supplementary Fig. 27. The diffusion barrier of Cr migration for the second is 0.83 V, but the total energy after 2 Cr atoms (25% Cr in 2 × 2 × 1 cell) migrated is 1.79 eV lower than the original structure, which could provide enough energy. The reduction of energy after Cr migration mainly comes from the bond energy released in the formation of S–S and the weakening of Van der Waals repulsion between S layers.
Discussion
The formation of S–S dimers is directly observed in a model layered NaxCr2/3Ti1/3S2 and the migration of Cr to Na vacancies is revealed. In previous works on O3 LiMO2 materials50, transition metal migration is considered to be hardly reversible, and exerts negative effects, such as capacity loss, lower ion diffusivity, and increased electronic resistance. The migration of transition metal ions may lead to complete conversion to spinel type structure upon de-intercalating the alkali metal ions. However, our results claimed that the migration of Cr in NaCr1−yTiyS2 is reversible from P3 to O1′. Instead, the loss of sulfur is the key factor responsible for the capacity loss. Based on in situ XRD, in situ XAS, ex situ STEM, SAED, Raman, XPS, and DFT results, various anionic redox chemistries are proposed,
We define S–S dimer as (S2)n− (broadly speaking, 2 ≤ n < 4) here, the disulfides are defined as exactly (S2)2−, which can be considered as a special case of S–S dimer. The localized electron holes (Sn−) are formed at the top of a given 3p band with Na ions removed at the P3 evolution process in order to compensate the charge balance as expressed in Eq. (1). The crystal-field stabilization of the sulfur sublattice is degraded upon the further removal of Na+ ions. Consequently, the bonding of an oxidized Sn− ion to its original lattice site is weakened, which leads to the local stacking faults of sulfur sublattice at the end of this process. When more than 0.5 Na ions are extracted, the part-misplacement of layer sulfur in the sublattice, along with the weakening of Cr–S electrostatic attraction due to the decrease of the charge on sulfur induce ~1/4 Cr ions to migrate to Na vacancy sites, resulting in the structural transformations from P3 to O1′. The formation of (S2)n− (Eq. (2)) dimers in O1′ phase is observed by STEM, which directly shows the shortening of the S–S distance to around 0.293 nm. In the meantime, the anion defects and the formation of (S2)2− (Eq. (3)) in O1′ structure are accompanied during the Cr migration process. The irreversible oxidation or disproportionation of S22− pairs to sulfur occurs near the crack surface as mentioned before (Eq. (4)). The structural flexibility of the sulfur network can be assumed, as well as repeated cationic migrations and the breathing of S–S dimers upon cycle process. In our case, S2−/Sn− (Eq. (1)) and Sn−/(S2)n− (Eq. (2)) redox processes are similar to two types of oxygen redox in LixMyOz that were reported previously, e.g., On− in Li1.2Ni0.13Co0.13Mn0.54O217 and (O2)n− in Li2IrO311. It can be concluded that localized electron holes on the anions and isolated non-bonding anion p orbitals are prerequisites to form (S2)n− species, which is in accord with the mechanism elucidated in oxide electrode systems17.
In this work, a novel layered NaCr2/3Ti1/3S2 is successfully synthesized and the capacity is boosted from ~100 mAh g−1 in NaCrS2 to ~186 mAh g−1 in this system. The extra capacity is attributed to a synergy effect on the anionic ((S2−/Sn−), (Sn−/(S2)n−)) and cationic (Ti3+/4+) redox. The electrochemical effect of Ti3+ dopants, which has 3d orbital above S 3p orbital, can provide ~1/3 of the capacity. In addition, it makes the structure transform to a more stable P3 phase in low Na content and the oxidized Ti4+ can stabilize the structure as well. The dimers in O1′ are stabilized by Cr3+/Ti4+ − (S2)n− covalent interactions as the bottom portion of S-3p band merges with the Fermi level. Various anionic redoxes of sulfur are controllable at different state of charge. Normally, it is difficult to distinguish different kinds of anionic redox activities because of the composition and structural complexity of the electrode. Our experimental results provide core evidence to reveal various charge compensation mechanisms, involving the formation of electron holes, anionic dimers, disulfide-like species, as well as the precipitation of elemental sulfur. Our results have demonstrated possible routes of anionic redox processes from electron holes to element. These results provide a insight to clarify the nature of an anionic redox process and help to establish a comprehensive scientific understanding for anionic chemistry.
