Abstract
Depression is associated with mortality in patients with cardiovascular risk factors. The frequency and severity of depression and the association between depression and cardiovascular events have sex-specific and ethnic differences. We conducted this study to evaluate the sex-specific difference in the association between depression and cardiovascular prognosis in patients with cardiovascular risk factors. We enrolled 4025 patients (64.7 ± 10.9 years, 53% women, 47% men) with cardiovascular risk factors in the Japan Morning Surge–Home Blood Pressure study. Depressive symptoms were assessed using the Beck Depression Inventory (BDI). The follow-up period was 47 ± 24 months. The primary end points were all-cause mortality and nonfatal cardiovascular events. The BDI scores and the prevalence of depression were significantly higher in women than in men. When a BDI score of 16 was the cutoff, the primary end points in the depression group (n = 217) were significantly higher than those in the nondepression group (n = 1677) among men (adjusted hazard ratio 1.76, 95% confidence interval: 1.17, 2.64; P = 0.007). In women, the primary end points in the depression and nondepression groups were similar when BDI scores of 16, 14, and 10 were the cutoffs. In conclusion, depression defined by a BDI score ≥16 was associated with cardiovascular events in men with cardiovascular risk factors.
Similar content being viewed by others
Introduction
Depression has a high prevalence and is associated with a decreased quality of life [1]. Depression is also associated with mortality in patients with cardiovascular disease and cardiovascular risk factors. Several studies have shown that a higher depression score is associated with a poor prognosis in patients after myocardial infarction [2,3,4]. A higher depression score has also been associated with mortality and cardiovascular events in hypertensive patients [5, 6] and patients with diabetes [7,8,9]. Therefore, the identification of depression can help predict cardiovascular events in individuals with cardiovascular disease or cardiovascular risk factors.
The association between baseline characteristics and the cardiovascular event rate differs between the genders [10], and a propensity for depression appears to be strong in women [11]. The prevalence of depression is more frequent in women than in men among patients with coronary artery disease, heart failure [12, 13], and hypertension [5]. However, the prognosis for depressive men with myocardial infarction is poor compared with that of depressive women [14]. In addition, the difference in prognosis between depressive men and women remains unclear in patients with cardiovascular risk factors (e.g., hypertension or diabetes). The frequency and severity of depression also show ethnic differences [15, 16].
The Beck Depression Inventory (BDI) is broadly used as an evaluation predictor of depression; however, various cutoff levels of the BDI may be used [17]. We conducted this study to evaluate the sex-specific difference in the association between depression and cardiovascular prognosis in patients with cardiovascular risk factors using various cutoff levels of the BDI score.
Methods
Study participants
This study was performed as a subanalysis part of the Japan Morning Surge–Home Blood Pressure (J-HOP) study [18]. The protocol of the J-HOP study has been registered at the University Hospital Medical Information Network Clinical Trials Registry website under the trial identifier UMIN000000894. The J-HOP study is a prospective, observational evaluation of the predictive values of home blood pressure (BP) for cardiovascular events in Japanese outpatients with any of the following cardiovascular risk factors identified at a clinic or hospital: hypertension, diabetes, hyperlipidemia, smoking habit (including patients with chronic obstructive pulmonary disease), chronic renal disease, atrial fibrillation, metabolic syndrome, and sleep apnea syndrome.
Hypertension was defined as an office systolic BP ≥ 140 mmHg, diastolic BP ≥ 90 mmHg, or both or current treatment with anti-hypertensive medication. Hyperlipidemia was defined as a total cholesterol level ≥240 mg/dL or current treatment with an oral lipid-lowering agent. Diabetes was defined as a fasting glucose level ≥126 mg/dL, a random nonfasting glucose level ≥200 mg/dL, or current treatment with an oral hypoglycemic agent or insulin. Smoking was defined as a current smoking habit.
Assessment of depression
Depressive symptoms were assessed using the BDI [19], which is a 21-item self-completed questionnaire that measures the characteristic symptoms of depression, including negative affect, cognition, and somatic symptoms. The BDI has been shown to have prognostic value in cardiac populations [20, 21]. The Japanese version of the BDI score has been validated [22] and used for clinical follow-up and depression therapy [23, 24]. We determined depression as a BDI score ≥10, ≥14, and ≥16 in accord with past reports [17, 25, 26].
