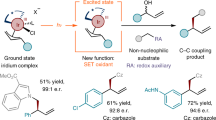
Abstract
Toluene and its derivatives are petroleum-derived raw materials produced from gasoline by catalytic reformation. These abundant chemical feedstocks are commonly used as solvents in organic synthesis. The C(sp3)−H functionalization of these unactivated substrates has been widely used to directly introduce benzylic motifs into diverse molecules to furnish important compounds. Despite these advances, progress in asymmetric catalysis remains underdeveloped. Here, we report photoinduced radical-based enantioselective C(sp3)−C(sp3) coupling reactions of activated ketones with toluene and its derivatives by means of chiral acid catalysis. With a La(OTf)3/pybox complex catalyst, a variety of chiral 3-hydroxy-3-benzyl-substituted 2-oxindoles, including many conventionally difficult-to-access variants, are obtained directly from isatins in high yields with good to excellent enantioselectivities. Acenaphthoquinone is also compatible with the use of a chiral phosphoric acid (CPA) catalyst, leading to another series of important enantioenriched tertiary alcohols.
Similar content being viewed by others
Introduction
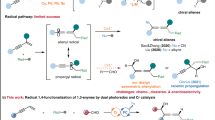
The use of abundant industrial feedstocks to generate value-added products is always highly desirable in organic synthesis1. A classic paradigm is the direct C(sp3)−H functionalization of petroleum-derived toluene and its derivatives2,3. To enable the C−H activation of these substrates, which are generally used as solvents, many effective methods involving the oxidative insertion of transition-metal catalysts4, hydrogen atom transfer (HAT)5,6,7 and single-electron-transfer (SET) oxidation8,9 have been developed. Nevertheless, asymmetric catalytic systems, which can serve to advance the development of chiral pharmaceuticals and chiral functional materials because of their ability to provide highly convenient and economical synthetic approaches, are still uncommon. The Davies group reported site-selective and enantioselective C–H functionalization of the secondary carbon10 or primary carbon11 in toluene derivatives by means of rhodium carbene-induced C–H insertion through using distinct chiral dirhodium catalysts (Fig. 1a). Recently, Melchiorre and co-workers skilfully took advantage of the high excited-state oxidation potential of iminium ion intermediates to perform SET with the aromatic rings of toluene and its electron-rich derivatives and furnish benzylic radical species (Fig. 1b)12. Covalent-bonding catalysis can be used to achieve good stereocontrol in the formation of C(sp3)–C(sp3) bonds. This elegant work further demonstrates that the use of a radical-based strategy offers improved regioselectivity with respect to transition-metal catalysed C–H activation procedures13,14, especially in regard to the functionalization of toluene derivatives, by differentiating C(sp3)–H from C(sp2)–H due to the greater stability of a benzylic radical compared to that of an aryl one. The development of radical approaches with compatible asymmetric catalytic strategies is thus an extremely desirable goal.
In 1909, Paternò and Chieffi reported the synthesis of oxetanes by the photoinduced [2 + 2] cycloaddition of ketones with olefins15. This radical transformation was later named the Paternò–Büchi reaction and was proven to be triggered by the generation of triplet-state ketones under photoactivation16,17. The capability of photoexcited ketones to activate alkenes, alkynes and alkanes via an SET redox or HAT mechanism has been revealed in recent decades18,19,20,21,22,23,24. Accordingly, we wondered if a similar photochemical mechanism could serve to perform the direct C(sp3)−H functionalization of toluene and its derivatives, most likely furnishing benzyl-substituted tertiary alcohols. The use of an extrinsic chiral catalyst25,26,27,28,29,30,31,32 to present efficient stereocontrol might provide a straightforward approach to synthesize their optically pure variants, such as the abundant and biologically important chiral 3-hydroxy-3-benzyl-substituted 2-oxindoles, from commercially available isatins33. Although the enantioselective α-oxygenation of 3-benzyl-substituted 2-oxindoles could furnish these entities34,35,36,37,38,39,40,41, the tedious preparation of the starting substrates and the limited types of benzyls, mainly owing to the expensive feedstocks or lack of viable synthetic methods, underscore the importance of exploring the feasibility of this challenging reaction.