Methods
Sample preparation
To synthesize the NaCr1−yTiyS2 series materials, the mixture of Na2S, Ti, S, and Cr powder in a stoichiometry of 3:2:9:4 was compressed and placed into carbon-coated quartz tubes. Then the mixture was slowly heated to 700 °C, kept for 10 h, and then cooled down slowly for over 5 h to room temperature. All preparation was under argon atmosphere unless otherwise noted.
Electrochemical characterization
A slurry of as-prepared NaCr1−yTiyS2 (70 wt%), conductive carbon black (20 wt%), and polyvinylidenefluoride (PVDF, Sigma-Aldrich, 10 wt%) dispersed in N-methyl-2-pyrrolidone (NMP, Sigma-Aldrich) was coated on aluminum foil. The electrolyte consisted of 1 M NaClO4 dissolved in 19:19:2 volume ethylene carbonate/dimethyl carbonate/fluoroethylene carbonate. Then 2032-type coin cell was assembled for electrochemical test. Electrochemical measurements were tested on LAND battery testing system.
XRD measurements
Synchrotron XRD data were recorded at Shanghai Synchrotron Sadiation Facility (SSRF, beamline BL14B1). The wavelength of the X-ray was 0.6884 Å. The angles were converted to the angles of Cu-Ka (λ = 1.54 Å), in order to be consistent with other data, then refined using the GSAS software based on the Rietveld method. The in situ XRD were tested at the Institute of Physics, Chinese Academy of Sciences, using X’Pert Pro MPD X-ray diffractometer (D8 Bruker) equipped with Cu-Ka radiation (λ = 1.5405 Å) in the scan range (2θ) of 10–60° under a current density of 0.2 C to avoid deterioration, using a cell with small windows sealed with Al foil.
XAS measurements
In situ Cr/Ti/S K-edge XAS spectra were measured at Taiwan Light Source (Beamline 16A1) at National Synchrotron Radiation Research Center. The bending magnet beamline delivers monochromatic photon beams with energies from 2 to 8 keV with a resolving power (E/ΔE) up to 7000, and a beam size of 0.5 mm (H) × 0.4 mm (V). The XAS were collected using total fluorescence yield method with the sample chamber filled with helium gas51. The XANES spectra were processed using Athena software packages52. 2D XRF images and ex situ S K-edge XAS spectra of the pristine and charged sample were collected at beamline 8-BM (TES) of the National Synchrotron Light Source II (NSLSII) at Brookhaven National Laboratory (BNL). 2*2 mm2 area from the middle of the the electrodes were selected for the XRF measurement. XRF images were collected in a continuous scan mode with a 20 μm of pixel size at a energy of 3000 eV, which is above the absorption edge of S and Cl. Ex situ XAS spectra at Ti and Cr K-edge were also collected at beamline 7-BM of the National Synchrotron Light Sources II (NSLS-II) at Brookhaven National Laboratory (BNL).
SEM measurements
The measurements were obtained using a field emission scanning electron microscope (Cambridge S-360) equipped with an energy-dispersive X-ray spectroscopy (EDS) detector for elements analysis. The images were obtained by 12 kV voltage.
STEM measurements
The measurements were obtained by a field-emission transmission electron microscope (JEOL ARM200F, 200 keV) with spherical aberration corrector for the probe-forming lenses, which was operated at 200 kV. The Cs corrector was optimized for STEM observations and the point-to-point resolution in the STEM mode is better than 1.0 Å. For HAADF-STEM imaging, the electron probe convergence angle of 25 mrad and an HAADF detector with an inner angle larger than 100 mrad were used. The HAADF detector was set to collect electrons scattered between 100 and 267 mrad, guaranteeing that the collected signals give an approximately incoherent atomic-number contrast in HAADF-STEM.
XPS characterization
XPS was carried out on a PHI 5000C ESCA System with monochromatic Al-Ka X-ray source. The C 1s peak at 285.0 eV from hydrocarbon contamination was used to calibrate the binding energy.
DFT + U calculations
Based on the projector-augmented wave (PAW) method within DFT theory53, conducted with the VASP program54, spin-polarized calculations were carried out. We used the Perdew–Burke–Ernzerhof functional for exchange correlation55. We set an effective Ueff value to 3.5 eV for Cr and 3.2 eV for Ti as discussed in electronic structure calculations on MCrS2 (M=Li, Na, K, and Ag)56,57. The plane wave cutoff energy and Monkhorst–Pack k-point mesh were set to 550 eV and 3 × 3 × 3 for NaxCr2/3Ti1/3S2 2 × 2 × 1 supercells. As for the calculation of the electronic DOSs, 5 × 5 × 3 k-point mesh for the conventional cell and the modified tetrahedron method were used. The above parameters made the total energy converged to 2 meV per atom. The calculated structural parameters of NaxCr2/3Ti1/3S2 are consistent with experimental ones as shown in Fig. 2a. To determine the energy barriers for Na or Cr ion diffusion in Na0Cr2/3Ti1/3S2 the CINEB method58 was employed for searching the minimum-energy path.
