Abstract
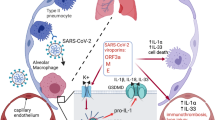
The utilization of host-cell machinery during SARS-CoV-2 infection can overwhelm the protein-folding capacity of the endoplasmic reticulum and activate the unfolded protein response (UPR). The IRE1α-XBP1 arm of the UPR could also be activated by viral RNA via Toll-like receptors. Based on these premises, a study to gain insight into the pathogenesis of COVID-19 disease was conducted using nasopharyngeal exudates and bronchioloalveolar aspirates. The presence of the mRNA of spliced XBP1 and a high expression of cytokine mRNAs were observed during active infection. TLR8 mRNA showed an overwhelming expression in comparison with TLR7 mRNA in bronchioloalveolar aspirates of COVID-19 patients, thus suggesting the presence of monocytes and monocyte-derived dendritic cells (MDDCs). In vitro experiments in MDDCs activated with ssRNA40, a synthetic mimic of SARS-CoV-2 RNA, showed induction of XBP1 splicing and the expression of proinflammatory cytokines. These responses were blunted by the IRE1α inhibitor MKC8866, the TLR8 antagonist CU-CPT9a, and knockdown of TLR8 receptor. In contrast, the IRE1α-XBP1 activator IXA4 enhanced these responses. Based on these findings, the TLR8/IRE1α system seems to play a significant role in the induction of the proinflammatory cytokines associated with severe COVID-19 disease and might be a druggable target to control cytokine storm.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data are available from the corresponding author on reasonable request.
References
Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383:2255–73.
Riva G, Nasillo V, Tagliafico E, Trenti T, Comoli P, Luppi M. COVID-19: More than a cytokine response. Crit Care. 2020;24:549.
Hetz C, Zhang K, Kaufman RJ. Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol. 2020;21:421–38.
Bettigole SE, Glimcher LH. Endoplasmic reticulum stress in immunity. Annu Rev Immunol. 2015;33:107–38.
Chen X, Cubillos-Ruiz JR. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat Rev Cancer. 2021;21:71–88.
Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–6.
Chopra S, Giovanelli P, Alvarado-Vazquez PA, Alonso S, Song M, Sandoval TA, et al. IRE1α-XBP1 Signaling in leukocytes controls prostaglandin biosynthesis and pain. Science. 2019;365:eaau6499.
Martinon F, Chen X, Lee AH, Glimcher LH. TLR Activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2011;11:411–18.
Zeng L, Liu YP, Sha H, Chen H, Qi L, Smith JA. XBP-1 couples endoplasmic reticulum stress to augmented IFN-β induction via a cis-acting enhancer in macrophages. J Immunol. 2010;185:2324–30.
Márquez S, Fernández JJ, Terán-Cabanillas E, Herrero C, Alonso S, Azogil A, et al. Endoplasmic reticulum stress sensor IRE1α enhances IL-23 expression by human dendritic cells. Front Immunol. 2017;8:639.
Mogilenko DA, Haas JT, L’homme L, Fleur S, Quemener S, Levavasseur M, et al. Metabolic and innate immune cues merge into a specific inflammatory response via the UPR. Cell. 2019;177:1201–16.
Awasthi D, Chopra S, Cho BA, Emmanuelli A, Sandoval TA, Hwang SM, et al. Inflammatory ER stress responses dictate the immunopathogenic progression of systemic candidiasis. J Clin Invest. 2023;133:e167359.
Prasad V, Greber UF. The endoplasmic reticulum unfolded protein response - homeostasis, cell death and evolution in virus infections. FEMS Microbiol Rev. 2021;45:fuab016.
Hrincius ER, Liedmann S, Finkelstein D, Vogel P, Gansebom S, Samarasinghe AE, et al. Acute lung injury results from innate sensing of viruses by an ER stress pathway. Cell Rep. 2015;11:1591–603.
Liu N, Jiang C, Cai P, Shen Z, Sun W, Xu HM, et al. Single-cell analysis of COVID-19, sepsis, and HIV infection reveals hyperinflammatory and immunosuppressive signatures in monocytes. Cell Rep. 2021;37:109793.
Ren X, Wen W, Fan X, Hou W, Su B, Cai P, et al. COVID-19 immune features revealed by a large-scale single-cell transcriptome atlas. Cell. 2021;184:1895–19.
Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;117:10970–5.
Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, Chopra S, Perales-Puchalt A, Song MS, et al. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell. 2015;161:1527–38.
Rosen DA, Seki SN, Fernández-Castañeda A, Beiter RM, Eccles JD, Woodfolk JA, et al. Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci Transl Med. 2019;11:eaau5266.
Echavarría-Consuegra L, Cook GM, Busnadiego I, Lefèvre C, Keep S, Brown K, et al. Manipulation of the unfolded protein response: a pharmacological strategy against coronavirus infection. PLoS Pathog. 2021;17:e1009644.