Under irradiation with visible light, the triplet excited state of isatins generally shows n−π* reactivity and is a strong electron acceptor due to its rather high reduction potential (Ered 1/2*)23,24. Our investigations also indicated that the introduction of distinct N-substituents can substantially alter the Ered 1/2* values of isatins (Supplementary Note 1). Given that the excited-state reduction potential of the N-acetyl isatin 1d (+2.06 V vs. SCE in CH3CN) is the same as that of [Acr+-Mes]ClO4 (Ered 1/2* = + 2.06 V vs. SCE in CH3CN), which as a photoredox catalyst allows the slow SET oxidation of toluene (Eox = +2.28 V vs. SCE in CH3CN)9, this system is likely able to practically oxidize toluene, furnishing benzylic radical II, radical anion III42 and a proton (Fig. 2a). Given the strong basicity of the oxygen anion of III, it might capture the chiral Brønsted acid27,30,32 or Lewis acid43 catalyst to form dioxindolyl or the dioxindolyl-like radical IV, which is a merostabilized carbon radical due to the capto-dative substitution of the radical centre by the electron-donating oxygen atom and the electron-withdrawing amide group44. According to the persistent radical effect45, the coupling of radical IV with the transient benzylic radical II is viable and thus furnishes oxindoles, possibly in an enantioselective manner. Mechanistically, an HAT process19 involving these photoexcited isatin species and toluene is also thermodynamically favourable owing to their high triplet-state energy (ET, Fig. 2a and Supplementary Note 1), which should be enough to overcome the gap between the bond-dissociation energies (BDEs) of the O-H bond of the generated dioxindolyl radical44 V (48.1–49.8 kcal × mol−1, Supplementary Note 1) and the sp3 C−H bond of toluene (88.5 ± 1.5 kcal × mol−1)46. The chiral acid catalyst also potentially provides stereocontrol for the radical coupling by interacting with the merostabilized dioxindolyl radical V to form the more electrophilic radical VI31,43. It is worth mentioning that neutral radicals II47 and IV/V48 readily undergo homocoupling, which can affect the achievement of a satisfactory yield. Meanwhile, the very low activation barrier of the radical coupling and competitive racemic background process constitute two crucial challenges for precise and absolute stereocontrol. Herein, we report the investigation and realization of this hypothetical scenario (Fig. 2b). A wide array of valuable and challenging-to-synthesize chiral tertiary alcohols were expediently prepared by the reaction of toluene and its electron-rich and electron-deficient derivatives with isatins and acenaphthoquinone by using chiral Lewis acid or Brønsted acid catalysis.
Results
Reaction optimization
The viability of the plan was first examined by the transformations of isatins 1a-e with toluene as the solvent and irradiation with a 3 W blue LED (entries 1−5 in Supplementary Table 1). Isatin 1a was found to undergo poor chemical conversion, and the reaction delivered a complex crude mixture. Furthermore, no reaction was observed for 1b or 1c. When using 1d and 1e, to our delight, the corresponding products, rac−2d and rac−2e, were obtained in 28 and 24% yields, respectively, after 24 h. Accordingly, we next explored a range of chiral acid catalysts and reaction parameters using 1d and 1e as substrates (Supplementary Table 1). The reaction of N-Ts isatin 1e in toluene at 25 °C for 50 h in the presence of 20 mol% La(OTf)3, 22 mol% s-Bu-pybox L1 and 70 mg of 4 Å molecular sieves (MS) as an additive provided chiral product 2e in 71% yield and 98% ee (entry 1, Table 1). It was found that the catalyst loading was crucial for achieving the best enantioselectivity result (entries 44 and 45, Supplementary Table 1). Under the same reaction conditions, product 2d was generated from N-Ac isatin 1d in a similar yield but with moderate enantioselectivity (entry 2). The ee value decreased slightly to 91% when ligand L2 was used, as it lacked the dimethylamino moiety at the 4-position of the pyridyl unit seen in L1 (entry 3). Other lanthanide metal triflate complexes, i.e., Ce(OTf)3 and Nd(OTf)3, also afforded 2e with excellent enantioselectivity (entries 4–5). The addition of 1.0 mol% [Acr+–Mes]ClO4 did not improve the reaction rate or yield (entry 6). The control experiments confirmed that the Lewis acid, visible light and an oxygen-free environment are all required for the transformation (entries 7–9). Although the use of toluene as a solvent would facilitate recycling, improving the industrial applicability of this methodology, a solvent screening was still performed to evaluate the reaction of toluene derivatives, especially solid variants (Supplementary Table 2). As a result, 2e was obtained in 57% yield and 98% ee when using chlorobenzene (PhCl) as the solvent (entry 10).
