Abstract
The purpose of this study was to evaluate the impact of prebifurcation renal denervation in a swine model and assess its safety through optical coherence tomography (OCT). Prebifurcation renal denervation with a multi-electrode catheter was performed in one renal artery of 12 healthy pigs, with the contralateral artery and kidney being used as controls. Angiograms and OCT pullbacks were obtained peri-procedurally and 1 month post procedure. Renal tissue catecholamines were quantified, and the arterial wall and peri-adventitial tissue were analyzed histologically. Intraluminal changes (endothelial swelling, spasm, and thrombus formation) were observed acutely by OCT in most of the treated arteries and were no longer visible at follow-up. Histology revealed a statistically significant accumulation of collagen (fibrosis) and a near absence of tyrosine hydroxylase labeling in the denervated artery, suggesting a clear reduction in nervous terminals. Renal tissue catecholamine levels were similar between both sides, probably due to the low number of ablation points and the renorenal reflex. The present study demonstrates that renal denervation is associated with acute intimal disruptions, areas of fibrosis, and a reduction in nervous terminals. The lack of difference in renal tissue catecholamine levels is indicative of the need to perform the highest and safest number of ablation points in both renal arteries. These findings are important because they demonstrate the histological consequences of radiofrequency energy application and its medium-term safety.
Similar content being viewed by others
Introduction
Sympathetic nervous system hyperactivation is associated with several pathological conditions, such as arterial hypertension [1]. Radiofrequency (RF) catheter-based renal denervation (RDN) has emerged as a minimally invasive percutaneous procedure, designed to potentially disrupt the sympathetic fibers of the renal vasculature and hence decrease the sympathetic drive. Several proof-of-concept randomized controlled trials have been conducted in resistant hypertensive patients [2,3,4,5], but the results have been inconsistent. Disbelief in the true efficacy of this technique has arisen, especially after the disclosure of the HTN-3 trial [6]. So far, the outcomes of RDN have been unpredictable, and several hypotheses, such as the Hawthorne effect, operator inexperience, an inability to achieve circumferential energy delivery, location inadequacy, and catheter design, have been extensively discussed as reasons for response variability [7]. Early investigations revealed that sympathetic fibers are mainly located in the adventitia of renal vessels with larger bundles in the proximal segment, compared to the thinner fibers found distally [8, 9]. However, more recent research on renal nerve distribution showed that even though sympathetic nerve fibers are concentrated in the proximal and middle arterial segments, the distance from the lumen is significantly higher than that of distal fibers, which are fewer but probably more vulnerable to RF injury [10]. Safety is a major concern after RDN, and the occurrence of renal stenosis has been described in a few patients. However, the actual incidence in the medium term and long term is not known. Currently, there is insufficient data regarding the correlation between prebifurcation RF-induced vessel injury, assessed by intra-arterial imaging, and the levels of fibrosis, density of nervous terminals, and effects on renal tissue catecholamine levels in the medium term.
The main purposes of this study are: (1) to evaluate the effect of RF energy application (using a multi-electrode RDN catheter) on the proximal/middle segment of the renal artery (prebifurcation) of a swine model, through histological analysis of the arterial wall and peri-adventitial tissue and to compare the levels of fibrosis with the untreated contralateral side (control) and (2) to assess the disruption of the arterial wall acutely (pre- and post procedure) and at 1 month of follow-up with intrarenal optical coherence tomography (OCT). A secondary endpoint is the assessment of renal tissue epinephrine (Ep) and norepinephrine (NE) levels in both sides.
Methods
Study design and animal procedures
All animal procedures were performed in the Veterinary Medical School of the Lisbon University, Portugal and were approved by the Ethical and Animal Welfare Commission (CEBEA) of the institution and by the Portuguese National Authority for Animal Health (DGAV).
This study was executed in two phases (Fig. 1).