Nguyen LC, Yang D, Nicolaescu V, Best TJ, Gula H, Saxena D, et al. Cannabidiol inhibits SARS-CoV-2 replication through induction of the host ER stress and innate immune responses. Sci Adv. 2022;8:eabi6110.
Nguyen LC, Renner DM, Silva D, Yang D, Parenti NA, Medina KM, et al. SARS-CoV-2 diverges from other betacoronaviruses in only partially activating the IRE1α/XBP1 endoplasmic reticulum stress pathway in human lung-derived cells. mBio 2022;20:e0241522.29.
Roach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD, et al. The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci USA. 2005;102:9577–82.
van der Made CI, Simons A, Schuurs-Hoeijmakers J, van den Heuvel G, Mantere T, Kersten S, et al. Presence of genetic variants among young men with severe COVID-19. JAMA. 2020;324:663–73.
van Essen MF, Schlagwein N, van Gijlswijk-Janssen GJ, Anholts JDH, Eikmans M, Ruben JM, et al. Culture medium used during small interfering RNA (siRNA) transfection determines the maturation status of dendritic cells. J Immunol Methods. 2020;479:112748.
Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–59.
Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–33.
Krähling V, Stein DA, Spiegel M, Weber F, Mühlberger E. Severe acute respiratory syndrome coronavirus triggers apoptosis via protein kinase R but is resistant to its antiviral activity. J Virol. 2009;83:2298–309.
Adachi Y, Yamamoto K, Okada T, Yoshida H, Harada A, Mori K. ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell Struct Funct. 2008;33:75–89.
Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, et al. ATF6α optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell. 2007;13:351–64.
Koshiba R, Yanai H, Matsuda A, Goto A, Nakajima A, Negishi H, et al. Regulation of cooperative function of the Il12b enhancer and promoter by the interferon regulatory factors 3 and 5. Biochem Biophys Res Commun. 2013;430:95–100.
Rodríguez M, Domingo E, Alonso S, Frade JG, Eiros J, Crespo MS, et al. The unfolded protein response and the phosphorylations of activating transcription factor 2 in the trans-activation of il23a promoter produced by β-glucans. J Biol Chem. 2014;289:22942–57.
Song R, Gao Y, Dozmorov I, Malladi V, Saha I, McDaniel MM, et al. IRF1 governs the differential interferon-stimulated gene responses in human monocytes and macrophages by regulating chromatin accessibility. Cell Rep. 2021;34:108891.
Karki R, Sharma BR, Tuladhar S, Williams EP, Zalduondo L, Samir P, et al. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 2021;184:149–68.
Mimura N, Fulciniti M, Gorgun G, Tai YT, Cirstea D, Santo L, et al. Blockade of XBP1 splicing by inhibition of IRE1α is a promising therapeutic option in multiple myeloma. Blood. 2012;119:5772–81.
Madhavan A, Kok BP, Rius B, Grandjean JMD, Alabi A, Albert VA, et al. Pharmacologic IRE1/XBP1s activation promotes systemic adaptive remodeling in obesity. Nat Commun. 2022;13:608.
Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, et al. XBP1 controls diverse cell-type and condition-specific transcriptional regulatory networks. Mol Cell. 2007;27:53–66.
Zhou Z, Ren L, Zhang L, Zhong J, Xiao Y, Jia Z, et al. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe. 2020;27:883–990.
Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;26:842–4.
Sarma A, Christenson SA, Byrne A, Mick E, Pisco AO, DeVoe C, et al. Tracheal aspirate RNA sequencing identifies distinct immunological features of COVID-19 ARDS. Nat Commun. 2021;12:5152.
Engel JJ, van der Made CI, Keur N, Setiabudiawan T, Röring RJ, Damoraki G, et al. Dexamethasone attenuates interferon-related cytokine hyperresponsiveness in COVID-19 patients. Front Immunol. 2023;14:1233318.
Reis G, dos Santos Moreira-Silva EA, Silva DCM, Thabane L, Milagres AC, Ferreira TS, et al. Effect of hearly treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health. 2022;10:e42–e51.
Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000.
Li T, Yang Y, Li Y, Wang Z, Ma F, Luo R, et al. Platelets mediate inflammatory monocyte activation by SARS-CoV-2 spike protein. J Clin Invest. 2022;132:e150101.
Assil S, Coléon S, Dong C, Décembre E, Sherry L, Allatif O, et al. Plasmacytoid dendritic cells and infected cells form an interferogenic synapse required for antiviral responses. Cell Host Microbe. 2019;25:730–45.
Yun TJ, Igarashi S, Zhao H, Perez OA, Pereira MR, Zorn E, et al. Human plasmacytoid dendritic cells mount a distinct antiviral response to virus-infected cells. Sci Immunol. 2021;6:eabc7302.
Marongiu L, Protti G, Facchini FA, Valache M, Mingozzi F, Ranzani V, et al. Maturation signatures of conventional dendritic cell subtypes in covid-19 suggest direct viral sensing. Eur J Immunol. 2022;52:109–22.