Substrate scope
The generality of this direct enantioselective coupling protocol was initially explored under the optimized conditions depicted in Table 1, entry 10 (Fig. 3). We evaluated toluene derivatives with a variety of substituents on the aryl ring in the reaction with isatin 1e. The transformations proceeded smoothly and provided products 2e-2s in 43 to 80% yields with 83 to 98% ee within 48–60 h. For xylene and its derivatives (2f-l), only one methyl group was functionalized. Derivatives such as 4-fluorotoluene, 4-chlorotoluene and 4-bromotoluene, which have electron-deficient substituents, provided products 2m-o in moderate yields with 92 to 97% ee. 4-Nitrotoluene and 4-(trifluoromethyl)toluene, which have stronger electron-withdrawing substituents on the aryl ring, were also evaluated, but nearly none of the desired product was obtained; instead, isatin 1a was completely transformed to its homocoupling product, i.e., N,N′-ditosylisatide 3 (0% ee, >19:1 dr). Inspiringly, the reaction of toluene-d8 and heteroaromatic ring-containing 2,5-dimethylfuran and 2,5-dimethylthiophene afforded chiral deuterated oxindole 2t and products 2u-v, respectively, in high enantioselectivities. Secondary benzylic derivatives were next tested in the developed reaction. Nonsymmetric acyclic substrates furnished products 2w-ab with 88 to 99% ee but low diastereomeric ratios. Diphenylmethane gave 2ac in 50% yield with 97% ee. Cyclic 9,10-dihydroanthracene, xanthene, fluorene and acenaphthene efficiently produced the corresponding oxindoles 2ad-ag. The formation of quaternary carbons was achieved using cumene; product 2ah was obtained in 92% ee. No regioselectivity was observed for p-cymene, and the two products 2ai and 2aj were isolated in a ratio of nearly 1:1. Distinct isatins were also tested in the reaction with toluene, and adducts 2ak-2ap with different substitution patterns on the aromatic ring of the oxindole were generated with 92 to 98% ee. Of note, many oxindoles with complex 3-benzyl groups, such as 2i-l, 2t-v and 2ac-ah, were successfully synthesized, whether in a racemic or in an optically pure manner.
Enantioselective coupling of N-tosyl isatins with toluene and its derivatives. Reaction conditions: 1 (0.1 mmol), toluene or its derivatives (5.0 mmol), PhCl (4.0 mL), 25 °C. The data in parentheses were obtained under neat conditions [1 (0.1 mmol), toluene or its derivatives (4.0 mL), 25 °C]. Yields were determined from the material isolated after chromatographic purification. Enantiomeric excesses were determined by HPLC analysis on a chiral stationary phase. aOn a 1.0 mmol scale, 72 h, yield of 2e = 69%, ee of 2e = 98%
We next performed these transformations neat in toluene or one of its derivatives (see the standard reaction conditions in Table 1). Except for several solid (2j, 2k, 2q, 2ab and 2ad-ag) and expensive (2 v and 2 y) feedstocks, toluene and all of its previously tested derivatives were shown to react efficiently with N-Ts isatins and achieve similar excellent enantioselectivities with the exception of 2 l, 2p and 2 u (see the data in parentheses in Fig. 3). Remarkably increased yields were obtained for many substrates. The reaction of 1e with toluene on a 1.0 mmol scale provided product 2e in a similar yield and enantioselectivity after 72 h (footnote a). Notably, the poor to moderate yields in all cases are due to the competition with isatin homocoupling and other resulting side transformations, given the observation of N,N′-ditosylisatide 3 and some unknown byproducts.