Data availability
Data supporting this study are available in the article and corresponding Supplementary Information files. Extra data or the source data are available from the corresponding authors.
References
Rijnsdorp, J. & Jellinek, F. The crystal structure of niobium trisulfide, NbS3. J. Solid State Chem. 25, 325–328 (1978).
Goodenough, J. B. & Fatseas, G. A. Mössbauer 57 Fe isomer shift as a measure of valence in mixed-valence iron sulfides. J. Solid State Chem. 41, 1–22 (1982).
Blandeau, L., Ouvrard, G., Calage, Y., Brec, R. & Rouxel, J. Transition-metal dichalcogenides from disintercalation processes. Crystal structure determination and Mössbauer study of Li2FeS2 and its disintercalates LixFeS2 (0.2 ≤ x ≤ 2). J. Phys. C 20, 4271–4281 (1987).
Vanbruggen, C. et al. CrSe2, a new layered dichalcogenide. Physica B+C 99, 166–172 (1980).
Jobic, S., Brec, R. & Rouxel, J. Occurrence and characterization of anionic bondings in transition metal dichalcogenides. J. Alloy. Compd. 178, 253–283 (1992).
Rouxel, J. Anion–cation redox competition and the formation of new compounds in highly covalent systems. Chemistry 2, 1053–1059 (2010).
Tarascon, J. M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001).
Zhao, C. et al. Review on anionic redox for high-capacity lithium- and sodium-ion batteries. J. Phys. D 50, 183001 (2017).
Li, B. & Xia, D. Anionic redox in rechargeable lithium batteries. Adv. Mater. 29, https://doi.org/10.1002/adma.201701054 (2017).
Assat, G. & Tarascon, J.-M. Fundamental understanding and practical challenges of anionic redox activity in Li-ion batteries. Nature. Energy 3, 373–386 (2018).
Pearce, P. E. et al. Evidence for anionic redox activity in a tridimensional-ordered Li-rich positive electrode beta-Li2IrO3. Nat. Mater. 16, 580–586 (2017).
Yang, W. & Devereaux, T. P. Anionic and cationic redox and interfaces in batteries: advances from soft X-ray absorption spectroscopy to resonant inelastic scattering. J. Power Sources 389, 188–197 (2018).
Johnson, C. S. et al. The significance of the Li2MnO3 component in ‘composite’ xLi2MnO3·(1−x)LiMn0.5Ni0.5O2 electrodes. Electrochem. Commun. 6, 1085–1091 (2004).
Thackeray, M. M. et al. Advances in manganese-oxide ‘composite’ electrodes for lithium-ion batteries. J. Mater. Chem. 15, 2257 (2005).
Thackeray, M. M. et al. Li2MnO3-stabilized LiMO2 (M=Mn, Ni, Co) electrodes for lithium-ion batteries. J. Mater. Chem. 17, 3112 (2007).
Koga, H. et al. Operando X-ray absorption study of the redox processes involved upon cycling of the Li-rich layered oxide Li1.20Mn0.54Co0.13Ni0.13O2 in Li ion batteries. J. Phys. Chem. C 118, 5700–5709 (2014).
Luo, K. et al. Charge-compensation in 3d-transition-metal-oxide intercalation cathodes through the generation of localized electron holes on oxygen. Nat. Chem. 8, 684–691 (2016).
Mccalla, E. et al. Visualization of O–O peroxo-like dimers in high-capacity layered oxides for Li-ion batteries. Science 350, 1516 (2015).
Seo, D. H. et al. The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials. Nat. Chem. 8, 692–697 (2016).
Grimaud, A., Hong, W. T., Shao-Horn, Y. & Tarascon, J. M. Anionic redox processes for electrochemical devices. Nat. Mater. 15, 121–126 (2016).
Sathiya, M. et al. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat. Mater. 12, 827–835 (2013).
Qiao, Y. et al. Reversible anionic redox activity in Na3RuO4 cathodes: a prototype Na-rich layered oxide. Energy Environ. Sci. 11, 299–305 (2018).
Grayfer, E. D., Pazhetnov, E. M., Kozlova, M. N., Artemkina, S. B. & Fedorov, V. E. Anionic redox chemistry in polysulfide electrode materials for rechargeable batteries. ChemSusChem 10, 4805–4811 (2017).