Blood pressure measurements
Office BP was measured by a nurse using a validated upper arm cuff oscillometric BP device [27] with the patient seated and after 5 min of rest. Three measurements were conducted at each of two visits. Three BP and pulse rate measurements were obtained continuously at 15-s intervals: the 1st measurement, a 15-s interval, the 2nd measurement, a 15-s interval, and the 3rd measurement. The values of office BP and pulse rate were defined by the average of six readings from each of the two clinic visits.
Blood and urine samples
Blood and spot urine samples were collected in the morning in a fasting state at enrollment. The blood samples were centrifuged at 3000×g for 15 min at room temperature. Plasma/serum samples after separation and urine samples were stored at 4 °C in refrigerated containers and sent to a commercial laboratory within 24 h of collection. Serum samples after separation were also stored at −80 °C. All assays were performed within 24 h of sample collection at the same single laboratory center.
We measured the levels of total cholesterol, triglycerides, fasting glucose, glycated hemoglobin (hemoglobin A1c), and brain natriuretic peptide (BNP). Urinary albumin levels were measured using a turbidimetric immunoassay and is expressed as the albumin-to-creatinine ratio (mg/gCr). Both the serum and urine creatinine were measured by enzymatic assay. All blood samples were measured at a single laboratory.
Pulse oximetry
The nocturnal oxygen saturation changes were evaluated by a pulse oximetry device (PULSOX-3Si, Minolta, Tokyo) in 973 patients. The device was attached to the left arm of the patient from the time that he or she went to bed until she/he rose in the morning. The sensor probe was fitted to the second, third or fourth finger and secured with tape or a finger glove to prevent detaching. The data recorded by the pulse oximeter were downloaded to a personal computer via an interface and analyzed using proprietary software supplied with the equipment (DS-Me ver. 2.1, Minolta). We used the value of oxygen desaturation per hour (i.e., the oxygen desaturation index (ODI)) as an indicator of sleep disorder. A 3% ODI was selected as an index of oxygen desaturation.
Follow-up and primary end points
The primary end points included all-cause mortality and nonfatal cardiovascular events. The outcomes were categorized as follows:
-
(1)
Fatal and nonfatal stroke, defined as the sudden onset of a neurologic deficit that persisted for ≥24 h in the absence of another disease that could account for the symptoms, with the findings of brain computed tomography or magnetic resonance imaging; transient ischemic attack was not included.
-
(2)
Fatal and nonfatal coronary artery disease, defined as acute myocardial infarction, angina pectoris that required percutaneous coronary intervention, and sudden death within 24 h of the abrupt onset of symptoms.
-
(3)
Fatal and nonfatal heart failure and artery disease that required admission.
-
(4)
Other fatal events (e.g., cancer or suicide).
Evidence regarding the previously described cardiovascular disease outcomes was ascertained by ongoing reports from a general physician at each institute. Incident fatal and nonfatal events were ascertained by means of annual or more frequent reviews of patient medical records. When patients failed to come to the hospital, we interviewed them and/or their families by telephone.
The end point committee adjudicated all events by reviewing patient files and source documents or requesting more detailed written information from investigators. The committee was blinded to the individual clinical characteristics, including home BP data. A final follow-up survey to reconfirm the clinical outcomes was performed from September 2014 to March 2015.
Statistical analysis
The χ2 test was used for the categorical data. Unpaired t-tests were used for the normally distributed data and comparisons between two groups, and an analysis of variance was used for comparisons of more than two groups. The hazard ratios were adjusted for age, sex, body mass index, drinking habit, current smoking habit, history of hypertension, dyslipidemia, diabetes, ischemic heart disease and stroke. We used Cox proportional hazard models to examine the associations between depression (defined as a BDI score ≥16) and the primary end points, including the clinic BP, the albumin-to-creatinine ratio, and the BNP level. To evaluate the effect size of depression (defined as a BDI score ≥16) on outcomes, we used the likelihood ratio values to evaluate the goodness-of-fit of the predictive models. Our comparison of the discriminative ability of each model was conducted with Harrell C statistics (with 95% confidence intervals (CIs) calculated by bootstrapping). The covariates included traditional risk factors: age, sex, body mass index, current smoking habit, history of hypertension, dyslipidemia, diabetes, prior ischemic heart disease, and prior stroke; the factors in model 1, clinic systolic BP, log urine albumin-to-creatinine ratio, and BNP were used in model 2. All statistical analyses were performed using the computer software package SPSS (ver. 20; SPSS, Chicago, IL) and the SAS system, ver. 9.4 (SAS Institute, Cary, NC). P-values <0.05 were considered significant.