Mechanistic studies
Based on the structures of the products and the control experiments (Table 1), it is reasonable that the transformation occurs via sp3 C–H abstraction followed by a radical coupling (Fig. 2). To elucidate the mechanism, UV-vis absorption spectroscopy analyses were performed for the reaction of isatin 1e with toluene;35 the results revealed that no electron donor–acceptor (EDA) complex49 was present in the mixture. It was also found that the Ered 1/2* of 1e could be increased to + 2.0 V in the presence of La(OTf)3 (Supplementary Note 1). As mentioned by Melchiorre12, even the photoexcited iminium ions (Ered 1/2* ≈ + 2.4 V), which have higher excited-state reduction potentials than 1e, were unable to oxidize the toluene derivatives that contained electron-deficient substituents, as these compounds feature higher oxidation potentials than toluene50. However, our reaction system did successfully generate adducts 2m-2o from 4-F-, 4-Cl- and 4-Br-toluene (Fig. 3). We also performed the reaction of 1e with THF under the established conditions (Fig. 4a). Although the BDE of an α-sp3 C–H bond in THF (92.0 ± 1.0 kcal × mol−1)51 is higher than that of the sp3 C–H bonds of toluene, the reaction provided coupling adduct 4 in 21% yield. These results suggest that an HAT process between the isatins and toluene and its derivatives does generate a benzylic radical species. We next attempted the reaction of 4-benzyl Hantzsch ester (5)52 with 1e, and adduct 2e was obtained in 89% yield with 87% ee (Fig. 4b). This SET-enabled radical coupling provided the same high enantioselectivity as the HAT reaction, suggesting that using chiral La-complex as a Lewis acid would provide similar stereocontrol in both catalytic systems. Therefore, the chiral La-complex should interact with the O atom of ketyl species III or dioxindolyl radical IV and the O atom of the amide simultaneously. For this reason and given the lack of regioselectivity with p-cymene53, we speculate that for toluene derivatives with lower oxidation potentials than toluene, the generation of the benzyl radicals can occur via HAT, HAT and SET, or SET depending on the difference between their required energies.
A competition deuterium kinetic isotope effect (KIE) study, using a mixture of toluene and toluene-d8, was then performed, and the results revealed a KIE of 3.8 (Fig. 4c). On the other hand, independent measurement of the reaction rate of these two substrates indicated a remarkable difference in rate (KIE = 3.77) when both transformations proceeded within 6 h (Fig. 4c and Supplementary Fig. 109). These values suggest that the C−H bond cleavage event is involved in the first irreversible step of the catalytic cycle and therefore does affect the rate of the transformation. Notably, although 1e was almost consumed within 30 h, the best yield of product 2e was obtained when the reaction was prolonged to 60 h (Supplementary Fig. 109). Meanwhile, the highest yield of 2t was achieved after the same reaction time. The observations reflect the fact that the overall efficiency of the transformation also depends on other competing processes. Given the demonstrated capability of isatide to produce a dioxindolyl radical via homolysis42,44, we speculated that the readily generated homocoupling byproduct N,N’-ditosylisatide 342,44 might reversibly participate in the transformations to provide adducts 2. In this context, the reaction of N,N’-ditosylisatide 3 with toluene was evaluated under the simulated reaction conditions, and as a result, product 2e was obtained in 73% yield with 90% ee (Fig. 4d). The formation of 2e indicates that some proportionation42,44 of dioxindolyl radical IV derived from 3 occurred during the course of the reaction. Importantly, the yields of 2 could not be further improved by prolonging the reaction time after 60 h, likely due to the decreased concentration of 3 or IV impeding the ability to undergo homolysis or proportionation.
Extension of the method to acenaphthoquinone
Encouraged by these successes, we extended this method to acenaphthoquinone (6) to directly access another series of potentially bioactive chiral tertiary alcohols54,55. The initial study on the neat reaction of 6 with toluene under standard conditions provided product 7a in 71% yield with 55% ee. Attempts to modify the reaction parameters could not produce better results. A further examination of the conditions was thus performed by investigating the catalytic system (Supplementary Table 3). Pleasingly, the reaction of 6 neat in toluene with 20 mol% 8H-SPINO CPA (CPA-1) at −5 °C with 80 mg of 4 Å MS as an additive furnished product 7a in 51% yield with 90% ee within 60 h (Fig. 5). Five toluene derivatives were then evaluated, and they produced adducts 7b-f in moderate yields with good enantioselectivities under slightly modified reaction conditions (Supplementary Methods).
Enantioselective coupling of acenaphthoquinone with toluene and its derivatives. 0.1 mmol scale at −5 oC under an argon atmosphere. Yields were determined from the material isolated after chromatographic purification. Enantiomeric excesses were determined by HPLC analysis on a chiral stationary phase
Discussion
In summary, we have developed a chiral acid-catalysed enantioselective direct C(sp3)−H functionalization of toluene and its derivatives with activated ketones under irradiation with visible light. In the presence of a chiral Lewis acid catalyst, a wide range of biologically important 3-hydroxy-3-benzyl-substituted 2-oxindoles including many difficult-to-access variants were obtained from isatins in a straightforward manner in high yields and with high ee. This challenging C−H abstraction and radical coupling strategy is also compatible with acenaphthoquinone when a CPA catalyst is used, furnishing access to another array of valuable enantioenriched tertiary alcohols. Given the tremendous achievements based on the Paternò–Büchi reaction and its variants, we believe that this result will inspire the further pursuit of asymmetric scaffolds and transformations, which will provide expedient and economical approaches for synthesizing numerous useful chiral compounds from diverse ketones and abundant chemical feedstocks.
