Abstract
The reactivity of photoexcited molecules has been extensively studied for decades but until today direct bond-forming reactions of such excited states in a catalytic and asymmetric fashion are restricted to the synthesis of cyclobutanes via [2 + 2] photocycloadditions. Herein, we demonstrate a previously elusive visible-light-induced catalytic asymmetric [2 + 3] photocycloaddition of alkenes with vinyl azides. A wide range of complex 1-pyrrolines are obtained as single diastereoisomers and with up to >99% enantiomeric excess using a simple reaction setup and mild reaction conditions. The reaction is proposed to proceed through the photoexcitation of a complex out of chiral rhodium catalyst coordinated to α,β-unsaturated N-acylpyrazole substrates. All reactive intermediates remain bound to the catalysts thereby providing a robust catalytic scheme (no exclusion of air necessary) with excellent stereocontrol. This work expands the scope of stereocontrolled bond-forming reactions of photoexcited intermediates by providing catalytic asymmetric access to a key nitrogen heterocycle in organic chemistry.
Similar content being viewed by others
Introduction
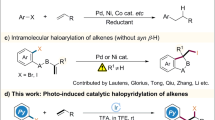
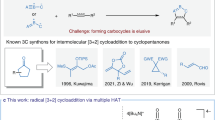
The induction or activation of chemical reactions by visible light is an extremely valuable tool for synthetic organic chemistry1,2. Typically, photochemically excited species engage in single electron transfer with a substrate or reagent to generate radical ions and/or free radicals, which then participate in a multitude of reaction schemes (Fig. 1a) 3. However, reactions through radical ions and free radicals have severe drawbacks. For example, radical ions are extremely sensitive to solvent effects, placing restriction to the reaction parameters, whereas the high reactivity of free radicals renders a control of their reaction pathway challenging, especially the stereocontrol, and often leads to a narrow substrate scope4,5. A highly attractive alternative are therefore bond-forming reactions that occur directly from a photoexcited state without prior charge transfer to circumvent the mentioned disadvantages arising from intermediate free radicals and radical ions6. Due to the demand of optically pure compounds in the chemical and pharmaceutical industry, catalytic stereocontrolled reactions are of particular interest. Unfortunately, currently available methods for catalytic asymmetric reactions that occur from a photochemically excited state without any intermediate charge separation are limited to [2 + 2] photocycloadditions7,8,9,10,11,12, probably owing to the lack of suitable, robust catalysts to enable new reaction patterns of such short-lived excited states and at the same time to control their stereochemistry. Although cyclobutanes built through [2 + 2] photocycloadditions are present in natural products, a direct access to more prevalent structural motifs, such as the important class of nitrogen hetereocycles, by direct stereocontrolled reactions from photoexcited states would be highly desirable and furnish new powerful synthetic methodology.
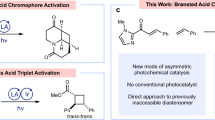
Different reactivity of photochemically excited states. a Charge transfer versus direct bond-forming reactions from a photoexcited state, whereas direct stereocontrolled reactions of excited states limited to [2 + 2] photocycloadditions. b Previous report on the photochemical construction of 1-pyrrolines via ground state 1,3-dipolar cycloaddition. c This work: visible-light-activated catalytic asymmetric [2 + 3] photocycloaddition via the direct reaction of a photochemically excited intermediate en route to enantioenriched 1-pyrrolines and functionalized pyrrolidines
1-Pyrrolines are widely found in biologically active molecules such as natural products and drugs, and constitute versatile synthetic intermediates by conversion to functionalized pyrrolidines13,14. Catalytic asymmetric methods to directly access this important heterocycle are little developed and limited to a few reports including the partial hydrogenation of pyrroles15, 1,3-cycloadditions of azlactones16,17 or α-isocyano esters18 with acceptor-substituted alkenes, and some other addition/cyclization protocols19,20. We envisioned to access this heterocycle in a completely different way by exploiting the photochemical reactivity of vinyl azides as shown in Fig. 1. Padwa reported in pioneering work21,22 that the UV-photolysis of aryl azirines, accessible from vinyl azides by photolysis or heat23,24, could form nitrile ylides which were subsequently trapped by ground-state electron-deficient alkenes to afford racemic 1-pyrrolines (Fig. 1b). Inspired by Padwa’s work and other reports on the diverse reactivity of vinyl azides25, we develop a herein reported direct [2 + 3] photocycloaddition of photoexcited acceptor-substituted alkenes with vinyl azides in a catalytic and asymmetric manner toward the synthesis of nonracemic chiral 1-pyrrolines with virtually perfect diastereoselectivities and enantioselectivities of up > 99% ee. Although vinyl azides are well-known to act as radical acceptors26,27,28 or triplet energy acceptors23,29, their ability to direct undergo cycloaddition with photoexcited alkenes has no precedence. All mechanistic experiments support a scenario in which a visible-light-excited complex out of α,β-unsaturated carbonyl compound and rhodium catalyst directly engages in a reaction with vinyl azides, thereby providing a convenient and unique access to nonracemic chiral 1-pyrrolines with a constitution that differs from related ground-state chemistry.