Phase 1: RF energy was delivered to one renal artery of twelve domestic swine (nine males Duroc X and three females F1 Large White X Landrace, 31 ± 1.2 kg) using the contralateral artery as the histological control and the contralateral kidney as the Ep/NE control. The animals were fasted 12 h prior to the procedure. Two certified veterinarians were present in every procedure and were responsible for the administration of anesthetic drugs, which included 2 mg/kg of azaperone, 0.04 mg/kg of atropine, and 0.2 mL/kg of ketamine, via an intramuscular route. In the catheterization laboratory, venous access was obtained from the marginal ear vein, followed by the administration of 6 mg/kg of 5% sodium thiopental with subsequent orotracheal intubation and mechanical ventilation. Anesthetic maintenance was obtained through the administration of volatile 2% isoflurane in a continuous oxygen flow. A 0.9% saline infusion was kept flowing until the end of the procedure, and a thermal blanket was used for temperature control. Anticoagulation with heparin was used to achieve an activation clotting time > 250 s. An 8-F sheath was placed in the right femoral artery, and selective catheterization of the renal arteries was performed under fluoroscopic guidance. A basal angiogram was obtained with prior administration of 0.5–1 mg isosorbide dinitrate to determine suitability of the artery. Suitable arteries were required to be ≥4 mm in diameter and ≥20 mm in length. OCT was performed pre- and post RDN. The multi-electrode catheter was placed prior to the bifurcation, and 6–8 ablation points were delivered. A final angiogram was obtained, and a percutaneous closure device was used in the femoral puncture for rapid hemostasis. Continuous hemodynamic monitoring was maintained during the procedure. Rimadyl ® was used in the following 3 days for analgesia. Acetylsalicylic acid (100 mg) and antibiotics were administered empirically in the following 7 days. No complications or adverse effects were observed in the animals in either of the two study phases.
Phase 2: Medium-term efficacy and safety were assessed by renal angiography and OCT evaluation 1 month after RDN. Subsequently, the animals were euthanized according to the standard protocols, with intravenous sodium thiopental. Both kidneys, including the renal vasculature with peri-adventitial tissue, and abdominal aorta were harvested for histological purposes. The samples were embedded in optimal cutting temperature (Sakura® Finetek Inc, Torrance, CA, USA) compound and transported frozen in dry ice. The animals underwent a brief necropsy to exclude any pathological conditions (none found).
Technique description
Renal denervation system
The EnligHTNTM Multi-Electrode RDN system (St. Jude Medical, MN, USA) was used in this study. The ablation catheter consists of an expandable basket with four electrodes, providing a 60-s ablation time per electrode, set with the purpose of obtaining a circumferential ablation pattern. The system allows the independent selection of electrodes. It is compatible with an 8-F sheath and is powered by the EnligHTN TM generator. Final OCT evaluation and a renal angiogram were obtained.
Optical coherence tomography
OCT was performed pre- and post RDN and at 1-month follow-up, using the non-occlusive acquisition technique, to access the presence of intraluminal disruptions such as spasm, thrombus, or dissection. Images were acquired using the IlumienTM OptisTM System and the DragonflyTM Imaging Catheter (St. Jude, MN, USA). The catheter was advanced over a standard 0.014ʹʹ guidewire, positioned distally to the major bifurcation, and pulled back automatically with simultaneous manual injection of a non-ionic contrast medium. OCT analysis was done by two independent and experienced analysts. Minimum, mean and maximum vessel areas and diameters were calculated through the analysis of cross-sectional images frame by frame, using the main bifurcation as the distal reference point. Lumen volume was calculated based on the minimum lumen area.
