Abstract
Oxidative stress has been implicated in the pathophysiology of cerebral stroke. As NADPH oxidases (NOXs) play major roles in the regulation of oxidative stress, we hypothesized that reduction of NOX activity by depletion of p22phox, an essential subunit of NOX complexes, would prevent cerebral stroke. To investigate this, we used the stroke-prone spontaneously hypertensive rat (SHRSP) and the p22phox-deleted congenic SHRSP. Although p22phox depletion reduced blood pressure under salt loading, it did not ameliorate oxidative stress or reduce the incidence of salt-induced stroke in SHRSPs. Additional pharmacological reduction of oxidative stress using antioxidant reagents with different mechanisms of action was necessary to prevent stroke, indicating that NOX was not the major target in salt-induced stroke in SHRSPs. On the other hand, oxidative stress measured based on urinary isoprostane levels showed significant correlations with blood pressure, stroke latency and urinary protein excretion under salt loading, suggesting an important role of oxidative stress per se in hypertension and hypertensive organ damage. Overall, our results imply that oxidative stress from multiple sources influences stroke susceptibility and other hypertensive disorders in salt-loaded SHRSPs.
Similar content being viewed by others
Introduction
In recent years, reactive oxygen species (ROS) have emerged as key mediators in the pathogenesis of cardiovascular diseases such as stroke [1,2,3,4]. In addition, several experimental studies have reported that increased RO2S production is associated with neuronal damage, which might be one of the important mechanisms underlying stroke [5, 6]. As the stroke-prone spontaneously hypertensive rat (SHRSP) is well characterized by its unique stroke susceptibility and high ROS levels in vivo [7], new insights regarding the roles of ROS in the pathogenesis of stroke may be obtained through studies on this rat model.
ROS levels in vivo are determined through a delicate balance between ROS-generating and ROS-degrading systems [1, 8, 9]. There are several ROS-generating enzymes, including mitochondrial respiratory enzymes, NADPH oxidases (NOXs), xanthine oxidase (XO) and uncoupled NO synthases [10,11,12]. NOXs are considered to play major roles in ROS production. In contrast to enzymes generating ROS as byproducts, the NOX family exclusively produces ROS, which is important in the regulation of various cellular functions [5,6,7,8,9,10,11,12,13]. So far, the NOX family includes 7 isoforms: NOX1–5 and the dual oxidases 1 and 2 [5, 14]. Through the generation of ROS, NOX has been shown to modulate redox-sensitive targets in intracellular signaling pathways that control cell growth, cell differentiation, gene expression, oxygen sensing, protein modification and innate immunity [14,15,16]. Most isoforms of NOX contain six subunits that form an active NOX complex [5, 15]. Of the six subunits, the small docking P22PHOX is thought to be obligatory for ROS production by NOX1–414 [15]. As NOX1, 2, and 4 are found abundantly in the vasculature and the central nervous system [5, 13, 17], genetic depletion of p22phox seems a good strategy to evaluate the importance of NOX-dependent ROS generation in cardiovascular diseases, including stroke.
In this regard, we employed the p22phox-depleted congenic SHRSP (SHRSP.MES-Cybames/Izm; abbreviated SP.MES) in this study [18]. SP.MES rats were established by introducing a mutated Cyba allele (coding p22phox) from Matsumoto Eosinophilia Shinshu (MES) rats and have been demonstrated to have lower oxidative stress and lower blood pressure (BP) than SHRSPs [7].
The present study revealed that P22PHOX deprivation was not sufficient to ameliorate stroke susceptibility in SHRSPs. However, further reduction of ROS using different antioxidant reagents in SP.MES rats could reduce stroke occurrence, and oxidative stress per se was suggested to be important in the pathogenesis of stroke. It is therefore suggested that NOXs do not play a major role in stroke susceptibility in SHRSPs.