Tanibata, N., Matsuyama, T., Hayashi, A. & Tatsumisago, M. All-solid-state sodium batteries using amorphous TiS3 electrode with high capacity. J. Power Sources 275, 284–287 (2015).
Takeuchi, T. et al. Improvement of cycle capability of FeS2 positive electrode by forming composites with Li2S for ambient temperature lithium batteries. J. Electrochem. Soc. 159, A75–A84 (2012).
Sun, R. et al. Vanadium sulfide on reduced graphene oxide layer as a promising anode for sodium ion battery. ACS Appl. Mater. Interfaces 7, 20902–20908 (2015).
Rout, C. S. et al. Synthesis and characterization of patronite form of vanadium sulfide on graphitic layer. J. Am. Chem. Soc. 135, 8720–8725 (2013).
Sakuda, A. et al. Amorphous metal polysulfides: electrode materials with unique insertion/extraction reactions. J. Am. Chem. Soc. 139, 8796–8799 (2017).
Lindic, M. H. et al. XPS investigations of TiOySz amorphous thin films used as positive electrode in lithium microbatteries. Solid State Ionics 176, 1529–1537 (2005).
Dubois, V., Pecquenard, B., Soule, S., Martinez, H. & Le Cras, F. Dual cation- and anion-based redox process in lithium titanium oxysulfide thin film cathodes for all-solid-state lithium-ion batteries. ACS Appl. Mater. Interfaces 9, 2275–2284 (2017).
Shadike, Z. et al. Antisite occupation induced single anionic redox chemistry and structural stabilization of layered sodium chromium sulfide. Nat. Commun. 8, 566 (2017).
Maitra, U. et al. Oxygen redox chemistry without excess alkali-metal ions in Na2/3[Mg0.28Mn0.72]O2. Nat. Chem. 10, 288–295 (2018).
Rong, X. et al. Structure-induced reversible anionic redox activity in Na layered oxide cathode. Joule 2, 125–140 (2018).
Lee, E., Sahgong, S., Johnson, C. S. & Kim, Y. Comparative electrochemical sodium insertion/extraction behavior in layered NaxVS2 and NaxTiS2. Electrochim. Acta 143, 272–277 (2014).
Totir, D. A., Antonio, M. R., Schilling, P., Tittsworth, R. & Scherson, D. A. In situ sulfur K-edge X-ray absorption near edge structure of an embedded pyrite particle electrode in a non-aqueous Li-based electrolyte solution. Electrochim. Acta 47, 3195–3200 (2003).
Prange, A. et al. In situ analysis of sulfur in the sulfur globules of phototrophic sulfur bacteria by X-ray absorption near edge spectroscopy. Biochim. Biophys. Acta 1428, 446–454 (1999).
Sugiura, C. & †, S. M. Sulfur K X-ray absorption-edge structures and electronic states of transition-metal sulfides MS and MS 2 (M=Fe, Co, Ni). Phys. Status Solidi 129, K157–K161 (1985).
Takeuchi, T. et al. Preparation of Li2S-FeSx composite positive electrode materials and their electrochemical properties with pre-cycling treatments. J. Electrochem. Soc. 162, A1745–A1750 (2015).
Liu, X. et al. Phase transformation and lithiation effect on electronic structure of Li(x)FePO4: an in-depth study by soft X-ray and simulations. J. Am. Chem. Soc. 134, 13708–13715 (2012).
Li, X. et al. Direct visualization of the reversible O(2−)/O(−) redox process in Li-rich cathode materials. Adv. Mater. 30, e1705197 (2018).
Wu, H. L., Huff, L. A. & Gewirth, A. A. In situ Raman spectroscopy of sulfur speciation in lithium–sulfur batteries. ACS Appl. Mater. Interfaces 7, 1709–1719 (2015).
Jr, W. E. S., Wynne, K. J. & Hercules, D. M. X-ray photoelectron spectroscopic investigation of Group VIA elements. Anal. Chem. 43, 1884 (1971).
Ye, H. et al. Amorphous MoS3 as the sulfur-equivalent cathode material for room-temperature Li–S and Na–S batteries. Proc. Natl Acad. Sci. USA 114, 13091–13096 (2017).
Yabuuchi, N. et al. P2-type Nax [Fe1/2Mn1/2] O2 made from earth-abundant elements for rechargeable Na batteries. Nat. Mater. 11, 512 (2012).
Yan, P. et al. Intragranular cracking as a critical barrier for high-voltage usage of layer-structured cathode for lithium-ion batteries. Nat. Commun. 8, 14101 (2017).