Methods
General information
For the NMR spectra of compounds in this manuscript, see Supplementary Figs. 1−46. For the HPLC spectra of compounds in this manuscript, see Supplementary Figs. 47−92. For details of the optimization of reaction conditions, see Supplementary Note 1. For details of the mechanistic studies, see Supplementary Note 2. For the determination of the absolute configuration of products, see Supplementary Note 3. For the Gaussian data, see Supplementary Data. For general information, general experimental procedures and analytic data of compounds synthesized, see Supplementary Methods. For references on DFT caculations, see Supplementary Reference.
Preparation of 2 with PhCl as the solvent
First, 1 (0.1 mmol, 1.0 equiv), L1 (0.022 mmol, 0.22 equiv), La(OTf)3 (0.02 mmol, 0.2 equiv) and 4 Å MS (140 mg) were added to a 25 mL Schlenk tube. Subsequently, toluene or one of its derivatives (5.0 mmol, 50.0 equiv) and PhCl (4.0 mL) were sequentially added and degassed three times by the freeze–pump–thaw method. The reaction mixture was stirred under an argon atmosphere at 30 °C (the temperature was maintained in an incubator) in the dark for 2 h and then irradiated with a 3 W blue LED (λ = 450–455 nm) from a distance of 3.0 cm for another 48–60 h at 25 °C. The reaction mixture was directly loaded onto a short silica gel column, followed by gradient elution with petroleum ether/ethyl acetate (20/1–5/1 ratio). Removing the solvent in vacuo afforded products 2e-an.
Preparation of 2 in neat toluene and its derivatives
First, 1 (0.1 mmol, 1.0 equiv), L1 (0.022 mmol, 0.22 equiv), La(OTf)3 (0.02 mmol, 0.2 equiv) and 4 Å MS (140 mg) were added to a 25 mL Schlenk tube. Subsequently, toluene or one of its derivatives (4.0 mL) was sequentially added and degassed three times by the freeze–pump–thaw method. The reaction mixture was stirred under an argon atmosphere at 30 °C (the temperature was maintained in an incubator) in the dark for 2 h and then irradiated with a 3 W blue LED (λ = 450−455 nm) from a 3.0 cm distance for another 48−60 h at 25 °C. The reaction mixture was directly loaded onto a short silica gel column, followed by gradient elution with petroleum ether/ethyl acetate (20/1‒5/1 ratio). Removing the solvent in vacuo afforded products 2e-i, 2l-p, 2r-2u, 2w-aa, 2ac and 2ag-ap.
Preparation of 7a
First, 6 (0.1 mmol, 1.0 equiv), CPA-1 (0.02 mmol, 0.20 equiv) and 4 Å MS (80 mg) were added to a 25 mL Schlenk tube. Subsequently, toluene (saturated aqueous solution) (2.0 mL) was added and degassed three times by the freeze–pump–thaw method. The reaction mixture was stirred under an argon atmosphere at −5 °C (the temperature was maintained in an incubator) for 30 min in the dark and then irradiated with 2*3 W blue LEDs (λ = 450−455 nm) from a distance of 6.0 cm for another 60 h at −5 °C. The reaction mixture was directly loaded onto a short silica gel column, followed by elution with a gradient of petroleum ether/dichloromethane (1/1 ratio) to petroleum ether/ethyl acetate (5/1 ratio). Removing the solvent in vacuo afforded product 7a.
Data availability
The X-ray crystallographic coordinates for structures that support the findings of this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC) with the accession codes CCDC 1896394 (3), 1877077 (7b), 1877066 (8). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. The authors declare that all other data supporting the findings of this study are available within the article and Supplementary Information files and are also available from the corresponding author upon reasonable request.
References
Li, C.-J. & Trost, B. M. Green chemistry for chemical synthesis. Proc. Natl Acad. Sci. USA 105, 13197–13202 (2008).
Lyons, T. W. & Sanford, M. S. Palladium-catalyzed ligand-directed C−H functionalization reactions. Chem. Rev. 110, 1147–1169 (2010).
Davies, H. M. L., Bois, J. D. & Yu, J.-Q. C−H functionalization in organic synthesis. Chem. Soc. Rev. 40, 1855–1856 (2011).