Methods
Animal procedures
Eight-week-old adult male SHRSPs and SP.MES rats were used in all experiments. The p22phox-depleted congenic strain SP.MES was generated in an accelerated fashion by using a marker-assisted accelerated backcrossing (MAX-BAX®) strategy as previously described [7]. Rats were fed a stroke-permissive diet (Funabashi Farm Co., Ltd., Chiba, Japan) and given drinking water ad libitum. Then, for 1 week, the rats were given regular drinking water with or without tempol (3 mmol/L), febuxostat dissolved in 0.05 N NaOH [19] (30 mg/L) or coenzyme Q10 (CoQ10, 200 mg/L). To measure stroke latency, the water was then switched to 1% salt water with or without one of the three reagents above, and the rats were closely checked every day until at least one suggestive sign of stroke (e.g., paralytic gait, seizures and diminished motor activity) developed [20, 21]. Magnetic resonance imaging was performed on some of the examined rats to confirm and obtain representative images of cerebral edema and hemorrhage (MRmini SA 1508, 1.5 T, DS Pharma Biomedical. Co., Ltd., Tokyo, Japan). The period (days) between the start of salt loading and the first day when stroke signs were observed was considered the stroke latency [22].
Another set of rats was given plain water ± tempol/febuxostat/CoQ10 for 1 week and then 1% salt water ± one of the three reagents for 2 weeks. Twenty-four hour urine samples were collected by using individual metabolic cages before salt loading (i.e., after 1 week of treatment with or without one of the three reagents) and after 2 weeks of reagent + 1% salt. The rats were then sacrificed, and kidneys and serum samples were harvested. The collected urine samples were centrifuged at 2000 rpm and 4 °C for 10 min. Blood samples were withdrawn from the abdominal aorta and then centrifuged at 3000 rpm and 4 °C for 5 min to obtain serum samples. Both urine and serum samples were stored at −20 °C until analysis. Figure 1 shows a summary of the experimental procedures.
Schematic diagram of the experimental procedures indicating the time points of the treatments and experiments The experiments were started on 8-week-old male rats. In the first week, the rats were given regular drinking water with or without tempol, febuxostat or CoQ10. To evaluate stroke latency, the drinking water was then switched to 1% salt water with or without one of the three reagents above. The rats were then closely checked every day for suggestive signs of stroke, upon which MRI was also used for confirmation. Twenty-four-hour urine samples were collected by using metabolic cages before salt loading (i.e., after 1 week of treatment with or without one of the three antioxidant reagents) and after two weeks of treatment with or without one of the reagents + 1% salt
As tempol is light-sensitive, the water bottles were wrapped with aluminum foil. All solutions used in this experiment were replaced with fresh solutions every 48 h.
The SHRSP/Izm model was provided by the Disease Model Cooperative Research Association (Kyoto, Japan). All animal procedures were conducted after review and approval of the Local Committee of Animal Research at Shimane University and were performed in compliance with the regulations and guidelines for animal experiments at Shimane University.
BP measurement
BP was measured by using a computerized tail cuff BP-98A machine (Softron Corp., Tokyo, Japan). BP was measured at baseline, after 1 week of treatment with or without one of the three reagents, and after 1 week of treatment with 1% salt ± one of the three reagents. At each time point, the average of 5 readings was taken for each measurement. In addition, BP measurement under salt loading was performed by radiotelemetry (TA11PA-C40; Data Sciences Intl., St. Paul, MN) on SHRSPs and SP.MES rats as previously reported [7].
Assessment of biochemical parameters
The urine samples were allowed to thaw at room temperature and then centrifuged at 860 × g for 15 min at 4 °C. The supernatants were collected, and the levels of isoprostane, a stable and sensitive oxidative stress marker [23], were quantified by ELISA (urinary isoprostane kit, JaICA, Nikken SEIL Co., Ltd, Tokyo, Japan) according to a protocol provided in the kit. Urinary protein was determined using a BCA protein assay kit (Nakalai Tesque, Kyoto, Japan). Serum uric acid levels were quantified with a Spotchem EZ SP-4430 (Arkray Inc., Kyoto, Japan) according to the manufacturer’s protocol.