Histological analysis
The samples were removed from the dry ice, sectioned at 5 µm, and stored at −80 °C. Three artery regions, prebifurcation, were evaluated and compared with the contralateral side (proximal, medium, and distal). Fibrosis area was assessed in frozen sections with Masson’s trichrome staining, according to the instructions of the Trichrome Stain kit (Abcam, Cambridge, MA, USA). Briefly, frozen slides were fixed with Bouin’s solution. After incubation in Weigert’s iron hematoxylin solution, the slides were stained with Biebrich Scarlet-Acid Fuchsin and Aniline Blue and dehydrated in ethanol and xylene. Extensive washes were done between each staining. The collagen fibers were stained blue, the nuclei were stained black and blue, and the cytoplasm was stained red. Artery sections were immunostained as follows: frozen sections were fixed with 4% paraformaldehyde, washed with phosphate-buffered saline, permeabilized with 0.2% (vol/vol) Triton X-100, and incubated in 2.5% bovine serum albumin to block unspecific staining. For TH immunodetection, sections were incubated with primary antibody (ab137869, Abcam, Cambridge, MA, USA) overnight at 4 °C. Thereafter, the samples were incubated with the secondary antibody for an additional hour at room temperature. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). The specimens were mounted with MOWIOL 4–88 Reagent (Calbiochem®). Fluorescence microscopy images were collected using a Zeiss Axio HXP IRE 2 microscope (Carl Zeiss AG, Jena, Germany).
Norepinephrine and epinephrine quantification in the renal cortex and medulla
NE and Ep were quantified in the renal cortex and medulla by an experienced researcher who was blinded to the treated artery. From each kidney, 100 mg of renal cortex and medulla were collected, distributed in Eppendorf tubesTM, sonicated in ice-cold 0.2 M perchloric acid and centrifuged. Supernatants were filtered using 0.2 µm Nylon microfilters (Costar® Spin-X® Centrifuge Tube Filter) and stored at −80 °C until further analyses. The pellet was resuspended in 1 M NaOH and stored at −80 °C for total protein quantification by the bicinchoninic acid protein assay (Thermo Fisher Scientific, MA, USA). A reversed-phase high-performance liquid chromatography method using isocratic elution and electrochemical detection was applied to quantify kidney NE and Ep levels, as described previously [11,12,13]. NE was quantified by an analytical cell (model 5011, ESA Analytical, Dorton Aylesbury, Buckinghamshire, UK) set at a potential of 0.25 V and attached to a Coulochem-II electrochemical detector. Ep was quantified by a glassy carbon electrode vs. Ag/AgCl reference electrode set at a potential of 0.75 V and attached to an amperometric electrochemical detector. A flow rate of 0.5 mL/min and a sensitivity of 100 nA were used to detect renal cortical and medulla NE (coulometric detector). A flow rate of 0.3 mL/min and a sensitivity of 0.5 nA were used to detect renal cortical and medulla Ep (amperometric detector). Catecholamine concentration in each sample was determined by comparison with peak areas of standards and is expressed in nanograms per milligram of protein.
Statistical analysis
This study was planned to evaluate independent cases, with one control per case, with a previously determined sample size. The statistical tests used in the different sets of data were Student’s t-test and the non-parametric Friedman test. All reported p values < 0.05 were considered statistically significant. Analyses were performed using SPSS statistics version 22 (IBM SPSS Statistics, IBM Corporation, Armonk, NY, USA) and GraphPad Prism version 5 (GraphPad Software, Inc.).
Results
All animals survived the procedure and were in good health after 1 month. A weight change from a baseline of 31 ± 1.2 to 43.8 ± 4.5 kg at follow-up was documented. No vascular complications on the renal angiogram or at the femoral level were observed. RDN was performed in 12 renal arteries (six in the left and six in the right); a total of 6–8 ablations were delivered to each artery (mean 6.3 ± 0.8).