Results
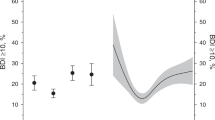
We enrolled 4025 patients from the J-HOP study. The average age was 64.7 ± 10.9 years, and the percentage of men was 47%. The average BDI score was 9.2, and the median BDI score was 7 (25th–75th percentiles: 4–13). The BDI scores and prevalence of depression were significantly higher in the women than in the men (BDI scores: 10.3 ± 7.5 vs. 7.6 ± 6.4, respectively; P < 0.001; Fig. 1).
The characteristics of the four subgroups of patients divided by depression (defined as a BDI score ≥16) and nondepression in the men and women are shown in Table 1. In both sexes, the depression group had significantly lower values of the body mass index and a higher office pulse rate and BNP than in the group without depression. Among the men, the depression group had a significantly higher percentage of patients with a smoking habit (27.8% vs. 20.9%, respectively; P = 0.021) and a significantly higher albumin-to-creatinine ratio (median 15.7 vs. 10.5 mg/gCr, respectively; P = 0.006) than in the group without depression. Among the women, the depression group was significantly older (67.1 ± 10.5 vs. 65.1 ± 10.3, respectively; P < 0.001) than the group without depression.
The mean duration of follow-up was 47 ± 24 months. During the follow-up period, 239 primary end points were recorded. We evaluated the interaction between sex and depression status (Table 2) and confirmed a sex interaction between depression and the primary end point.
The differences in the rates of the primary end point between the groups by the different BDI thresholds are shown in Fig. 2. When a BDI score ≥16 was used as the cutoff, the number of primary end points in the depression group (n = 217) was significantly higher than that in the nondepression group (n = 1677) among the men (adjusted hazard ratio (HR) 1.75, 95% CI: 1.17, 2.64; P = 0.007; Fig. 2). However, the primary end points in the depression and nondepression groups were similar when BDI score ≥14 and ≥10 were used as the cutoffs in the men (BDI 14: adjusted HR 1.34, 95% CI: 0.91, 1.95; P = 0.137; BDI 10: adjusted HR 1.38, 95% CI: 1.00, 1.92; P = 0.051).
Hazard ratios of depression in the primary end points by the different Beck Depression Inventory (BDI) thresholds in men and women. The hazard ratios were adjusted for age, sex, body mass index, drinking habit, current smoking habit, history of hypertension, dyslipidemia, diabetes, ischemic heart disease and stroke. Plots represent hazard ratios and 95% CIs (confidence intervals)
In contrast, among the women, the primary end points in the depression and nondepression groups were similar when BDI scores ≥16, ≥14, and ≥10 were used as the cutoffs (BDI score ≥16: adjusted HR 0.98, 95% CI: 0.59, 1.65; P = 0.951; BDI score ≥14: adjusted HR 0.89, 95% CI: 0.55, 1.44; P = 0.629; BDI score ≥10: adjusted HR 1.49, 95% CI: 0.97, 2.29; P = 0.070; Fig. 2). We also conducted Cox proportional hazard modeling to adjust the clinic BP, albumin-to-creatinine ratio, and BNP. Among the men, depression defined as a BDI score ≥16 was an independent predictor of the primary end point after adjusting the clinic systolic BP, albumin-to-creatinine ratio, and BNP (HR 1.56, 95% CI: 1.03, 2.36; P = 0.036, Table 3).
To evaluate the effect size of depression (defined as a BDI score ≥16) on outcomes, we used the likelihood ratio values to evaluate the goodness-of-fit of the predictive models (Table 4). In the cardiovascular event prediction models, the model fit assessed by a likelihood test was significantly improved when we added depression to the models for the men (likelihood test in model 1: 1016.9 vs. 1013.7, P = 0.011; model 2: 1003.0 vs. 1001.0, P = 0.044), but not for the women (likelihood test in model 1: 566.3 vs. 566.3, P = 0.95; model 2: 557.8 vs. 557.8, P = 0.99). The details of the primary end points are summarized in the Supplemental table. Among the men, the fatal and nonfatal cardiovascular events were higher in the depression group (defined as BDI ≥ 16) than in the nondepression group (BDI < 16; fatal cardiovascular event: 1.8% vs. 0.6%; P = 0.044, nonfatal cardiovascular event: 8.8% vs. 4.2%; P = 0.003). Two suicide events occurred in the depressive women group.