Shimada, S., Batsanov, A. S., Howard, J. A. K. & Marder, T. B. Formation of aryl- and benzylboronate esters by rhodium-catalyzed C−H bond functionalization with pinacolborane. Angew. Chem. Int. Ed. 40, 2168–2171 (2001).
Qrareya, H., Ravelli, D., Fagnoni, M. & Albini, A. Decatungstate photocatalyzed benzylation of alkenes with alkylaromatics. Adv. Synth. Catal. 355, 2891–2899 (2013).
Aihara, Y., Tobisu, M., Fukumoto, Y. & Chatani, N. Ni(II)-catalyzed oxidative coupling between C(sp2)−H in benzamides and C(sp3)−H in toluene derivatives. J. Am. Chem. Soc. 136, 15509–15512 (2014).
Xiang, M., Xin, Z.-K., Chen, B., Tung, C.-H. & Wu, L.-Z. Exploring the reducing ability of organic dye (Acr+-Mes) for fluorination and oxidation of benzylic C(sp3)−H bonds under visible light irradiation. Org. Lett. 19, 3009–3012 (2017).
Xia, J.-B., Zhu, C. & Chen, C. Visible light-promoted metal-free C−H activation: diarylketone-catalyzed selective benzylic mono- and difluorination. J. Am. Chem. Soc. 135, 17494–17500 (2013).
Zhou, R., Liu, H., Tao, H., Yu, X. & Wu, J. Metal-free direct alkylation of unfunctionalized allylic/benzylic sp3 C−H bonds via photoredox induced radical cation deprotonation. Chem. Sci. 8, 4654–4659 (2017).
Davies, H. M. L., Jin, Q., Ren, P. & Kovalevsky, A. Y. Catalytic asymmetric benzylic C−H activation by means of carbenoid-induced C−H insertions. J. Org. Chem. 67, 4165–4169 (2002).
Qin, C. & Davies, H. M. L. Role of sterically demanding chiral dirhodium catalysts in site-selective C−H functionalization of activated primary C−H bonds. J. Am. Chem. Soc. 136, 9792–9796 (2014).
Mazzarella, D., Crisenza, G. E. M. & Melchiorre, P. Asymmetric photocatalytic C−H functionalization of toluene and derivatives. J. Am. Chem. Soc. 140, 8439–8443 (2018).
Davies, H. M. L. & Morton, D. Guiding principles for site selective and stereoselective intermolecular C−H functionalization by donor/acceptor rhodium carbenes. Chem. Soc. Rev. 40, 1857−1869 (2011).
Neufeldt, S. R. & Sanford, M. S. Controlling site selectivity in palladium-catalyzed C−H bond functionalization. Acc. Chem. Res. 45, 936–946 (2012).
Paternὸ, E. & Chieffi, G. Sintesi in chimica organica per mezzo della luce. nota II. Compositi degli idrocarburi N on stauri con aldeidi e chetoni. Gazz. Chim. Ital. 39, 341 (1909).
Büchi, G., Inman, C. G. & Lipinsky, E. S. Light-catalyzed organic reactions. I. The reaction of carbonyl compounds with 2-methyl-2-butene in the presence of ultraviolet light. J. Am. Chem. Soc. 76, 4327–4331 (1954).
Freilich, S. C. & Peters, K. S. Observation of the 1,4 biradical in the Paternò–Büchi reaction. J. Am. Chem. Soc. 103, 6255–6257 (1981).
Inbar, S., Linschitz, H. & Cohen, S. G. Primary quantum yields of ketyl radicals in photoreduction by amines. Abstr. Hydrog. nitrogen. J. Am. Chem. Soc. 102, 1419–1421 (1980).
Mattay, J., Gersdorf, J., Leismann, H. & Steenken, S. Photoreaction of biacetyl and 1,3-dioxole derivatives: modification of the mechanism of the photoreaction of carbonyl compounds with electron-rich olefins. Angew. Chem. Int. Ed. Engl. 23, 249–250 (1984).
Gersdorf, J., Mattay, J. & Görner, H. Radical cations. 3. Photoreactions of biacetyl, benzophenone, and benzil with electron-rich alkenes. J. Am. Chem. Soc. 109, 1203–1209 (1987).
Hu, S. & Neckers, D. C. Photochemical reactions of alkenyl phenylglyoxylates. J. Org. Chem. 62, 6820–6826 (1997).
Cho, D. W. et al. Photoaddition reactions of 1,2-diketones with silyl ketene acetals. Formation of β-hydroxy-γ-ketoesters. J. Org. Chem. 73, 4539–4547 (2008).