Results
Optimization of reaction conditions
Recently, we introduced a visible-light-activated intermolecular [2 + 2] photocycloaddition of photoexcited substrate-bound rhodium-based Lewis acid complexes with alkenes providing a practical synthetic route to enantioenriched cyclobutanes12. However, our previous work and related reports from other groups8,9,10,11 did not address the prevalent restriction to cyclobutanes as structural motifs for such stereocontrolled bond-forming reactions out of a photoexcited state. To explore new reaction manifolds of photoexcited molecules, we tested the reaction of N-acyl-3,5-dimethylpyrazole 1a with vinyl azide 2a catalyzed by a single-chiral bis-cyclometalated rhodium complex (Λ–RhS)30 developed in our group (Table 1). Gratifyingly, the expected 1-pyrroline 3a formed as a single diastereoisomer, albeit in only 54% yield with an unsatisfactory enantioselectivity of 86% ee and conditions of high catalyst loading (8.0 mol%) (Table 1, entry 1). Subsequently, we found that substituents within the pyrazole auxiliary have a profound influence on the reaction performance (entries 1–3). With a single phenyl substituent at the 3-position (R1) of the pyrazole, the yield of 1-pyrroline 3c was increased to 83% with 87% ee (entry 3). Optimization of solvent and concentration led to an improved outcome of 3c with 98% yield and 95% ee in CDCl3 under more diluted conditions (entries 3–6). We continued to evaluate the effect of the auxiliary in order to reduce the catalyst loading (entries 7–10, for more details see Supplementary Tables 1–3). As a result, 3-(4-methoxyphenyl) pyrazole provide the best choice for the present [2 + 3] photocycloaddition, allowing to reduce the catalyst loading to 4 mol% (entry 10). No differences were found between CDCl3, which was used to conveniently determine NMR yields, and CHCl3 (entry 11). Importantly, this simple and mild protocol can be conducted under air without the need for inert conditions, which renders it very practical (entry 12). It is noteworthy that the iridium analog Λ–IrS 31 also provided the 1-pyrroline 3f (50% yield) albeit without any enantioselectivity (entry 13). Control experiments demonstrated that the current transformation relies both on rhodium catalyst (entry 14) and visible light (entry 15).
Mechanistic investigation
The proposed mechanism for this [2 + 3] photocycloaddition is shown in Fig. 2. The coordination of substrate 1 to the chiral rhodium catalyst generates the substrate/catalyst complex I, which upon visible-light excitation generates the excited state I*, most likely in its triplet excited state. The following direct reaction of the excited state I* with the vinyl azide cosubstrate 2 affords the catalyst-bound diradical intermediate II under generation of a new C–C bond, with the stereochemistry of this bond formation being controlled by the metal-centred chirality of the rhodium complex. Subsequent extrusion of dinitrogen delivers the catalyst-bound iminyl radical intermediate III 32, which then undergoes a stereoselective cyclization generating the product coordinated complex IV. Release of product from the rhodium catalyst and coordination of new substrate 1 then initiates a new catalytic cycle. Since the reaction is insensitive to air, it can be assumed that the diradical intermediates II and III are very short-lived and thus cannot engage in any side reactions, thus revealing the advantages of such direct bond-forming reactions from a photoexcited state over processes that occur through initial electron transfer.