OCT analysis
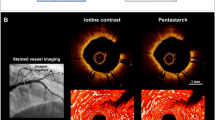
A total of 36 pullbacks were evaluated by two independent operators. All peri-procedural pullbacks had excellent quality, with good lumen and vessel layer visualization. At follow-up, 75% of the pullbacks had excellent quality, but 25% (three animals) were not analyzed due to the greater artery dimensions, which prevented full lumen/artery visualization. The lumen area, diameter and volume quantification are displayed in Table 1. Statistically significant differences were found between the minimum area, diameter, and volume in the three timings, mainly due to differences between post-procedural and follow-up values, probably indicating a higher degree of spasm at sites where the vessel is narrower. A normal and physiological vessel growth and healing after 1 month was observed. Post-procedurally, angiographically clear vessel notches were distinguished and correlated with the sites where RF energy was delivered. In OCT cross-sections, endothelial edema and vessel spasm were present in 100% of the arteries, without associated flow compromise. Intraluminal thrombus formation was present in 11 of the 12 treated arteries. One minor dissection was visualized (2 frames). At 1-month follow-up, all arteries appeared to be completely healed, as none of the previously described lesions or renal artery stenosis was present (Figs. 2 and 3).
Histological analysis
The presence of neuronal enervation was assessed by TH immunostaining in three zones of the renal artery, according to the distance from the bifurcation and the RF delivery. The images clearly showed that TH immunostaining in denervated arteries was significantly lower than in the non-denervated controls, in all three zones analyzed, suggesting a near absence of nerve terminals in the treated artery. The presence of fibrosis was evaluated using Masson's trichrome staining (in which blue color stains for collagen and red stains for muscle fibers). The images show that in denervated arteries, more collagen was embedded in the media, suggesting an increase in fibrosis (with the red layer (media) becoming less red and more blue). Collagen accumulation was quantified and revealed a statistically significant increase in collagen in the treated artery vs the control (Figs. 4–6).
Tyrosine hydroxylase immunostaining (THI) in three zones of the renal artery (a–f: control; g–l: denervated artery. THI is clearly lower in denervated areas than the control, translating a reduction in nervous terminals. Masson’s trichrome staining is shown in the right columns (a–c: control; d–f: denervated artery). Blue stain is collagen, whereas red stain is muscle fibers. In denervated areas, the amount of collagen embedded in the media is higher, translating into an increase in fibrosis (the red layer (media) becomes less red and more blue). Please refer HTML version for color figure
Norepinephrine and epinephrine assessment
NE and Ep levels in the renal cortex and medulla were similar when comparing the treated side to the contralateral untreated control. Overall, NE was significantly higher in the medulla than in the renal cortex, while Ep was similar in both regions (Fig. 7).
Discussion
Our study demonstrated several main findings: (1) RDN performed in the proximal/medial segment of the renal artery (prebifurcation), with a multi-electrode device, causes acute vessel wall changes, revealed by OCT, such as intimal disruption (edema/spasm) and intraluminal thrombus formation. (2) RDN appears to be safe in the medium term. At the 1-month follow-up, OCT revealed a completely healed vessel and the absence of ‘‘de novo’’ stenosis. The only dissection detected was no longer visible at this point in time. (3) The histological analysis revealed nearly absent TH immunostaining and a statistically significant increase in the amount of collagen fibers in the denervated artery, compatible with a decrease in nerve terminals and an increase in fibrosis, compared to the control, suggesting an efficacious delivery of the RF energy to the vessel wall. No differences were found in the NE or Ep renal tissue levels between the treated and contralateral kidney.
The recent HTN-3 trial [6, 7] raised several questions regarding the efficacy of RDN in the proximal main renal artery but is virtually the only negative trial among numerous randomized trials. Several aspects of the procedure and the Hawthorne effect are thought to have contributed to the unexpected results [14], and research is currently focusing on updating the present knowledge to achieve experimental and subsequent clinical success. Recent Ambulatory blood pressure monitoring (ABPM) data from the Simplicity Spyral Global registry are very encouraging (presented at EuroPCR 2016) and suggest distal (targeting the branches) RDN may be effective. Augmented sympathetic activity can also exert deleterious effects on the heart and the potential benefits of RDN in this setting are promising. Watanabe et al. [15] showed that, in hypertensive rats, RDN suppressed the development of left ventricular hypertrophy and was protective against renal damage, resulting in prolonged survival.