Xue, J., Zhang, Y., Wang, X.-L., Fun, H. K. & Xu, J.-H. Photoinduced reactions of 1-acetylisatin with phenylacetylenes. Org. Lett. 2, 2583–2586 (2000).
Wang, L., Zhang, Y., Hu, H.-Y., Fu, H.-K. & Xu, J.-H. Photoreactions of 1-acetylisatin with alkynes: regioselectivity in oxetene formation and easy access to 3-alkylideneoxindoles and dispiro[oxindole[3,2′]furan[3′,3′′]oxindole]s. J. Org. Chem. 70, 3850–3858 (2005).
Brimioulle, R., Lenhart, D., Maturi, M. M. & Bach, T. Enantioselective catalysis of photochemical reactions. Angew. Chem. Int. Ed. 54, 3872–3890 (2015).
Bauer, A., Westkämper, F., Grimme, S. & Bach, T. Catalytic enantioselective reactions driven by photoinduced electron transfer. Nature 436, 1139–1140 (2005).
Rono, L. J., Yayla, H. G., Wang, D. Y., Armstrong, M. F. & Knowles, R. R. Enantioselective photoredox catalysis enabled by proton-coupled electron transfer: development of an asymmetric aza-pinacol cyclization. J. Am. Chem. Soc. 135, 17735–17738 (2013).
Espelt, L. R., McPherson, I. S., Wiensch, E. M. & Yoon, T. P. Enantioselective conjugate additions of α-amino radicals via cooperative photoredox and Lewis acid catalysis. J. Am. Chem. Soc. 137, 2452–2455 (2015).
Uraguchi, D., Kinoshita, N., Kizu, T. & Ooi, T. Synergistic catalysis of ionic Brønsted acid and photosensitizer for a redox neutral asymmetric α-coupling of N-arylaminomethanes with aldimines. J. Am. Chem. Soc. 137, 13768–13771 (2015).
Lin, L. et al. Organocatalytic enantioselective protonation for photoreduction of activated ketones and ketimines induced by visible light. Angew. Chem. Int. Ed. 56, 13842–13846 (2017).
Li, J. et al. Formal enantioconvergent substitution of alkyl halides via catalytic asymmetric photoredox radical coupling. Nat. Commun. 9, 2445 (2018).
Liu, Y. et al. Catalytic enantioselective radical coupling of activated ketones with N-aryl glycines. Chem. Sci. 9, 8094–8098 (2018).
Zhou, F., Liu, Y.-L. & Zhou, J. Catalytic asymmetric synthesis of oxindoles bearing a tetrasubstituted stereocenter at the C-3 position. Adv. Synth. Catal. 352, 1381–1407 (2010).
Yang, Y. et al. Pentanidium−catalyzed enantioselective α-hydroxylation of oxindoles using molecular oxygen. Org. Lett. 14, 4762–4765 (2012).
Liao, Y.-H., Wu, Z.-J., Han, W.-Y., Zhang, X.-M. & Yuan, W.-C. Organocatalytic enantioselective stereoablative hydroxylation of 3-halooxindoles: an effective method for the construction of enantioenriched 3-substituted 3-hydroxy-2-oxindoles. Chem. Eur. J. 18, 8916–8920 (2012).
Bai, X. et al. L-Amino acid based urea−tertiary amine-catalyzed chemoselective and asymmetric stereoablative carboxylation of 3-bromooxindoles with malonic acid half thioesters. J. Org. Chem. 80, 12686–12696 (2015).
Li, B., Xu, Z.-J. & Han, J. Chiral iminophosphorances organocatalyzed asymmetric hydroxylation of 3-substituted oxindoles with oxaziridines. Tetrahedron Lett. 59, 2412–2417 (2018).
Ošeka, M. et al. Asymmetric organocatalytic wittig [2+3]-rearrangement of oxindoles. Org. Lett. 18, 1358–1361 (2016).
He, R. et al. Iridium-catalyzed enantioselective and diastereoselective allylation of dioxindoles: a one-step synthesis of 3-allyl-3-hydroxyoxindoles. Org. Lett. 20, 6183–6187 (2018).
Zhu, W.-M. et al. TEMPO-promoted C(sp3)−H hydroxylation of 2-oxindoles at room temperature. Asian J. Org. Chem. 7, 337–340 (2018).
Chen, W.-T. & Wei, W.-T. Recent development in the C(sp3)−H functionalization of 2-oxindoles through radical reactions. Asian J. Org. Chem. 7, 1429–1438 (2018).