We evaluated the association between sleep disorder and depression (defined as a BDI score ≥16). Among the men, the 3% ODI was similar in the patients with depression (n = 63) and nondepression (n = 434) (7.4 ± 7.3 vs. 10.3 ± 30.5, P = 0.46). Among the women, the 3% ODI was also similar in the patients with depression (n = 96) and nondepression (n = 380) (7.5 ± 27.3 vs. 6.8 ± 8.2, P = 0.66).
Discussion
The major finding of this study is that when a BDI score ≥16 was used as the cutoff, the rate of primary end points in the depression group was higher than that in the nondepression group among the men in this Japanese population.
Previous studies have reported the association between depression and cardiovascular events in patients with previous cardiovascular events or cardiovascular risk factors. A high BDI score has been associated with poor prognosis in patients after myocardial infarction [2,3,4], and depression assessed by the Hospital Anxiety and Depression Scale was associated with the worsening of heart failure in patients with previous heart failure [28]. In hypertensive patients, two studies showed that depression assessed by the Center for Epidemiologic Studies Depression Scale was associated with high mortality [5, 29]. In patients with diabetes, depression was associated with a 1.52-fold increase in the likelihood of total death in a 10-year prospective cohort study [30]. Depression assessed by the Patient Health Questionnaire-9 was also associated with a 2.24-fold increase in mortality in The Action to Control Cardiovascular Risk in Diabetes (ACCORD) study, in which diabetic patients were enrolled [31]. However, the association between depression and prognosis was not evaluated by sex in these previous reports.
In the present study, depression defined by a BDI score ≥16 was associated with a 1.7-fold increase in cardiovascular events among the men. Two studies have shown a sex-specific difference in the association between depression and cardiovascular prognosis. Larsen and colleagues investigated the association between depression and cardiovascular prognosis in 892 patients after myocardial infarction [32], and they observed that the incidence of cardiovascular events in depression was 1.89-fold in the men and 1.59-fold in the women; however, the sex difference was not significant. In the MINDMAPS study [14], a meta-analysis of depression patients after myocardial infarction, the frequency was higher in women; however, the prognosis was poor in men. Depression is more common among women with acute or chronic heart failure [33, 34]; however, depression was not associated with mortality in patients of either sex [33].
To the best of our knowledge, there are no data on sex differences in the association between depression and cardiovascular events in patients with hypertension or diabetes; however, three studies indicated an association between depression and cardiovascular events by dividing the sexes in a general population. Wyman et al. [35] investigated 2762 general subjects, and their analysis showed that depression defined by a Center for Epidemiological Studies-Depression score >16 was associated with an increase in cardiovascular disease (by 2.99-fold) in elderly (≥65 years) men during a 15-year follow-up. In a survey in Taiwan [36], 1764 elderly (≥65 years) subjects were assessed, and depression was associated with a higher risk of cardiovascular mortality (2.71-fold) in men only. Our findings are similar to these results.
However, Gasse et al. [37] showed that among 15- to 59-year-old subjects in a population-based cohort, the increase in cardiovascular events in women with depression was 1.64-fold compared to 1.39-fold in men with depression. The association between depression and cardiovascular events may be different in individuals of different ages and ethnicities.
In the present study, the BDI scores and the prevalence of depression were significantly higher in the women than in the men. Kojima et al. [22] validated the Japanese version of the BDI score, and the mean women’s score was significantly higher than that of the men in their study (women: 9.9 ± 6.4, men: 8.3 ± 6.5, P < 0.001). Our corresponding findings are concordant with the report by Kojima et al. We suspect that the mechanisms that underlie the association between women and depression may involve the following: (1) patient characteristics, including psychosocial mediators and physical activity, might be different by gender [38, 39], and (2) gender differences in serotonin metabolism (which affects depression) might be associated with the gender difference in depression [40].