Renal histopathology
The rats were euthanized by CO2 overdose after 2 weeks of salt loading, and then the collected kidneys were fixed and preserved in 10% formalin. Hematoxylin and eosin (H&E) staining was performed on 5 µm sections for histological evaluation. Glomeruli were categorized as normal, partially sclerotic or completely sclerotic according to the histological criteria shown in Fig. 2a–c. The number of glomeruli in each category were counted from digital images of kidneys. Approximately 400 glomeruli per rat were evaluated, and the percentage of partially + completely sclerotic glomeruli among the total glomeruli was calculated for each group (n = 3). We obtained the same result when partially sclerotic glomeruli were excluded from the analysis (data not shown). The chi-square (X2) test was employed for comparisons between groups.
Effects of tempol on oxidative stress, blood pressure and stroke latency in SHRSPs and SP.MES rats (a) Effects of salt and tempol on BP. BP was measured at baseline (8 weeks of age), after a 1-week treatment with/without 3 mM tempol, and then after an additional 1-week treatment with salt ± tempol. #P < 0.01 vs. SHRSPs and *P < 0.05 vs. SP.MES rats without tempol (n = 8–10/group). b Isoprostane levels in 24-h urine after 2 weeks of salt loading. *P < 0.05 vs. corresponding rats without tempol treatment and #P < 0.05 vs. corresponding SHRSPs (n = 8–10/group). c Left panel: stroke latency in salt-loaded SP.MES rats and SHRSPs. Each line represents the same group of rats as in (a). *P < 0.05 vs. control SHRSPs and SP.MES rats by the log-rank test; significant after Bonferroni’s correction (n = 12–14). Right panel: representative T2-weighted brain MRI image of a cerebral lesion (arrow) obtained immediately after stroke signs were observed. d–f Correlations between urinary isoprostane levels and blood pressure after 1 week of salt loading (d), stroke latency (e) and urinary protein levels after 2 weeks of salt loading (f) (see Fig. 2e). Each symbol represents the same group of rats as in (a). Pearson’s correlation coefficient is indicated in each panel with the respective P-value
Chemicals
1-Oxyl-2, 2,6,6-tetramethyl-4-hydroxypiperidine (tempol) and febuxostat were purchased from Sigma Aldrich Chemical Company (St. Louis, MO, USA). CoQ10 (Kaneka QHTM) was obtained from Kaneka Corporation (Osaka, Japan).
Data presentation and statistical analysis
All results are presented as the mean ± standard deviation. Unless otherwise stated, statistical analyses for intergroup differences were performed with either Student’s t-test or one-way ANOVA followed by Bonferroni’s post hoc test. Stroke onset was compared among the groups with the Bonferroni-adjusted log-rank test. Correlations between variables were tested with Pearson’s correlation coefficient. Analyses of data were conducted with Prism version 7.00 (GraphPad Prism Software Inc., CA, USA). Statistical significance was set at P < 0.05 (two-tailed).
Results
Effects of p22phox depletion on oxidative stress, blood pressure, and stroke latency
SP.MES rats exhibited significantly lower baseline BP than age-matched SHRSPs (148 ± 9 and 179 ± 13 mmHg, respectively, Fig. 2a). After 1 week of salt loading, the difference in BP between SHRSPs and SP.MES rats remained significant (Fig. 2a). The interstrain difference in BP was confirmed by the radiotelemetry measurement as well (Supplementary Fig. 1). Administration of tempol at the maximal dose (3 mM) failed to reduce BP or urinary isoprostane levels in SHRSPs with or without salt loading (Fig. 2a, b). Tempol did not affect stroke latency in SHRSPs (Fig. 2c). Unlike in SHRSPs, tempol significantly reduced BP and urinary isoprostane in SP.MES rats (Fig. 2a, b). It is of interest that, despite their lower BPs, stroke latency was not significantly ameliorated in SP.MES rats (median latencies: 28.5 and 29 days for SP.MES rats and SHRSPs, respectively, Fig. 2c). However, SP.MES rats treated with tempol demonstrated significantly increased stroke latencies compared with untreated SP.MES rats and SHRSPs (Fig. 2c). These results indicated that, even though BP was significantly reduced, the p22phox deletion in SP.MES rats was not enough to decrease stroke susceptibility; rather, additional ROS scavenging was needed to rescue SHRSPs from stroke. In Pearson’s correlation analysis, oxidative stress estimated based on urinary isoprostane showed a significant correlation with BP, stroke latency and urinary protein excretion under salt loading (Fig. 2d–f), suggesting that oxidative stress was indeed important in hypertension and hypertensive organ damage.