Renal anatomy and the sympathetic nerve distribution in the proximity of the renal vasculature was the pathophysiological basis for the development of RDN. Particularly important was the distance of the renal nerves to the vessel lumen and how they were distributed around the artery. Atherton et al. [9] demonstrated that there were fewer but larger nervous bundles in the proximal segment, which were distributed throughout the artery and became progressively smaller but more numerous in the distal segments. Therefore, it has been hypothesized that if the ablation points are closer to the ostium and directed to the superior side of the artery, the effectiveness of RDN is increased [16]. These recommendations were difficult to implement with the first-generation single-electrode devices, as this region is associated with higher catheter instability, which makes contact with the arterial wall difficult to achieve. However, more recent findings and an improved understanding of the renal anatomy refined the original technique. Roy et al. [17] examined three sections of post-mortem human renal arteries and found that although 77% and 22.5% of the nerves are located between 0.5–2.5 mm and 2.5–5 mm from the intimal layer, respectively, variations do occur, and in larger arteries with thicker parenchyma, the nerves are concentrated even further away from the lumen. Additionally, as nerve bundles have a three-dimensional distribution along the vessel, the authors suggest that a circumferential denervation pattern is preferential to an interrupted one in order to produce tissue damage beyond 2.5 mm. Sakakura et al. [10] showed that >75% of sympathetic nerves are located within 4.28 mm from the lumen and less frequently in a dorsal location compared to the ventral, superior and inferior regions. Furthermore, the authors found that even though there are fewer nerves in the distal segments compared to the proximal and middle segments, they are closer to the lumen. They proposed a diagram that reflects the circumferential peri-arterial nerve location. In pigs, Mahfoud et al. [18] demonstrated that delivering RF to the branches resulted in greater NE reductions than treating the main renal artery alone; the later significantly reduced NE concentrations, but without a clear dose response to the increasing number of RF lesions. The greatest decline in NE concentrations and reduction of axon density observed in this analysis was obtained from a combined treatment of the main artery and branches, an approach currently being investigated by two major clinical trials [19, 20]. The 3-month results of the proof-of-concept, sham-controlled randomized trial SPYRAL HTN-OFF MED were recently published [21] and revealed a significant decrease in office and 24-h blood pressure in the RDN group compared to the sham control, suggesting RDN is effective in the treatment of hypertension.
NE, a neurotransmitter of the sympathetic nervous system, has been used in several human (NE spillover) and experimental (renal tissue quantification) studies to evaluate the efficacy and magnitude of RDN. Henegar et al. [22] evaluated whether RDN performed in different segments of the renal artery in pigs led to variable NE renal tissue concentrations and concluded that RDN performed in the branches, closer to the kidney, produced the greatest NE reductions. However, this study did not evaluate procedural safety.
RDN appears to be safe, although concerns regarding the rare occurrence of renal stenosis during follow-up have been raised by a few published case reports [23, 24]. In the last 15 years, OCT has become an important technology in the evaluation of coronary artery structure, by overcoming the limited spatial resolution and drawbacks of Intravascular ultrasound (IVUS). Not only is it utilized in the assessment of coronary vulnerable plaques, lumen geometry, and to guide coronary intervention, but it can also evaluate intraluminal repercussions of RDN, particularly the presence of spasm, intimal injury, or thrombus formation. The evaluation of human renal arteries with OCT is clearly more challenging; due to vessel size, a complete removal of blood cells is difficult to achieve, and proper visualization of layers is limited by an insufficient depth penetration. Nevertheless, a few small observational studies have disclosed some data concerning this subject. Templin et al. [25] performed RDN with two different denervation systems (Symplicity® and EnligHTNTM) in 16 patients, with OCT evaluation pre- and post procedure; spasm and edema occurred with both devices, although the greatest amount of thrombus formation occurred with the EnligHTNTM and one dissection with the Symplicity®.
