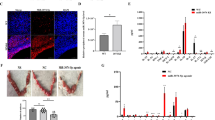
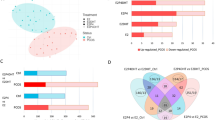
Abstract
Repeated implantation failure (RIF) is one of the most prominent problems in the field of assisted reproduction. Neu5Gc on the surface of decidual macrophages (dMΦ) leads to different activation patterns of dMΦ, which affects embryo implantation and development. Cmah−/− (Neu5Gc-deficient) mice induced to produce anti-Neu5Gc antibodies in vivo were given a special diet rich in Neu5Gc and their fertility was monitored. The long-term diet rich in Neu5Gc induced the decrease of endometrial receptivity of female mice. The pregnancy rate of female mice fed the normal diet was 63.6% (n = 11) and the average number of embryos was 9.571 ± 1.272, while the pregnancy rate of female mice fed the diet rich in Neu5Gc was 36.4% (n = 11) and the average number of embryos in pregnant mice was 5.750 ± 3.304. The intake of Neu5Gc and the production of anti-Neu5Gc antibody led to M1 polarization of endometrial dMΦ and abnormal embryo implantation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Coughlan C, Yuan X, Nafee T, Yan J, Mariee N, Li TC. The clinical characteristics of women with recurrent implantation failure. J Obstet Gynaecol. 2013;33:494–8.
Chen X, Diao L, Lian R, Qi L, Yu S, Liu S, et al. Potential impact of maternal vitamin D status on peripheral blood and endometrium cellular immunity in women with recurrent implantation failure. Am J Reprod Immunol. 2020;84:e13243.
Zhuang B, Shang J, Yao Y. HLA-G: an important mediator of maternal–fetal immune-tolerance. Front Immunol. 2021;12:744324.
Semmes EC, Coyne CB. Innate immune defenses at the maternal–fetal interface. Curr Opin Immunol. 2022;74:60–67.
Li Y, Zhang D, Xu L, Dong L, Zheng J, Lin Y, et al. Cell–cell contact with proinflammatory macrophages enhances the immunotherapeutic effect of mesenchymal stem cells in two abortion models. Cell Mol Immunol. 2019;16:908–20.
Sun F, Wang S, Du M. Functional regulation of decidual macrophages during pregnancy. J Reprod Immunol. 2021;143:103264.
Robertson SA, Care AS, Moldenhauer LM. Regulatory T cells in embryo implantation and the immune response to pregnancy. J Clin Investig. 2018;128:4224–35.
Meng YH, Zhou WJ, Jin LP, Liu LB, Chang KK, Mei J, et al. RANKL-mediated harmonious dialogue between fetus and mother guarantees smooth gestation by inducing decidual M2 macrophage polarization. Cell Death Dis. 2017;8:e3105.
Liao HQ, Han MT, Cheng W, Zhang C, Li H, Li MQ, et al. Decidual-derived RANKL facilitates macrophages accumulation and residence at the maternal–fetal interface in human early pregnancy. Am J Reprod Immunol. 2021;86:e13406.
Ono Y, Yoshino O, Hiraoka T, Sato E, Fukui Y, Ushijima A, et al. CD206+ M2-like macrophages are essential for successful implantation. Front Immunol. 2020;11:557184.
Eastman AJ, Vrana EN, Grimaldo MT, Jones AD, Rogers LM, Alcendor DJ, et al. Cytotrophoblasts suppress macrophage-mediated inflammation through a contact-dependent mechanism. Am J Reprod Immunol. 2021;85:e13352.
Okerblom J, Varki A. Biochemical, cellular, physiological, and pathological consequences of human loss of N-glycolylneuraminic acid. ChemBioChem. 2017;18:1155–71.
Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, et al. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci USA. 1998;95:11751–6.
Irie A, Koyama S, Kozutsumi Y, Kawasaki T, Suzuki A. The molecular basis for the absence of N-glycolylneuraminic acid in humans. J Biol Chem. 1998;273:15866–71.
Martin MJ, Rayner JC, Gagneux P, Barnwell JW, Varki A. Evolution of human–chimpanzee differences in malaria susceptibility: relationship to human genetic loss of N-glycolylneuraminic acid. Proc Natl Acad Sci USA. 2005;102:12819–24.
Kawanishi K, Dhar C, Do R, Varki N, Gordts PLSM, Varki A. Human species-specific loss of CMP-N-acetylneuraminic acid hydroxylase enhances atherosclerosis via intrinsic and extrinsic mechanisms. Proc Natl Acad Sci USA. 2019;116:16036–45.
Martin PT, Kawanishi K, Ashbrook A, Golden B, Samraj A, Crowe KE, et al. Serum antibodies to N-glycolylneuraminic acid are elevated in Duchenne muscular dystrophy and correlate with increased disease pathology in Cmah−/−mdx mice. Am J Pathol. 2021;191:1474–86.
Haller M, Yin Y, Ma L. Development and utilization of human decidualization reporter cell line uncovers new modulators of female fertility. Proc Natl Acad Sci USA. 2019;116:19541–51.
Angata T. Possible influences of endogenous and exogenous ligands on the evolution of human Siglecs. Front Immunol. 2018;9:2885.
Martin PT, Golden B, Okerblom J, Camboni M, Chandrasekharan K, Xu R, et al. A comparative study of N-glycolylneuraminic acid (Neu5Gc) and cytotoxic T cell (CT) carbohydrate expression in normal and dystrophin-deficient dog and human skeletal muscle. PLoS ONE. 2014;9:e88226.