Effects of p22phox deletion on renal pathology in SHRSPs
In previous studies, increased susceptibility to kidney injury and proteinuria have been observed in SHRSPs [24]. We therefore evaluated protein excretion in 24-h urine samples and renal histopathological changes in SP.MES rats and SHRSPs after 2 weeks of salt loading. SP.MES rats exhibited significantly lower numbers of sclerotic glomeruli and lower levels of proteinuria than SHRSPs (Fig. 3d, e). Interestingly, treatment with tempol significantly reduced glomerulosclerosis and proteinuria in SP.MES rats but not in SHRSPs (Fig. 3e).
Renal injury in salt-loaded SHRSPs and SP.MES rats. a–c Histological appearance of glomeruli showing no (a), partial (b) and complete (c) sclerotic changes. d Percentage of sclerotic glomeruli [%, (completely + partially sclerotic)/total glomeruli] after 2 weeks of salt loading. *P < 0.05 vs. SHRSPs by the X2 test (the combined numbers of glomeruli from three rats in each group were used for analysis). e Urinary protein levels; protein was measured in 24-h urine samples after 2 weeks of salt loading. #P < 0.05 vs. corresponding SHRSPs and *P < 0.05 vs. SP.MES without tempol (by Student’s t-test, n = 8/group)
Effects of other antioxidant treatments on blood pressure and stroke latency in SHRSPs and SP.MES rats
The results above clearly indicated that depletion of p22phox alone was insufficient to ameliorate oxidative stress and stroke latency in salt-loaded SHRSPs and that further reduction of oxidative stress with tempol was necessary. Accordingly, we examined the effects of other antioxidant reagents with different mechanisms of action on SP.MES rats. We used febuxostat (a xanthine oxidase (XO) inhibitor) and CoQ10 (an essential cofactor in the mitochondrial electron transport chain; supplementation with CoQ10 is known to reduce ROS in mitochondria [25]) to target two major sources of ROS in cells.
Both febuxostat and CoQ10 reduced urinary isoprostane levels in SP.MES rats (Fig. 4a). Febuxostat reduced urinary isoprostane levels in SHRSPs, while CoQ10 could not (Fig. 4b). Administration of febuxostat significantly decreased serum uric acid in SP.MES rats and tended to decrease it in SHRSPs (P = 0.08) (Fig. 4c). Serum uric acid levels were significantly reduced under salt loading in both strains (Fig. 4d), which is consistent with a recent finding in a Chinese population indicating that high salt intake decreases plasma uric acid levels [26].
Effects of febuxostat and CoQ10 on oxidative stress and uric acid in SHRSPs and SP.MES rats. a, b Febuxostat and CoQ10 were given for 2 weeks with 1% salt water to SP.MES rats (a) and SHRSPs (b). Isoprostane was measured in 24-h urine samples. *P < 0.05 vs. control (n = 5–6/group) by Bonferroni’s post hoc test. c Effects of febuxostat on serum uric acid concentrations in SHRSPs and SP.MES rats. *P < 0.05 vs. control (n = 4–5/group). d Effect of 2 weeks of salt loading on serum uric acid levels in both strains. *P < 0.05 vs. control (n = 5/group)
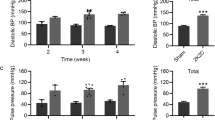
Administration of the two antioxidants resulted in significant decreases in BP both under salt loading and at baseline in SP.MES rats (Fig. 5a). Furthermore, stroke latency was significantly extended in all tempol, CoQ10, and febuxostat-treated SP.MES rats (median stroke-free survival days: 37, 52, and 93, respectively, Fig. 5c). This result is likely not due to reduced intake of salt water by the rats treated with the three reagents (Supplementary Fig. 2). Of interest, only febuxostat showed significantly reduced BP and stroke latency in salt-loaded SHRSPs (Figs. 5b, d).