In this study, we used a multi-electrode device to achieve a more circumferential ablation pattern. Pre-clinical anatomical studies performed in pigs [26], whose anatomical similarities with the human cardiovascular system have proven to be useful for the development of several techniques, revealed a much higher concentration of nervous bundles in the proximal arterial segments, and predominantly in the right side. Several studies have concentrated on determining the exact position of peri-arterial nerves, on identifying the ones involved in the pathophysiology of resistant HT and on determining the depth range of ablation devices. Vink et al. [27] evaluated histopathologically a human patient and determined the RF damage did not penetrate deeper than 2 mm. Therefore, an incomplete denervation potentially leads to a lack of renal tissue catecholamine reduction. Our study demonstrated similar NE and Ep concentrations between both sides, which we hypothesize was due to the following explanations: (1) there was not enough contact with the arterial wall, and RF energy was not delivered properly. Evidence against this is that the OCT analysis showed a clear edematous swelling in this segment, with a reduction in the area and diameter acutely (spasm) and thrombus formation evident in most of the cases. Additionally, the histological analysis revealed a significant increase in collagen concentration in the denervated sections, a finding consistent with the intra-arterial imaging. (2) The RF energy was adequately delivered (TH immunostaining showed a near absence of nervous terminals in the denervated side), but the distance from the larger nerves to the arterial lumen was higher than previously described. (3) The number of ablations (mean 6.3 per artery) was not enough and was therefore unable to achieve a circumferential pattern in the artery, leading to incomplete denervation. In SPYRAL HTN-OFF MED, a mean of 43.8 ablations were performed. (4) Afferent sensory nerve fibers communicate with the contralateral kidney to maintain diuresis and natriuresis despite unilateral disturbances (renorenal reflex) [28]. Since RF energy was only delivered to one renal artery, after 1 month of follow-up, adaptive mechanisms could have brought NE and Ep levels back to normal, which validates the need to denervate both sides systematically. (5) A combination of the described mechanisms is probably the most likely explanation.
Implications of the current evidence in daily practice
RDN with a multi-electrode device leads to fibrosis and reduced nerve terminals and appears to have a favorable medium-term safety profile.
The authors hypothesize, according to the current study and the available evidence, that a higher number of ablations, with a more even and circumferential distribution throughout the artery, should be performed to achieve a favorable hemodynamic outcome.
These results provide imaging and histopathological evidence of the effect of unilateral RDN, in a small group of subjects, with the previously described method.
Limitations
Several limitations are present in this study. First, this was an observational study in which RDN was performed in a small number of animals. The distance between nerve fascicles to the arterial lumen was not evaluated. NE spillover was not assessed but could have been useful to compliment renal tissue measurements. Regarding safety, even though microthrombi were present peri-procedurally in most of the cases, the impact of this finding on renal function is unknown, as it was not assessed. Our study was performed in healthy, normotensive pigs, and hence, clinical outcomes were not analyzed.
Conclusions
Our study shows that RDN, when performed in the proximal/medial segment of the renal artery (prebifurcation) of a swine model, is associated with clear intraluminal disruptions, as assessed with optical coherence tomography, which are not present 1 month after the procedure. A statistically significant reduction in nervous terminals and an increase in areas of fibrosis were demonstrated by histological analysis, suggesting an efficacious application of the radiofrequency energy. No differences in NE or Ep renal tissue levels were found, probably due to an insufficient number of ablations and to the study design (unilateral treatment). Current research is very promising and suggests that a circumferential ablation pattern in both the main renal artery and the branches may yield more advantageous clinical/hemodynamic consequences.
References
Grassi G,Mark A,Esler M, The sympathetic nervous system alterations in human hypertension. Circ Res. 2015;116:976–90. https://doi.org/10.1161/CIRCRESAHA.116.303604.
Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicenter safety and proof-of-principle cohort study. Lancet. 2009;373:1275–81.
Krum H, Schlaich MP, Sobotka PA, Böhm M, Mahfoud F, Rocha-Singh K et al. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet. 2014;383:622–9.