There are various reports regarding BDI cutoffs [20, 21, 25, 26]. In our study, the median BDI score in women was >10; thus, the cutoff level of a BDI score ≥10 (which is widely used) may be inadequate as a predictor of cardiovascular events in the Japanese population. When the cutoff BDI score is 16, the negative predictive value is not low, and the positive predictive value is increased compared with the cutoff BDI score of 10. Further studies are needed to identify the optimal cutoff value of depression scores as a cardiovascular event predictor in patients with cardiovascular risk factors.
In this study, depressive men had higher BP values, pulse rates, percentages of current smoking, and albumin-to-creatinine ratios. The effect of emotional stress on the increase of BP and pulse rate is greater in men than in women [41]. An increase in the double product (i.e., the product of the BP and pulse rate) may affect cardiovascular events. It has been shown that there is a higher rate of smoking among individuals with depression [42]. Depressive patients with cardiovascular risk factors should be advised to stop smoking.
The association between depression and an increase in the albumin-to-creatinine ratio is associated with asymptomatic target organ damage, which may result in cardiovascular events. Moreover, depression has been associated with both macro- and microvascular events [43]; thus, clinicians should take heed of an increase in the albumin-to-creatinine ratio in depressive patients with cardiovascular risk factors.
This study has limitations. First, the enrolled subjects in this study were Japanese patients with cardiovascular risk factors. Second, we did not assess education, social status, financial status, and marital status.
In conclusion, depression defined by a BDI score ≥16 was associated with cardiovascular events in men with cardiovascular risk factors in this practical population study. Our results emphasize the importance of the assessment of depression to identify individuals who may be at risk for the development of cardiovascular events. The optimal cutoff BDI score should be investigated in further clinical trials.
References
Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223.
Frasure-Smith N, Lespérance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA. 1993;270:1819–25.
Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146538 participants in 54 observational studies. Eur Heart J. 2006;27:2763–74.
Frasure-Smith N, Lespérance F. Depression and other psychological risks following myocardial infarction. Arch Gen Psychiatry. 2003;60:627–36.
Axon RN, Zhao Y, Egede LE. Association of depressive symptoms with all-cause and ischemic heart disease mortality in adults with self-reported hypertension. Am J Hypertens. 2010;23:30–7.
Wassertheil-Smoller S, Applegate WB, Berge K, Chang CJ, Davis BR, Grimm R Jr, et al. Change in depression as a precursor of cardiovascular events. SHEP Cooperative Research Group (systolic hypertension in the elderly). Arch Intern Med. 1996;156:553–61.
Katon WJ, Rutter C, Simon G, Lin EH, Ludman E, Ciechanowski P, et al. The association of comorbid depression with mortality in patients with type 2 diabetes. Diabetes Care. 2005;28:2668–72.
Bruce DG, Davis WA, Starkstein SE, Davis TM. A prospective study of depression and mortality in patients with type 2 diabetes: the Fremantle Diabetes Study. Diabetologia. 2005;48:2532–9.
Egede LE, Zheng D, Simpson K. Comorbid depression is associated with increased health care use and expenditures in individuals with diabetes. Diabetes Care. 2002;25:464–70.
Teramukai S, Okuda Y, Miyazaki S, Kawamori R, Shirayama M, Teramoto T. Dynamic prediction model and risk assessment chart for cardiovascular disease based on on-treatment blood pressure and baseline risk factors. Hypertens Res. 2016;39:113–8.
Silverstein B, Edwards T, Gamma A, Ajdacic-Gross V, Rossler W, Angst J. The role played by depression associated with somatic symptomatology in accounting for the gender difference in the prevalence of depression. Soc Psychiatry Psychiatr Epidemiol. 2013;48:257–63.
Frasure-Smith N, Lespérance F, Juneau M, Talajic M, Bourassa MG. Gender, depression, and one-year prognosis after myocardial infarction. Psychosom Med. 1999;61:26–37.
Gottlieb SS, Khatta M, Friedmann E, Einbinder L, Katzen S, Baker B, et al. The influence of age, gender, and race on the prevalence of depression in heart failure patients. J Am Coll Cardiol. 2004;43:1542–9.
Doyle F, McGee H, Conroy R, Conradi HJ, Meijer A, Steeds R, et al. Systematic review and individual patient data meta-analysis of sex differences in depression and prognosis in persons with myocardial infarction: a MINDMAPS Study. Psychosom Med. 2015;77:419–28.