Effects of febuxostat and CoQ10 on blood pressure and stroke latency. a, b SBP in SP.MES rats (a) and SHRSPs (b) treated with febuxostat or CoQ10 with 1% salt for 2 weeks. *P < 0.05 vs. control (n = 5–8). c, d Stroke-free survival in SP.MES rats (c) and SHRSPs (d) treated with tempol, febuxostat or CoQ10 in 1% salt water. The results for the control (given 1% salt water alone) and tempol-treated rats were the same as those shown in Fig. 1c. *P < 0.05 vs. control by the log-rank test; significant after Bonferroni’s correction for multiple comparisons (n = 7–13)
Discussion
In this study, using p22phox-deficient congenic SHRSPs, we showed that depletion of NOX activity per se was not sufficient to improve stroke susceptibility in SHRSPs. This result was consistent with a previous report by Yao H. et al., which revealed that the size of infarcts produced by distal middle cerebral artery occlusion was not mitigated in SP.MES rats despite decreased ROS production and lower blood pressure [18]. Intriguingly, we found that additional suppression of oxidative stress using an antioxidant reagent, tempol, was necessary to prevent stroke in SP.MES rats. Other antioxidative reagents with different mechanisms (i.e., febuxostat and CoQ10) were also effective in preventing stroke in SP.MES rats (Fig. 5c), indicating that, although reduction of oxidative stress could indeed ameliorate stroke susceptibility in SHRSPs, inhibition of NOX activity alone was not sufficient.
In contrast, examination of renal pathology in SP.MES rats and SHRSPs indicated that inhibition of NOX by p22phox depletion was sufficient to ameliorate salt-induced renal injury in SHRSPs, which was further improved by treatment with tempol (see Fig. 3). This suggests that the kidney is more vulnerable to oxidative stress than the brain in terms of stroke susceptibility in SHRSPs.
Tempol has been used to reduce oxidative stress in many studies. This reagent is generally thought to be potent enough to achieve substantial reductions in oxidative stress and prevention of subsequent biological reactions. In fact, previous studies have shown that 1 mM tempol in drinking water prevents remodeling of the cerebral vasculature and changes in blood-brain barrier permeability in SHRSPs [27, 28]. The present study could not reproduce these results in terms of delaying stroke onset even with 3 mM tempol in drinking water (Fig. 2c). We have no explanations for this discrepancy so far; differences in experimental conditions between the present study and previous studies are possible reasons. However, the addition of tempol elicited a significant effect in SP.MES rats, which implied that the combined effect of the inhibition of NOX activity and ROS scavenging was necessary to reduce stroke risk in SHRSPs. Correspondingly, renal injury was also markedly reduced in SP.MES rats treated with tempol. Whether there is a causal relationship between the two pathologies (i.e., kidney injury and stroke) is still unclear despite the fact that some attempts have been made to explore this possibility [24].
Several systems generate and degrade ROS in vivo to cooperatively regulate oxidative stress. In addition to the NOX system, which is assumed to be a major source of ROS, XO and the mitochondrial electron transport system are proposed to be additional major sources of ROS [11].
Mitochondria are important sources of ROS, where CoQ10 acts as a powerful endogenous antioxidant. CoQ10 supplementation has neuroprotective effects in ischemia/reperfusion-induced cerebral injury [25, 29, 30]. In the present study, however, CoQ10 did not improve stroke susceptibility or urinary isoprostane levels in salt-loaded SHRSPs (Fig. 4b and Fig. 5d). This observation suggests that, as in the case of tempol, CoQ10 was not enough to achieve a substantial decrease in oxidative stress in SHRSPs.