Esler MD, Krum H, Schlaich M, Schmieder RE, Bohm M, Sobotka PA. Symplicity HTN-2 Investigators. Renal sympathetic denervation for treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126:2976–82.
Worthley SG, Tsioufis CP, Worthley MI, Sinhal A, Chew DP, Meredith IT et al. Safety and efficacy of a multi-electrode renal sympathetic denervation system in resistant hypertension: the EnligHTN I trial. Eur Heart J. 2013;34:2132–40.
Bakris GL, Townsend RR, Flack JM, Brar S, Cohen SA,D’Agostino R et al. SYMPLICITY HTN-3 Investigators. 12-Month blood pressure results of catheter-based renal artery denervation for resistant hypertension the SYMPLICITY HTN-3 Trial. J Am Coll Cardiol. 2015;65:1314–21.
Silva JD, Costa M, Gersh BJ, Gonçalves L. Renal denervation in the era of HTN-3. Comprehensive review and glimpse into the future. J Am Soc Hypertens. 2016;10:656–70. https://doi.org/10.1016/j.jash.2016.05.009.
Tsioufis C, Kordalis A, Flessas D, Anastasopoulos I, Tsiachris D, Papademetriou V et al. Pathophysiology of resistant hypertension: the role of sympathetic nervous system. Int J Hypertens. 2011;2011:642416 https://doi.org/10.4061/2011/642416.
Atherton DS, Deep NL, Mendelsohn FO. Micro-anatomy of the renal sympathetic nervous system: a human postmortem histologic study. Clin Anat. 2012;25:628–33. https://doi.org/10.1002/ca.21280.
Sakakura K, Ladich E, Cheng Q, Otsuka F, Yahagi K, Fowler DR et al. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J Am Coll Cardiol. 2014;64:635–43. https://doi.org/10.1016/j.jacc.2014.03.059.
Pereira FC, Lourenço ES, Borges F, Morgadinho T, Ribeiro CF, Macedo TR et al. Single or multiple injections of methamphetamine increased dopamine turnover but did not decrease tyrosine hydroxylase levels or cleave caspase-3 in caudate-putamen. Synapse. 2006;60:185–93.
Pereira FC, Gough B, Macedo TR, Ribeiro CF, Ali SF, Binienda ZK. Buprenorphine modulates methamphetamine-induced dopamine dynamics in the rat caudate nucleus. Neurotox Res. 2011;19:94–101. https://doi.org/10.1007/s12640-009-9143-9.
Reis F, Rocha L, Ponte L, Alcobia T, Almeida L, Costa-Almeida C et al. Effect of preventive and regressive isosorbide 5-mononitrate treatment on catecholamine levels in plasma, platelets, adrenals, left ventricle and aorta in cyclosporin A-induced hypertensive rats. Life Sci. 2005;77:2514–28. https://doi.org/10.1016/j.lfs.2005.01.032
Pocock SJ, Gersh BJ. Do current clinical trials meet society’s needs?: a critical review of recent evidence. J Am Coll Cardiol. 2014;64:1615–28.
Watanabe H, Iwanaga Y, Miyaji Y, Yamamoto H, Miyazaki S. Renal denervation mitigates cardiac remodeling and renal damage in Dahl rats: a comparison with β-receptor blockade. Hypertens Res. 2016;39:217–26.
Bertog SC, Blessing E, Vaskelyte L, Hofmann I, Id D, Sievert H. Renal denervation: tips and tricks to perform a technically successful procedure. EuroIntervention . 2013;9:R83–8.
Roy AK, Fabre A, Cunningham M, Buckley U, Crotty T, Keane D. Post mortem study of the depth and circumferential location of sympathetic nerves in human renal arteries--implications for renal denervation catheter design. Catheter Cardiovasc Interv. 2015;86:E32–7. https://doi.org/10.1002/ccd.26035.
Mahfoud F, Tunev S, Ewen S, Cremers B, Ruwart J, Schulz-Jander D et al. Impact of lesion placement on efficacy and safety of catheter-based radiofrequency renal denervation. J Am Coll Cardiol. 2015;66:1766–75. https://doi.org/10.1016/j.jacc.2015.08.018.