The proposed mechanism is supported by a series of experiments. First, a crystal structure of the pyrazole substrate 1f bound to the (racemic) catalyst RhS shown in Fig. 3a (RhS–1f) confirms the proposed formation of intermediate I of the catalytic cycle. Interestingly, it further reveals a π–π stacking between the cyclometalating benzothiazole moiety of the rhodium catalyst and the p-methoxyphenyl moiety of the pyrazole auxiliary, which might explain the superiority of this auxiliary by modulating binding constant, steric and/or electronic effects33. Second, UV-Vis absorption spectra demonstrate that the visible-light absorption of substrate 1f is greatly enhanced upon binding the rhodium catalyst (RhS–1f) so that in the reaction mixture the intermediate I is selectively photoexcited to generate the photoexcited intermediate I* from which the reaction (I* + 2 → II) occurs (Fig. 3b). This is crucial in order to suppress any background reaction resulting from the photoactivation of the free α,β-unsaturated N-acylpyrazole substrate, which would deteriorate the overall enantioselectivity. On the other hand, the excited intermediate I* apparently undergoes a direct cycloaddition instead of triplet energy transfer to the vinyl azide cosubstrate 2, so that no undesired photoinduced rearrangement23 to 2H-azirine 4 can occur. In fact, we ruled out that such azirines are viable intermediates in this catalytic cycle since independently synthesized 2H-azirine 4 was demonstrated to not react with 1f in the presence of RhS and visible light (Fig. 3c). Furthermore, the quantum yield for the reaction 1f + 2a → 3f was determined as Φ = 0.19, supporting our proposal that the photoreaction does not involve a chain process and instead a single photon is required for each formed product molecule (Fig. 3d). Finally, the problem remained why the iridium congener IrS cannot provide any enantioselectivity (Table 1, entry 13). Computational studies revealed that there is a significant difference between the spin distribution of excited RhS–1f and IrS–1f. As shown in Fig. 3e, most of the spin density in the triplet state of RhS–1f is localized at the alkene carbons of 1f, at which position the chemoselective cycloaddition occurs, whereas in the triplet state of IrS–1f, the iridium center possesses the majority of the spin density. This distinct difference in the nature of the excited state might account for the reactivity differences. Photoexcited IrS–1f is not capable of undergoing a direct reaction with vinyl azides, but instead serves as a photosensitizer to transfer its triplet energy to free substrate 1, and thereby serving as a photosensitizer for the formation of racemic [2 + 3] photocycloaddition product.
Mechanistic studies. a Crystal structure of the key intermediate RhS–1f. b UV/Vis absorption spectra of RhS (0.05 mM), RhS–1f (0.05 mM), 1f (1.0 mM), and 2a (1.5 mM) in CH2Cl2. c Control experiment which rules out 2H-azirines as reaction intermediates. d Determination of quantum yield of 0.19. e Calculated spin distribution and energy of the excited triplet states of 1f, RhS–1f, and IrS–1f
Scope of the methodology
With the mechanistic picture in mind, we next evaluated the generality of the [2 + 3] photocycloaddition with a single chiral rhodium catalyst using 3-(4-methoxyphenyl)pyrazole as the auxiliary. As shown in Fig. 4, different patterns of substitution at the β-aryl moiety of N-acylpyrazoles 1 were well tolerated, regardless of electronic nature (3g–l) or position (3g, m, n) of the substituents. Heteroaryl frameworks were proved to be compatible as demonstrated by the effective formation of thienyl- and indolyl- substituted 1-pyrrolines (3o, p). Notably, this protocol is amenable to the construction of complex 1-pyrrolines bearing a quaternary stereocenter (3q–s), including a spiro center (3s), reflecting the robustness of this single rhodium catalysis. Additionally, α,β-unsaturated N-acylpyrazoles (E)-1q and (Z)-1q gave the product 3q in similar yields with identical stereochemistry as an indicator for the involvement of the diradical intermediates II/III. To our delight, various functionalized vinyl azides were readily accommodated, including not only aryl vinyl azides (3t–x) but also more challenging alkyl vinyl azides (3z–ab). Although vinyl azides bearing an electron-rich substitution at phenyl group gave a decreased ee value (3t, 90% ee), the reaction outcome was not influenced by steric hindrance (3v–x). Furthermore, a free hydroxyl group (3aa) as well as C = C double bonds (3ab) were tolerated, thereby highlighting the good functional group compatibility of the current protocol.