In contrast to the observations above, febuxostat elicited substantial reductions in salt-induced stroke, oxidative stress and BP in SHRSPs with and without salt loading. This result implied that XO was the most potent generator of oxidative stress in the SHRSPs, which is supported by the results of a previous study showing that XO accounts for most ROS production in SHRs [31].
XO is a molybdenum metalloenzyme that catalyzes electron transfer from hypoxanthine to uric acid (UA), producing superoxide anions as a byproduct in the process [1]. Because of its participation in oxidative stress, XO has been regarded as an essential player in the pathogenesis of oxidant-induced cardiovascular diseases [32]. Furthermore, inhibitors of XO such as allopurinol and febuxostat have been shown to improve endothelial function by reducing vascular oxidative stress as well as circulating UA levels [11, 33, 34].
However, in rodents, serum UA levels are low compared with those in humans because rodents, but not humans, harbor uricase, the enzyme that further metabolizes UA to allantoin [35]. Therefore, we must carefully determine whether febuxostat prevented hypertension and hypertensive organ damage through direct reduction of ROS generation or through reduction of UA production (or both) in SHRSPs. The UA levels seemed high in SHRSPs and SP.MES rats with and without salt loading in this study (Fig. 4d) compared with the unpublished data of Tsuchikura et al. (1.1 ± 0.1 mg/dL at 12 weeks of age, n = 10). We thus far do not have a good explanation for this discrepancy, but the present results indicate a therapeutic potential of febuxostat with regards to cardiovascular events in this model that is mediated by unknown molecular mechanisms.
This study has potential limitations. First, it is still controversial whether oxidative stress is a major cause of salt-induced stroke in SHRSPs. Inhibition of XO activity decreased urinary isoprostane levels and effectively reduced stroke susceptibility in both SP.MES rats and SHRSPs (see Fig. 4 and Fig. 5). However, as discussed above, we have no evidence indicating that ROS scavenging itself contributed to the reduction in stroke susceptibility. Regarding this matter, we cannot exclude the possibility that increased urinary isoprostane levels are the result of renal injury. Indeed, a positive correlation was found between urinary isoprostane levels and proteinuria (Fig. 2f). Serum isoprostane or different types of oxidative stress markers should also be analyzed in future studies to evaluate systemic redox conditions in vivo.
Second, since p22phox depletion abolished the activity of both NOX2 and 4, which have been argued to exert opposite effects on the cardiovascular system [2, 5], we cannot exclude the possibility that p22phox depletion resulted in a mixture of conflicting effects on stroke susceptibility. Focusing on individual NOX subtypes with recently developed genome editing technology using the CRISPR/Cas9 system [36] may unravel the roles of the subtypes in salt-induced cerebral stroke.
In summary, our study confirmed that oxidative stress is an important factor in the pathophysiology of SHRSPs, although NOXs do not seem to play a major role. Instead, our study highlighted the roles of multiple sources of ROS in hypertension and hypertensive organ damage in SHRSPs. In particular, the role of XO in hypertensive disorders should be explored in future studies.
References
Li W, Yang S. Targeting oxidative stress for the treatment of ischemic stroke: Upstream and downstream therapeutic strategies. Brain Circ. 2016;2:153.
Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, et al. Post-stroke inhibition of induced NADPH Oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010;8:e1000479.
Domínguez C, Delgado P, Vilches A, Martín-Gallán P, Ribó M, Santamarina E, et al. Oxidative stress after thrombolysis-induced reperfusion in human stroke. Stroke. 2010;41:653–60.
Moon GJ, Shin DH, Im DS, Bang OY, Nam HS, Lee JH, et al. Identification of oxidized serum albumin in the cerebrospinal fluid of ischaemic stroke patients. Eur J Neurol. 2011;18:1151–8.