Van der Sluis RM, Holm CK, Jakobsen MR. Plasmacytoid dendritic cells during COVID-19: Ally or adversary? Cell Rep. 2022;40:111148.
Laurent P, Yang C, Rendeiro AF, Nilsson-Payant BE, Carrau L, Chandar V, et al. Sensing of SARS-CoV-2 by pDCs and their subsequent production of IFN-I contribute to macrophage-induced cytokine storm during COVID-19. Sci Immunol. 2022;75:eadd4906.
Alameh MG, Tombácz I, Bettini E, Lederer K, Sittplangkoon C, Wilmore JR, et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity. 2021;54:2877–7.
Li Y, Chen M, Cao H, Zhu Y, Zheng J, Zhou H. Extraordinary GU-rich single-strand RNA identified from SARS coronavirus contributes an excessive innate immune response. Microbes Infect. 2013;15:88–95.
Moreno-Eutimio MA, López-Macías C, Pastelin-Palacios R. Bioinformatic analysis and identification of single-stranded RNA sequences recognized by TLR7/8 in the SARS-CoV-2, SARS-CoV, and MERS-CoV genomes. Microbes Infect. 2020;22:226–9.
Campbell GR, To RK, Hanna J, Spector SA. SARS-CoV-2, SARS-CoV-1, and HIV-1 derived ssRNA sequences activate the NLRP3 inflammasome in human macrophages through a non-classical pathway. iScience. 2021;24:102295.
Lee J, Sun C, Zhou Y, Lee J, Gokalp D, Herrema H, et al. p38 MAPK-mediated regulation of Xbp1s is crucial for glucose homeostasis. Nat Med. 2011;17:1251–60.
Liu J, Ibi D, Taniguchi K, Lee J, Herrema H, Akosman BP, et al. Inflammation improves glucose homeostasis through IKKβ-XBP1s interaction. Cell. 2016;167:1052–66.
Temesgen Z, Burger CD, Baker J, Polk C, Libertin CR, Kelley CF, et al. LIVE-AIR Study Group. Lenzilumab in hospitalised patients with COVID-19 pneumonia (LIVE-AIR): a phase 3, randomised, placebo-controlled trial. Lancet Respir Med. 2022;10:237–46.
Acknowledgements
Biobanco del Centro de Hemoterapia y Hemodonación de Castilla y León is thanked for providing buffy coats. Staff from the Intensive Care Unit of Hospital Clínico Universitario de Valladolid is thanked for the effort devoted to patient follow-up care and sample collection. BioRender.com software was used in some figures.
Funding
This work was supported by Junta de Castilla y León/Fondo Social Europeo. Valladolid Section of Asociación Española contra el Cáncer. Fondo COVID-19 del Instituto de Salud Carlos III/Junta de Castilla y León. European Commission-NextGenerationEU, (Regulation EU 2020/2094), through CSIC’s Global Health Platform (PTI Salud Global). Plan Nacional de Salud y Farmacia Grant SAF2017-83079-R and Grant PID2020-113751RB-I00 funded by MCIN/AEI/ 10.13039/501100011033. Junta de Castilla y León/Fondo Social Europeo Grants. CSI035P17 and VA175P20. Proyecto SEAHORSE INFRARED: IR2020-1-UVA05.
Author information
Authors and Affiliations
Contributions
JJF, CM, SG, GM, YA, SA, TA, and OM designed and performed experiments and interpreted data. CM and TAS carried out real-time metabolic analysis. YA performed flow cytometry assays. JJF and SA carried out ChIP assays. LI, JB, AO, OM, JRCR, EBM, NF, and MSC guided the overall project design and assisted in data interpretation and writing of the manuscript. All authors approved the content of the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
JRCR holds patents on the use of IRE1α modulators for the treatment of disease and serves as scientific consultant for Immagene B.V., NextRNA Therapeutics, Inc., and Autoimmunity Biologic Solutions, Inc. All other authors declare no potential conflicts of interest.
Ethical approval
The clinical part of the study was approved by the Ethics Committee of Area de Salud Valladolid Este (ref. PI-GR-20-2011 COVID). For in vitro experiments, MDDCs were obtained from human mononuclear cells collected from pooled buffy coats of healthy donors provided by Centro de Hemoterapia y Hemodonación de Castilla y León Biobank. The study was approved by the Bioethical Committee of the Spanish Council of Research (CSIC) and the written informed consent of all healthy donors was obtained at Centro de Hemoterapia y Hemodonación de Castilla y León Biobank. The process is documented by the Biobank authority according to the specific Spanish regulations. The ethics committee approved this procedure before starting the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fernández, J.J., Mancebo, C., Garcinuño, S. et al. Innate IRE1α-XBP1 activation by viral single-stranded RNA and its influence on lung cytokine production during SARS-CoV-2 pneumonia. Genes Immun 25, 43–54 (2024). https://doi.org/10.1038/s41435-023-00243-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41435-023-00243-6