Lindberg, B. J. et al. Molecular spectroscopy by means of ESCA II. Sulfur compounds. Correlation of electron binding energy with structure. Phys. Scr. 1, 286 (1970).
Yang, Y. et al. Inspired by “tip effect”: a novel structure design strategy of the cathode in advanced lithium-sulfur batteries. J. Mater. Chem. A 5, 3140–3144 (2016).
Ryu, H. S. et al. Electrochemical properties and discharge mechanism of Na/TiS2 cells with liquid electrolyte at room temperature. J. Electrochem. Soc. 160, A338–A343 (2012).
Vinckevičiu̅tė, J., Radin, M. D. & Van der Ven, A. Stacking-sequence changes and Na ordering in layered intercalation materials. Chem. Mater. 28, 8640–8650 (2016).
Kim, S., Ma, X., Ong, S. P. & Ceder, G. A comparison of destabilization mechanisms of the layered Na(x)MO2 and Li(x)MO2 compounds upon alkali de-intercalation. Phys. Chem. Chem. Phys. 14, 15571–15578 (2012).
Dann, T. E. et al. A high-performance double-crystal monochromator soft X-ray beamline. J. Synchrotron Radiat. 5, 664–666 (1998).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
PE, B. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. ERRATA: generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1998).
Wang, L., Maxisch, T. & Ceder, G. Oxidation energies of transition metal oxides within the GGA+U framework. Phys. Rev. B 73, https://doi.org/10.1103/PhysRevB.73.195107 (2006).
Anisimov, V. I., Zaanen, J. & Andersen, O. K. Band theory and Mott insulators: Hubbard U instead of Stoner I. Phys. Rev. B 44, 943 (1991).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21773037, 21473235, 11227902, 11704245 and U1632269); the National Key Scientific Research Project (Grant No. 2016YFB090150); Shanghai Pujiang Program (17PJ1403700). The work at Taiwan Light Source (beamline 16A1) was supported by National Synchrotron Radiation Research Center. The work at Brookhaven National Laboratory was supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Vehicle Technology Office of the U.S. Department of Energy through the Advanced Battery Materials Research (BMR) Program, including Battery500 Consortium under contract DE-SC0012704. This research used resources at beamlines 7-BM (QAS), 8-BM (TES) of the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract no. DE-SC0012704. DFT calculation was performed in National Supercomputing Center in Shenzhen (Shenzhen Cloud Computing Center). P.L. is supported by the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning. The authors also thank beamline BL14B1 of the Shanghai Synchrotron Radiation Facility (SSRF). G.-X.R. also acknowledges the support from the UCAS Joint Ph.D. Training Program. The authors gratefully acknowledge the help by Prof. Xi-Qian Yu for his help on in situ XRD measurements, Bo-Yuan Ning, and Hong-Kun Zhu at Fudan University for their help on DFT calculations, Peng-Fei Yu, Xin-Yang Yue, Qin-Chao Wang, Ding-Ren Shi, Jing-Ke Meng, Wei-Wen Wang, Si-Yu Yang and He-Yi Xia for their help on experiments.
Author information
Authors and Affiliations
Contributions
Z.-W.F. and X.-S.L. supervised the whole project. Z.-W.F. and Z.S. designed the experiment. T.W., X.-Q.Y., X.-S.L., and Z.-W.F. wrote the manuscript. T.W. and M.-H.C. tested the electrochemical performance. J.-N.Z. performed the in situ XRD measurements. T.W. and J.-L.Y. carried out the DFT calculations. G.-X.R. and X.-S.L. performed the K-edge XANES experiments as well as processed the data. Z.S., S.-M.B., X.-Q.Y. and P.N. measured the 2D XRF images and ex situ S K-edge XAS data. P.L. and M.-W.C. conducted STEM imaging.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Vicky Doan-Nguyen and other, anonymous, reviewers for their contributions to the peer review of this work. Peer review reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, T., Ren, GX., Shadike, Z. et al. Anionic redox reaction in layered NaCr2/3Ti1/3S2 through electron holes formation and dimerization of S–S. Nat Commun 10, 4458 (2019). https://doi.org/10.1038/s41467-019-12310-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-019-12310-6
This article is cited by
-
Covalent Organic Frameworks for Photocatalytic Organic Transformation
Chemical Research in Chinese Universities (2022)
-
Is there a common reaction pathway for chromium sulfides as anodes in sodium-ion batteries? A case study about sodium storage properties of MCr2S4 (M = Cr, Ti, Fe)
Journal of Solid State Electrochemistry (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.