Zhang L, Wu J, Duan X, Tian X, Shen H, Sun Q, et al. NADPH oxidase: a potential target for treatment of stroke. Oxid Med Cell Longev. 2016;2016:1–9.
Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–568.
Zahid HM, Ferdaus MZ, Ohara H, Isomura M, Nabika T. Effect of p22phox depletion on sympathetic regulation of blood pressure in SHRSP: evaluation in a new congenic strain. Sci Rep. 2016;6:36739.
Shirley R, Ord E, Work L. Oxidative stress and the use of antioxidants in stroke. Antioxidants. 2014;3:472–501.
Watts LT, Lloyd R, Garling RJ, Duong T. Stroke neuroprotection: targeting mitochondria. Brain Sci. 2013;3:540–60.
Di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS sources in physiological and pathological conditions. Oxid Med Cell Longev. 2016;2016:1245049.
Nomura J, Busso N, Ives A, Matsui C, Tsujimoto S, Shirakura T, et al. Xanthine oxidase inhibition by febuxostat attenuates experimental atherosclerosis in mice. Sci Rep. 2014;4:4554.
Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: Implications in hypertension. Histochem Cell Biol. 2004;122:339–52.
Guzik TJ, Sadowski J, Guzik B, Jopek A, Kapelak B, Przybylowski P, et al. Coronary artery superoxide production and nox isoform expression in human coronary artery disease. Arterioscler Thromb Vasc Biol. 2006;26:333–9.
Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313.
Kawahara T, Ritsick D, Cheng G, Lambeth JD. Point mutations in the proline-rich region of p22phox are dominant inhibitors of Nox1- and Nox2-dependent reactive oxygen generation. J Biol Chem. 2005;280:31859–69.
Chen F, Haigh S, Barman S, Fulton DJR. From form to function: the role of Nox4 in the cardiovascular system. Front Physiol. 2012;3:412.
Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, et al. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation. 2005;112:2677–85.
Yao H, Ferdaus MZ, Zahid HM, Ohara H, Nakahara T, Nabika T. Focal ischemic injury with complex middle cerebral artery in stroke-prone spontaneously hypertensive rats with loss-of-function in NADPH oxidases. PLoS ONE. 2015;10:e0138551.
Komers R, Xu B, Schneider J, Oyama TT. Effects of xanthine oxidase inhibition with febuxostat on the development of nephropathy in experimental type 2 diabetes. Br J Pharmacol. 2016; 2573–88.
Ishikawa N, Harada Y, Maruyama R, Masuda J, Nabika T. Genetic effects of blood pressure quantitative trait loci on hypertension-related organ damage: evaluation using multiple congenic strains. Hypertens Res. 2008;31:1773–9.
Nakamura T, Yamamoto E, Kataoka K, Yamashita T, Tokutomi Y, Dong YF, et al. Pioglitazone exerts protective effects against stroke in stroke-prone spontaneously hypertensive rats, independently of blood pressure. Stroke. 2007;38:3016–22.
Gandolgor TA, Ohara H, Cui ZH, Hirashima T, Ogawa T, Saar K, et al. Two genomic regions of chromosomes 1 and 18 explain most of the stroke susceptibility under salt loading in stroke-prone spontaneously hypertensive Rat/Izm. Hypertension. 2013;62:55–61.
Milatovic D, Montine TJ, Aschner M. Measurement of isoprostanes as markers of oxidative stress. Method Mol Biol. 2011;758:195–204.
Churchill PC, Churchill MC, Griffin KA, Picken M, Webb RC, Kurtz TW, et al. Increased genetic susceptibility to renal damage in the stroke-prone spontaneously hypertensive rat. Kidney Int. 2002;61:1794–1800.
El-Aal SAA, El-Fattah MAA, El-Abhar HS. CoQ10 augments rosuvastatin neuroprotective effect in a model of global ischemia via inhibition of NF-κB/JNK3/Bax and activation of Akt/FOXO3A/Bim cues. Front Pharmacol. 2017;8:735.