Global Clinical Study of Renal Denervation With the Symplicity Spyral™ Multi-electrode Renal Denervation System in Patients With Uncontrolled Hypertension on Standard Medical Therapy (SPYRAL HTN-ON MED): https://clinicaltrials.gov/ct2/show/NCT02439775.
Global Clinical Study of Renal Denervation With the Symplicity Spyral™ Multi-electrode Renal Denervation System in Patients With Uncontrolled Hypertension in the Absence of Antihypertensive Medications (SPYRAL HTN-OFF MED): https://clinicaltrials.gov/ct2/show/NCT02439749.
Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017 Nov 11;390(10108):2160-2170. https://doi.org/10.1016/S0140-6736(17)32281-X. Epub 2017 Aug 28.
Henegar JR, Zhang Y, Hata C, Narciso I, Hall ME, Hall JE. Catheter-based radiofrequency renal denervation: location effects on renal norepinephrine. Am J Hypertens. 2015;28:909–14. https://doi.org/10.1093/ajh/hpu258.
Diego-Nieto A, Cruz-Gonzalez I, Martin-Moreiras J, Rama-Merchan JC, Rodriguez-Collado J, Sanchez-Fernandez PL. Severe renal artery stenosis after renal sympathetic denervation. JACC Cardiovasc Interv. 2015;8:e193–4. https://doi.org/10.1016/j.jcin.2015.05.022.
Jaén Águila F, Mediavilla García JD, Molina Navarro E, Vargas Hitos JA, Fernández-Torres C. Bilateral renal artery stenosis after renal denervation. Hypertension. 2014;63:e126–7. https://doi.org/10.1161/HYPERTENSIONAHA.113.03065.
Templin C, Jaguszewski M, Ghadri JR, Sudano I, Gaehwiler R, Hellermann JP et al. Vascular lesions induced by renal nerve ablation as assessed by optical coherence tomography: pre- and post-procedural comparison with the Simplicity catheter system and the EnligHTN multi-electrode renal denervation catheter. Eur Heart J. 2013;34:2141–8. https://doi.org/10.1093/eurheartj/eht141.
Tellez A, Rousselle S, Palmieri T, Rate WR 4th, Wicks J, Degrange A et al. Renal artery nerve distribution and density in the porcine model: biologic implications for the development of radiofrequency ablation therapies. Transl Res. 2013;162:381–9. https://doi.org/10.1016/j.trsl.2013.07.002.
Vink EE, Goldschmeding R, Vink A, Weggemans C, Bleijs RL, Blankestijn PJ. Limited destruction of renal nerves after catheter-based renal denervation: results of a human case study. Nephrol Dial Transplant. 2014;29:1608–10. https://doi.org/10.1093/ndt/gfu192.
Bertog SC, Sobotka PA, Sievert H. Renal denervation for hypertension. JACC Cardiovasc Interv. 2012;5:249–58.
Acknowledgements
We thank the interventional cardiologist Dr. António Fiarresga, the veterinarians Prof. Belmira Carrapiço and Prof. Sandra Cavaco Gonçalves, the biostatistician Dr. Adriana Belo, and the Cardiopulmonary Technician Paula Neves for significantly contributing to the completion of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Delgado-Silva, J., Fernandes, R., Pita, I.R. et al. Intravascular imaging, histopathological analysis, and catecholamine quantification following catheter-based renal denervation in a swine model: the impact of prebifurcation energy delivery. Hypertens Res 41, 708–717 (2018). https://doi.org/10.1038/s41440-018-0072-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0072-y
This article is cited by
-
Laparoscopic-based perivascular unilateral renal sympathetic nerve denervation for treating resistant hypertension: a case report
Hypertension Research (2019)
-
Observation of renal sympathetic nerves by intravascular ultrasound
Hypertension Research (2019)