Taylor RE, Gregg CJ, Padler-Karavani V, Ghaderi D, Yu H, Huang S, et al. Novel mechanism for the generation of human xeno-autoantibodies against the nonhuman sialic acid N-glycolylneuraminic acid. J Exp Med. 2010;207:1637–46.
Ma F, Deng L, Secrest P, Shi L, Zhao J, Gagneux P. A mouse model for dietary xenosialitis: antibodies to xenoglycan can reduce fertility. J Biol Chem. 2016;291:18222–31.
Kawanishi K, Coker JK, Grunddal KV, Dhar C, Hsiao J, Zengler K, et al. Dietary Neu5Ac intervention protects against atherosclerosis associated with human-like Neu5Gc loss—brief report. Arterioscler Thromb Vasc Biol. 2021;41:2730–9.
Sroga JM, Wu DH, Ma F, Tecle E, Reynoso HS. Detection of the dietary xenoglycan N-glycolylneuraminic acid (Neu5Gc) and anti-Neu5Gc antibodies within reproductive tracts of male and female infertility subjects. Clin Obstet Gynecol Reprod Med. 2015;1:72–78.
Altman MO, Gagneux P. Absence of Neu5Gc and presence of anti-Neu5Gc antibodies in humans—an evolutionary perspective. Front Immunol. 2019;10:789.
Samraj AN, Pearce OM, Läubli H, Crittenden AN, Bergfeld AK, Banda K, et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci USA. 2015;112:542–7.
Alisson-Silva F, Kawanishi K, Varki A. Human risk of diseases associated with red meat intake: analysis of current theories and proposed role for metabolic incorporation of a non-human sialic acid. Mol Asp Med. 2016;51:16–30.
Ghaderi D, Springer SA, Ma F, Cohen M, Secrest P, Taylor RE, et al. Sexual selection by female immunity against paternal antigens can fix loss of function alleles. Proc Natl Acad Sci USA. 2011;108:17743–8.
Läubli H, Varki A. Sialic acid–binding immunoglobulin-like lectins (Siglecs) detect self-associated molecular patterns to regulate immune responses. Cell Mol Life Sci. 2020;77:593–605.
Meyer SJ, Linder AT, Brandl C, Nitschke L. B cell Siglecs—news on signaling and its interplay with ligand binding. Front Immunol. 2018;9:2820.
Brinkman-Van der Linden EC, Sjoberg ER, Juneja LR, Crocker PR, Varki N, Varki A. Loss of N-glycolylneuraminic acid in human evolution. Implications for sialic acid recognition by Siglecs. J Biol Chem. 2000;275:8633–40.
Duan S, Paulson JC. Siglecs as immune cell checkpoints in disease. Annu Rev Immunol. 2020;38:365–95.
Harris LK, Benagiano M, D’Elios MM, Brosens I, Benagiano G. Placental bed research: II. Functional and immunological investigations of the placental bed. Am J Obstet Gynecol. 2019;221:457–69.
Pérez S, Rius-Pérez S. Macrophage polarization and reprogramming in acute inflammation: a redox perspective. Antioxidants. 2022;11:1394.
Yang D, Yang L, Cai J, Hu X, Li H, Zhang X, et al. A sweet spot for macrophages: focusing on polarization. Pharmacol Res. 2021;167:105576.
Zhao Y, Mahajan G, Kothapalli CR, Sun XL. Sialylation status and mechanical properties of THP-1 macrophages upon LPS stimulation. Biochem Biophys Res Commun. 2019;518:573–8.
Song Y, Pan Q, Xiao J, Li W, Ma H, Chen H, et al. Sialidase of Glaesserella parasuis augments inflammatory response via desialylation and abrogation of negative regulation of Siglec-5. Infect Immun. 2021;89:e00696-20.
Zhu H, Hou CC, Luo LF, Hu YJ, Yang WX. Endometrial stromal cells and decidualized stromal cells: origins, transformation and functions. Gene. 2014;551:1–14.
Bany BM, Hamilton GS. Assessment of permeability barriers to macromolecules in the rodent endometrium at the onset of implantation. Methods Mol Biol. 2011;763:83–94.
Funding
This work was supported by National Natural Science Foundation of Fujian (2021J02036) and Medical Innovation in Fujian Province (2022CXA018). These funding sources played key supportive role for sample collection, molecular analysis of patient samples, and bioinformatics analysis.
Author information
Authors and Affiliations
Contributions
Q-CL and FG planned the project. Y-XL, H-LZ, LW, YW, and Q-CL conceived of and designed the study. Q-CL performed the sample collection. YW, FG, and Q-CL performed immunhistochemistry. Q-CL performed the expression analysis. All authors reviewed the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All experimental procedures in animal work were approved by the Ethical Committee of Fujian Medical University. This study was carried out in accordance with the recommendations of Medical ethics committee of the First Affiliated Hospital of Fujian Medical University. The protocol was approved by the Medical ethics committee of the First Affiliated Hospital of Fujian Medical University [2023] 521.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yue, W., Huiling, Z., Yuxin, L. et al. Neu5Gc regulates decidual macrophages leading to abnormal embryo implantation. Genes Immun 25, 149–157 (2024). https://doi.org/10.1038/s41435-024-00268-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41435-024-00268-5