Patel NC, Delbello MP, Strakowski SM. Ethnic differences in symptom presentation of youths with bipolar disorder. Bipolar Disord. 2006;8:95–9.
Angold A, Erkanli A, Farmer EM, Fairbank JA, Burns BJ, Keeler G, et al. Psychiatric disorder, impairment, and service use in rural African American and white youth. Arch Gen Psychiatry. 2002;59:893–901.
Ren Y, Yang H, Browning C, Thomas S, Liu M. Performance of screening tools in detecting major depressive disorder among patients with coronary heart disease: a systematic review. Med Sci Monit. 2015;21:646–53.
Ishikawa J, Haimoto H, Hoshide S, Eguchi K, Shimada K, Kario K, et al. An increased visceral-subcutaneous adipose tissue ratio is associated with difficult-to-treat hypertension in men. J Hypertens. 2010;28:1340–6.
Furlanetto LM, Mendlowicz MV, Romildo Bueno J. The validity of the Beck Depression Inventory-Short Form as a screening and diagnostic instrument for moderate and severe depression in medical inpatients. J Affect Disord. 2005;86:87–91.
Davidson KW, Reickman N, Lespérance F. Psychological theories of depression: potential application for the prevention of acute coronary syndrome recurrence. Psychosom Med. 2004;66:165–73.
Frasure-Smith N, Lespérance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation. 1995;91:999–1005.
Kojima M, Furukawa TA, Takahashi H, Kawai M, Nagaya T, Tokudome S. Cross-cultural validation of the Beck Depression Inventory-II in Japan. Psychiatry Res. 2002;110:291–9.
Hiroe T, Kojima M, Yamamoto I, Nojima S, Kinoshita Y, Hashimoto N, et al. Gradations of clinical severity and sensitivity to change assessed with the Beck Depression Inventory-II in Japanese patients with depression. Psychiatry Res. 2005;135:229–35.
Imamura K, Kawakami N, Furukawa TA, Matsuyama Y, Shimazu A, Umanodan R, et al. Effects of an internet-based cognitive behavioral therapy (iCBT) program in Manga format on improving subthreshold depressive symptoms among healthy workers: a randomized controlled trial. PLoS ONE. 2014;9:e97167.
Veerman JL, Dowrick C, Ayuso-Mateos JL, Dunn G, Barendregt JJ. Population prevalence of depression and mean Beck Depression Inventory score. Br J Psychiatry. 2009;195:516–9.
Huffman JC, Doughty CT, Januzzi JL, Pirl WF, Smith FA, Fricchione GL. Screening for major depression in post-myocardial infarction patients: operating characteristics of the Beck Depression Inventory-II. Int J Psychiatry Med. 2010;40:187–97.
Anwar YA, Giacco S, McCabe EJ, Tendler BE, White WB. Evaluation of the efficacy of the Omron HEM-737 IntelliSense device for use on adults according to the recommendations of the Association for the Advancement of Medical Instrumentation. Blood Press Monit. 1998;3:261–5.
Gustad LT, Laugsand LE, Janszky I, Dalen H, Bjerkeset O. Symptoms of anxiety and depression and risk of heart failure: the HUNT study. Eur J Heart Fail. 2014;15:861–70.
Kuo PL, Pu C. The contribution of depression to mortality among elderly with self-reported hypertension: analysis using a national representative longitudinal survey. J Hypertens. 2011;29:2084–90.
Coleman SM, Katon W, Lin E, Von Korff M. Depression and death in diabetes; 10-year follow-up of all-cause and cause-specific mortality in a diabetic cohort. Psychosomatics. 2013;54:428–36.
Sullivan MD, O’Connor P, Feeney P, Hire D, Simmons DL, Raisch DW, et al. Depression predicts all-cause mortality: epidemiological evaluation from the ACCORD HRQL substudy. Diabetes Care. 2012;35:1708–15.
Larsen KK, Christensen B, Søndergaard J, Vestergaard M. Depressive symptoms and risk of new cardiovascular events or death in patients with myocardial infarction: a population-based longitudinal study examining health behaviors and health care interventions. PLoS ONE. 2013;8:e74393.