Substrate scope. Reaction conditions: 1 (0.10 mmol), 2 (0.125 mmol) and Λ–RhS (4.0 mol%) in CDCl3 (1.0 mL) were assembled under air and stirred at room temperature under irradiation with blue LEDs (24 W). *Performed with 8.0 mol% of Λ–RhS under nitrogen atmosphere. †Performed in CDCl3 (2.0 mL). Configurations were assigned with crystal structure of 3k
Synthetic applications
With the practical conditions and broad substrate scope in hand, we next evaluated the synthetic potential of this protocol (Fig. 5). We were delighted to find a highly complex steroid substituted 1-pyrroline 6 could be obtained by the [2 + 3] photocycloaddition with ethisterone derived vinyl azide 5 in 86% yield with almost a single stereoisomer (Fig. 5a). Besides, a gram scale synthesis without loss of efficiency under air conditions highlights the practicality of this developed transformation without the requirement of inert conditions (Fig. 5b). Although the present methodology relies on a bidentate coordination mode, N-acylpyrazoles constitute very desirable synthons which can easily be transformed into other functionalities as shown in Fig. 5c for a clean and mild conversion to the amide 7 and ester 9. Importantly, the auxiliary 3-(4-methoxyphenyl)pyrazole was fully recovered in these conversions. Furthermore, 1-pyrrolines with a prochiral cyclic imine group is applicable for further stereoselective synthetic manipulation as highlighted by the synthesis of a carbapenem analog 8 and pyrrolidine 10.
Synthetic applications. a Late-stage modification of ethisterone derivative. b Gram scale synthesis. c Functional group conversions. Reaction conditions are as follows: (i) L-Leucin-tert-butylester hydrochloride, Et3N, HOBt, toluene, 50 °C; (ii) 2-Methoxyacetyl chloride, Et3N, CH2Cl2, 50 °C; (iii) LiCl, Et3N, EtOH/THF (4:1), r.t.; (iv) Pd/C, H2, EtOAc, r.t.
Discussion
In summary, we here introduced an important expansion of catalytic asymmetric reactions occurring via direct stereocontrolled bond-forming reactions of photoexcited states (Fig. 1c). Previously restricted to the class of cyclobutanes, we here reveal an economical34 access to the highly relevant class of chiral 1-pyrrolines based on a visible-light-activated [2 + 3] photocycloaddition of alkenes with vinyl azides catalyzed by a chiral rhodium complex. The employed robust rhodium-based chiral Lewis acid catalyst is key to the successful implementation of this methodology. Upon binding to the alkene substrate, it provides a unique handle for selective visible-light excitation of the substrate/catalyst complex with the triplet spin being mainly localized at the alkene carbons so that an efficient reaction with the vinyl azide cosubstrate can occur, while the metal-centered chirality provides outstanding stereocontrol. In addition to the conceptual appeal, the synthetic usefulness of this protocol is indicated by the simple reaction setup with a single catalyst and no necessity for inert reaction conditions, exceptional stereoselectivity with virtual perfect diastereoselectivity and up to >99% ee, a broad substrate scope that gives access to a large variety of chiral 1-pyrrolines, including quaternary stereocenters, and the ability to use these 1-pyrrolines as building blocks for conversions into biologically relevant chiral pyrrolidines. We anticipate that this report will stimulate the further discovery of new types of stereocontrolled reactions with electronically excited molecules.
Methods
General
For the emission spectrum of the blue LEDs lamp and the exemplary reaction setup, see Supplementary Figs 1 and 2. For more information about conditions screening and substrate scope, see Supplementary Tables 1–4. For the product analysis with HPLC on chiral stationary phase, see Supplementary Figs 8–38. For 1H and 13C NMR spectra of the compounds in this article, see Supplementary Figs 39–146, and more experimental details and compound characterization data, see Supplementary Methods. For details of the computational study see, Supplementary Fig. 5, Supplementary Table 7, Supplementary Methods and Supplementary Data. For crystallographic information, see the Supplementary Figs 3 and 4; Supplementary Tables 5 and 6; and Supplementary Methods. For configuration assignment of compounds 8 and 10, see Supplementary Figs 6 and 7, Supplementary Table 8 and Supplementary Methods.
General procedure for the reaction
Exemplary, to an oven-dried 10 mL Schlenk tube with a stirring bar, α,β-unsaturated N-acylpyrazole 1g (33.4 mg, 0.10 mmol), Λ–RhS (3.5 mg, 4 mol%), CDCl3 (1.0 mL) and vinyl azide 2a (18.2 mg, 0.125 mmol) were added in sequence under open air conditions. Then, the tube was sealed and positioned at ~8 cm from a 24 W blue LEDs lamp. After stirred at room temperature for 24 h (monitored by TLC), the mixture was directly subjected to 1H NMR to determine the diastereoselectivity of 3g as only a single diastereoisomer formed. Then, all the mixture was collected and purified by flash chromatography on silica gel (n-hexane/EtOAc) to afford the product 3g as a white solid (40.2 mg, 89% yield). The enantiomeric excess of 3g was determined as 99% ee by HPLC using a Chiralpak OD-H column (mobile phase = n-hexane/isopropanol 95:5).