Russell, G. A. et al. Semidione radical anions derived from indan-2,3-dione, coumaran-2,3-dione, thianaphthalenequinone, isatin, and N-hydroxyisatin. Nitroxide radicals derived from isatin, dioxindole, oxindole, and other indole derivatives. J. Am. Chem. Soc. 92, 2762–2769 (1970).
Yoon, T. P. Photochemical stereocontrol using tandem photoredox-chiral Lewis acid catalysis. Acc. Chem. Res. 49, 2307–2315 (2016).
Bennett, R. W., Wharry, D. L. & Koch, T. H. Formation kinetics of amino carboxy type merostablilized free radical. J. Am. Chem. Soc. 102, 2345–2349 (1980).
Studer, A. The persistent radical effect in organic synthesis. Chem. Eur. J. 7, 1159–1164 (2001).
Berkowitz, J., Barney Ellison, G. & Gutman, D. Three methods to measure RH bond energies. J. Phys. Chem. 98, 2744–2765 (1994).
Fagnoni, M., Dondi, D., Ravelli, D. & Albini, A. Photocatalysis for the formation of the C–C bond. Chem. Rev. 107, 2725–2756 (2007).
Wang, C.-M. et al. Photoredox-catalyzed reductive dimerization of isatins and isatin-derived ketimines: diastereoselective construction of 3,3’-disubstituted bisoxindoles. J. Org. Chem. 82, 3895–3900 (2017).
Arceo, E., Jurberg, I. D., Alvarez-Fernandez, A. & Melchiorre, P. Photochemical activity of a key donor−acceptor complex can drive stereoselective catalytic α-alkylation of aldehydes. Nat. Chem. 5, 750–756 (2013).
Wendt, H. & Bitterlich, S. Anodic synthesis of benzaldehydes−I. Voltammetry of the anodic oxidation of toluenes in non-aqueous solutions. Electrochim. Acta 37, 1951−1958 (1992).
McMillen, D. F. & Golden, D. M. Hydrocarbon bond dissociation energies. Ann. Rev. Phys. Chem. 33, 493–532 (1982).
Chen, W. et al. Building congested ketone: substituted Hantzsch ester and nitrile as alkylation reagents in photoredox catalysis. J. Am. Chem. Soc. 138, 12312–12315 (2016).
Bloom, S., McCann, M. & Lectka, T. Photocatalyzed benzylic fluorination: shedding ‘light’ on the involvement of electron transfer. Org. Lett. 16, 6338–6341 (2014).
Barakat, K. H. et al. Virtual screening and biological evaluation of inhibitors targeting the XPA-ERCC1 interaction. PLoS ONE 7, e51329 (2012).
Roche, M. et al. Synthesis, biological activity and structure-activity relationship of 4,5-dimethoxybenzene derivatives inhibitor of rhinovirus 14 infection. Eur. J. Med. Chem. 76, 445–459 (2014).
Acknowledgements
Grants from the NSFC (21672052) and Henan University are gratefully acknowledged. We also appreciate Prof. Qiang Liu (Lanzhou University) for providing constructive discussions.
Author information
Authors and Affiliations
Contributions
Z.J. conceived and designed the experiments. F.L., D.T. and Y.F. performed the experiments. R.L. performed DFT calculations on the triplet-state energies of compounds. G.L. performed DFT calculations on BDEs. Y.Y., B.Q., X.Z. and Z.X. helped isolate compounds and analyse the data. Z.J. wrote the paper. F.L., D.T. and Y.F. contributed equally to this work. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Journal peer review information: Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, F., Tian, D., Fan, Y. et al. Chiral acid-catalysed enantioselective C−H functionalization of toluene and its derivatives driven by visible light. Nat Commun 10, 1774 (2019). https://doi.org/10.1038/s41467-019-09857-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-019-09857-9
This article is cited by
-
Catalytic enantioselective C(sp3)–H functionalization involving radical intermediates
Nature Communications (2021)
-
Photocatalytic three-component asymmetric sulfonylation via direct C(sp3)-H functionalization
Nature Communications (2021)
-
Asymmetric C(sp3)–H functionalization of unactivated alkylarenes such as toluene enabled by chiral Brønsted base catalysts
Communications Chemistry (2021)
-
Multi-layer 3D chirality: new synthesis, AIE and computational studies
Science China Chemistry (2020)
-
Photocatalytic regio- and stereoselective C(sp3)–H functionalization of benzylic and allylic hydrocarbons as well as unactivated alkanes
Nature Catalysis (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.