Wang Y, Chu C, Wang KK, Hu JW, Yan Y, Lv YB, et al. Effect of salt intake on plasma and urinary uric acid levels in Chinese adults: an interventional trial. Sci Rep. 2018;8:1434.
Kim-Mitsuyama S, Yamamoto E, Tanaka T, Zhan Y, Izumi Y, Izumiya Y, et al. Critical role of angiotensin II in excess salt-induced brain oxidative stress of stroke-prone spontaneously hypertensive rats. Stroke. 2005;36:1083–8.
Pires PW, Deutsch C, McClain JL, Rogers CT, Dorrance AM. Tempol, a superoxide dismutase mimetic, prevents cerebral vessel remodeling in hypertensive rats. Microvasc Res. 2010;80:445–52.
Horecky J, Gvozdjakova A, Kucharska J, E. Obrenovich M, H. Palacios H, Li Y, et al. Effects of coenzyme Q and creatine supplementation on brain energy metabolism in rats exposed to chronic cerebral hypoperfusion. Curr Alzheimer Res. 2011;8:868–75.
Abd-El-Fattah AA, El-Sawalhi MM, Rashed ER, El-Ghazaly MA. Possible role of vitamin E, coenzyme Q10 and rutin in protection against cerebral ischemia/reperfusion injury in irradiated rats. Int J Radiat Biol. 2010;86:1070–8.
Suzuki H, DeLano FA, Parks DA, Jamshidi N, Granger DN, Ishii H, et al. Xanthine oxidase activity associated with arterial blood pressure in spontaneously hypertensive rats. Proc Natl Acad Sci USA. 1998;95:4754–9.
Taheraghdam AA, Sharifipour E, Pashapour A, Namdar S, Hatami A, Houshmandzad S, et al. Allopurinol as a preventive contrivance after acute ischemic stroke in patients with a high level of serum uric acid: a randomized, controlled trial. Med Princ Pract. 2014;23:134–9.
Higgins P, Ferguson LD, Walters MR. Xanthine oxidase inhibition for the treatment of stroke disease: a novel therapeutic approach. Expert Rev Cardiovasc Ther. 2011;9:399–401.
Dawson J, Quinn T, Harrow C, Lees KR, Weir CJ, Cleland SJ, et al. Allopurinol and nitric oxide activity in the cerebral circulation of those with diabetes. Diabetes Care. 2009;32:135–7.
Waring WS, Webb DJ, Maxwell SR. Uric acid as a risk factor for cardiovascular disease. QJM. 2000;93:707–13.
Ma Y, Shen B, Zhang X, Lu Y, Chen W, Ma J, et al. Heritable multiplex genetic engineering in rats using CRISPR/Cas9. PLoS ONE. 2014;9:e89413.
Acknowledgements
The authors thank Satoko Mishima, Masamichi Koike and Masaki Misumi for their skillful assistance in the histological evaluation.
Funding
This work was partly supported by JSPS KAKENHI 26293086 (to T.N.) and 17K08787 (to H.O.).
Author information
Authors and Affiliations
Contributions
TN conceived, designed and supervised this study. DN, MZF, HMZ, and HO performed the experiments. TN, KF, DN, and HO analyzed the data and discussed the results. DN wrote the manuscript in consultation with TN and HO. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ngarashi, D., Fujikawa, K., Ferdaus, M.Z. et al. Dual inhibition of NADPH oxidases and xanthine oxidase potently prevents salt-induced stroke in stroke-prone spontaneously hypertensive rats. Hypertens Res 42, 981–989 (2019). https://doi.org/10.1038/s41440-019-0246-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-019-0246-2
Keywords
This article is cited by
-
Inhibition of Maternal c-Src Ameliorates the Male Offspring Hypertension by Suppressing Inflammation and Neurotransmitters in the Paraventricular Nucleus
Cardiovascular Toxicology (2021)
-
Low doses of folic acid can reduce hyperhomocysteinemia-induced glomerular injury in spontaneously hypertensive rats
Hypertension Research (2020)