Data availability
The X-ray crystallographic coordinates for the structure of RhS–1f and 3k reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers CCDC 1568748 and 1568749, respectively. The data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information Files. All other data are available from the authors upon reasonable request.
References
Hoffmann, N. Photochemical reactions as key steps in organic synthesis. Chem. Rev. 108, 1052–1103 (2008).
Schultz, D. M. & Yoon, T. P. Solar synthesis: prospects in visible light photocatalysis. Science 343, 1239176 (2014).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: Applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013).
Zimmerman, J. & Sibi, M. P. Enantioselective radical reactions. Top. Curr. Chem. 263, 107–162 (2006).
Studer, A. & Curran, D. P. Catalysis of radical reactions: A radical chemistry perspective. Angew. Chem., Int. Ed. 55, 58–102 (2016).
Albini, A. Photosensitization in organic synthesis. Synthesis 249–264 (1981).
Poplata, S., Tröster, A., Zou, Y.-Q. & Bach, T. Recent advances in the synthesis of cyclobutanes by olefin [2+2] photocycloaddition reactions. Chem. Rev. 116, 9748–9815 (2016).
Brimioulle, R. & Bach, T. Enantioselective Lewis acid catalysis of intramolecular enone [2+2] photocycloaddition reactions. Science 342, 840–843 (2013).
Brimioulle, R., Bauer, A. & Bach, T. Enantioselective Lewis acid catalysis in intramolecular [2+2] photocycloaddition reactions: A mechanistic comparison between representative coumarin and enone substrates. J. Am. Chem. Soc. 137, 5170–5176 (2015).
Tröster, A., Alonso, R., Bauer, A. & Bach, T. Enantioselective intermolecular [2+2] photocycloaddition reactions of 2(1H)-quinolones induced by visible light irradiation. J. Am. Chem. Soc. 138, 7808–7811 (2016).
Blum, T. R., Miller, Z. D., Bates, D. M., Guzei, I. A. & Yoon, T. P. Enantioselective photochemistry through Lewis acid-catalyzed triplet energy transfer. Science 354, 1391–1395 (2016).
Huang, X. et al. Direct visible-light-excited asymmetric Lewis acid catalysis of intermolecular [2+2] photocycloadditions. J. Am. Chem. Soc. 139, 9120–9123 (2017).
Dannhardt, G. & Kiefer, W. 1‐Pyrrolines (3,4‐dihydro‐2H‐pyrroles) as a template for new drugs. Arch. Pharm. 334, 183–188 (2001).
Shvekhgeimer, M.-G. A. Methods for the synthesis of 3,4-2H-dihydropyrroles (Δ1-pyrrolines) and their chemical transformations. Chem. Heterocycl. Comp. 39, 405–448 (2003).
Wang, D.-S. et al. Highly enantioselective partial hydrogenation of simple pyrroles: A facile access to chiral 1-pyrrolines. J. Am. Chem. Soc. 133, 8866–8869 (2011).
Melhado, A. D., Luparia, M. & Toste, F. D. Au(I)-catalyzed enantioselective 1,3-dipolar cycloadditions of münchnones with electron-deficient alkenes. J. Am. Chem. Soc. 129, 12638–12639 (2007).
Melhado, A. D., Amarante, G. W., Wang, Z. J., Luparia, M. & Toste, F. D. Gold(I)-catalyzed diastereo- and enantioselective 1,3-dipolar cycloaddition and Mannich reactions of azlactones. J. Am. Chem. Soc. 133, 3517–3527 (2011).
Padilla, S., Adrio, J. & Carretero, J. C. Au-catalyzed asymmetric formal [3+2] cycloaddition of isocyanoacetates with maleimides. J. Org. Chem. 77, 4161–4166 (2012).
Kawai, H. et al. Partially saturated fluorinated heterocycles: Diastereo- and enantioselective synthesis of β-trifluoromethyl-pyrroline carboxylates. Chem. Commun. 48, 3632–3634 (2012).
Hashimoto, T. & Maruoka, K. Recent advances of catalytic asymmetric 1,3-dipolar cycloadditions. Chem. Rev. 115, 5366–5412 (2015).