Parissis JT, Mantziari L, Kaldoglou N, Ikonomidis I, Nikolaou M, Mebazaa A, et al. Gender-related differences in patients with acute heart failure: management and predictors of in-hospital mortality. Int J Cardiol. 2013;168:185–9.
Orszulak M, Mizia-Stec K, Siennicka A, Goscinska-Bis K, Waga K, Wojcik M, et al. Differences of psychological features in patients with heart failure with regard to gender and aetiology - results of a CAPS-LOCK-HF (Complex Assessment of Psychological Status Located in Heart Failure) study. Int J Cardiol. 2016;219:380–6.
Wyman L, Crum RM, Celentano D. Depressed mood and cause-specific mortality: a 40-year general community assessment. Ann Epidemiol. 2012;22:638–43.
Teng PR, Yeh CJ, Lee MC, Lin HS, Lai TJ. Change in depressive status and mortality in elderly persons: results of a national longitudinal study. Arch Gerontol Geriatr. 2013;56:244–9.
Gasse C, Laursen TM, Baune BT. Major depression and first-time hospitalization with ischemic heart disease, cardiac procedures and mortality in the general population: a retrospective Danish population-based cohort study. Eur J Prev Cardiol. 2014;21:532–40.
Leach LS, Christensen H, Mackinnon AJ, Windsor TD, Butterworth P. Gender differences in depression and anxiety across the adult lifespan: the role of psychosocial mediators. Soc Psychiatry Psychiatr Epidemiol. 2008;43:983–98.
Zhang J, Yen ST. Physical activity, gender difference, and depressive symptoms. Health Serv Res. 2015;50:1550–73.
Hiroi R, Carbone DL, Zuloaga DG, Bimonte-Nelson HA, Handa RJ. Sex-dependent programming effects of prenatal glucocorticoid treatment on the developing serotonin system and stress-related behaviors in adulthood. Neuroscience. 2016;320:43–56.
Samad Z, Boyle S, Ersboll M, Vora AN, Zhang Y, Becker RC, et al. Sex differences in platelet reactivity and cardiovascular and psychological response to mental stress in patients with stable ischemic heart disease: insights from the REMIT study. J Am Coll Cardiol. 2014;64:1669–78.
Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284:2606–10.
Lin EH, Rutter CM, Katon W, Heckbert SR, Ciechanowski P, Oliver MM, et al. Depression and advanced complications of diabetes: a prospective cohort study. Diabetes Care. 2010;33:264–9.
Acknowledgements
We thank the numerous study investigators, fellows, nurses, and research coordinators at each of the study sites who have participated in the J-HOP study. We also thank Ms. Kimiyo Saito for the coordination and data management of this study and Ms. Ayako Okura for the editorial assistance.
Funding
This study was financially supported in part, by a grant from the 21st Century Center of Excellence Project run by Japan’s Ministry of Education, Culture, Sports, Science and Technology; a grant from the Foundation for Development of the Community (Tochigi); a grant from Omron Healthcare Co., Ltd.; a Grant-in-Aid for Scientific Research (B) (21390247) from The Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, 2009–2013; and funds from the MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2011–2015 Cooperative Basic and Clinical Research on Circadian Medicine (S1101022) to K.K.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
K.K. has received research grants from Teijin Pharma Limited, Novartis Pharma K.K., Takeda Pharmaceutical Co., Ltd., Omron Healthcare Co., Ltd., and Fukuda Denshi, and honoraria from Mochida Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., and Sumitomo Dainippon Pharma Co., Ltd. The remaining authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kabutoya, T., Hoshide, S., Davidson, K.W. et al. Sex differences and the prognosis of depressive and nondepressive patients with cardiovascular risk factors: the Japan Morning Surge–Home Blood Pressure (J-HOP) study. Hypertens Res 41, 965–972 (2018). https://doi.org/10.1038/s41440-018-0103-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0103-8
Key words
This article is cited by
-
The association between depressive symptoms and masked hypertension in participants with normotension measured at research center
Hypertension Research (2024)
-
Evaluation of affective temperaments and arterial stiffness in different hypertension phenotypes
Hypertension Research (2021)
-
Depression in Patients with Heart Diseases: Gender Differences and Association of Comorbidities, Optimism, and Spiritual Struggle
International Journal of Behavioral Medicine (2021)