Padwa, A. & Smolanoff, J. Photocycloaddition of arylazirenes with electron-deficient olefins. J. Am. Chem. Soc. 93, 548–550 (1971).
Padwa, A., Dharan, M., Smolanoff, J. & Wetmore, S. I. Photochemical transformations of small ring heterocyclic compounds. XLVII. Electronic details of the photocycloaddition of arylazirines. J. Am. Chem. Soc. 95, 1954–1961 (1973).
Rajam, S. et al. Triplet sensitized photolysis of a vinyl azide: Direct detection of a triplet vinyl azide and nitrene. J. Org. Chem. 79, 9325–9334 (2014).
L’Abbé, G. & Mathys, G. Mechanism of the thermal decomposition of vinyl azides. J. Org. Chem. 39, 1778–1780 (1974).
Hu, B. & DiMagno, S. G. Reactivities of vinyl azides and their recent applications in nitrogen heterocycle synthesis. Org. Biomol. Chem. 13, 3844–3855 (2015).
Wang, Y.-F. & Chiba, S. Mn(III)-mediated reactions of cyclopropanols with vinyl azides: Synthesis of pyridine and 2-azabicyclo[3.3.1]non-2-en-1-ol derivatives. J. Am. Chem. Soc. 131, 12570–12572 (2009).
Sun, X. & Yu, S. Visible-light-promoted iminyl radical formation from vinyl azides: Synthesis of 6-(fluoro)alkylated phenanthridines. Chem. Commun. 52, 10898–10901 (2016).
Shu, W., Lorente, A., Gómez-Bengoa, E. & Nevado, C. Expeditious diastereoselective synthesis of elaborated ketones via remote Csp3–H functionalization. Nat. Commun. 8, 13832 (2017).
Hossain, A., Pagire, S. K. & Reiser, O. Visible-light-mediated synthesis of pyrazines from vinyl azides utilizing a photocascade process. Synlett 28, 1707–1714 (2017).
Ma, J., Shen, X., Harms, K. & Meggers, E. Expanding the family of bis-cyclometalated chiral-at-metal rhodium(III) catalysts with a benzothiazole derivative. Dalton Trans. 45, 8320–8323 (2016).
Huo, H. et al. Asymmetric photoredox transition-metal catalysis activated by visible light. Nature 515, 100–103 (2014).
Sun, X. et al. Mn-catalyzed highly efficient aerobic oxidative hydroxyazidation of olefins: A direct approach to β-azido alcohols. J. Am. Chem. Soc. 137, 6059–6066 (2015).
Neel, A. J., Hilton, M. J., Sigman, M. S. & Toste, F. D. Exploiting non-covalent π interactions for catalyst design. Nature 543, 637–646 (2017).
Newhouse, T., Baran, P. S. & Hoffmann, R. W. The economies of synthesis. Chem. Soc. Rev. 38, 3010–3021 (2009).
Acknowledgements
Funding from the Deutsche Forschungsgemeinschaft (ME 1805/13-1) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
E.M.: Coordinated the project. E.M. and X.H.: Conceived the project, designed the experiments, and wrote the manuscript. X.H.: Carried out the majority of the synthetic experiments. X.L.: Performed the computational study. X.X.: Assigned the relative configurations of compounds 8 and 10 by NMR spectroscopy. K.H. and R.R.: Collected the crystallographic data, solved and refined the X-ray crystal structure.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, X., Li, X., Xie, X. et al. Catalytic asymmetric synthesis of a nitrogen heterocycle through stereocontrolled direct photoreaction from electronically excited state. Nat Commun 8, 2245 (2017). https://doi.org/10.1038/s41467-017-02148-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-017-02148-1
This article is cited by
-
Asymmetric catalytic alkylation of vinyl azides with 3-bromo oxindoles: water-assisted chemo- and enantiocontrol
Science China Chemistry (2023)
-
Photocatalytic three-component asymmetric sulfonylation via direct C(sp3)-H functionalization
Nature Communications (2021)
-
Enantioselective synthesis of heterocyclic compounds using photochemical reactions
Photochemical & Photobiological Sciences (2021)
-
Alkyne–Alkene [2 + 2] cycloaddition based on visible light photocatalysis
Nature Communications (2020)
-
Divergent synthesis of chiral cyclic azides via asymmetric cycloaddition reactions of vinyl azides
Nature Communications (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.